Abstract
Context
Plyometric training has been successfully used in different sporting contexts. Studies that investigated the effect of plyometric training on muscle morphology are limited, and results are controversial with regard to which muscle fiber type is mainly affected.
Objective
To analyze the skeletal muscle structural and ultrastructural change induced by an acute bout of plyometric exercise to determine which type of muscle fibers is predominantly damaged.
Design
Descriptive laboratory study.
Setting
Research laboratory.
Patients or Other Participants
Eight healthy, untrained individuals (age = 22 ± 1 years, height = 179.2 ± 6.4 cm, weight = 78.9 ± 5.9 kg).
Intervention(s)
Participants completed an acute bout of plyometric exercise (10 sets of 10 squat-jumps with a 1-minute rest between sets).
Main Outcome Measure(s)
Blood samples were collected 9 days and immediately before and 6 hours and 1, 2, and 3 days after the acute intervention. Muscle samples were collected 9 days before and 3 days after the exercise intervention. Blood samples were analyzed for creatine kinase activity. Muscle biopsies were analyzed for damage using fluorescent and electron transmission microscopy.
Results
Creatine kinase activity peaked 1 day after the exercise bout (529.0 ± 317.8 U/L). Immunofluorescence revealed sarcolemmal damage in 155 of 1616 fibers analyzed. Mainly fast-twitch fibers were damaged. Within subgroups, 7.6% of type I fibers, 10.3% of type IIa fibers, and 14.3% of type IIx fibers were damaged as assessed by losses in dystrophin staining. Similar damage was prevalent in IIx and IIa fibers. Electron microscopy revealed clearly distinguishable moderate and severe sarcomere damage, with damage quantifiably predominant in type II muscle fibers of both the glycolytic and oxidative subtypes (86% and 84%, respectively, versus only 27% of slow-twitch fibers).
Conclusions
We provide direct evidence that a single bout of plyometric exercise affected mainly type II muscle fibers.
Key Words: eccentric exercise, sarcomere, Z-disk streaming, electron transmission microscopy, creatine kinase, dystrophin
Key Points
Plyometric exercise mainly affected the fast-twitch muscle fibers, damaging both the sarcolemma and the sarcomere at the site of the Z-disk.
Athletic trainers should avoid prescribing high-volume plyometric exercise bouts within quick succession or after other forms of high-intensity exercise that are known to stress the fast-twitch muscle fibers, so that athletes have sufficient time to regenerate damaged fibers.
Plyometrics refers to exercise that exploits the stretch-shortening cycle, which proceeds with the rapid stretch of a muscle (eccentric phase), followed by rapid (150-millisecond) shortening of the same muscle (concentric phase), which results in increased force and power output of the activated muscles.1 This type of training has been successfully used in different sporting contexts to improve strength, muscle power, coordination, and athletic performance by inducing greater motor-unit recruitment, enhanced reflex potentiation, and changes in the elastic properties of muscle and connective tissue.2,3 Studies investigating the effect of an acute bout of plyometric exercise on muscle morphology are limited, and the results are controversial with regard to which muscle fiber type is mainly affected, although research focused on muscle mechanics has indicated that a plyometric exercise bout is most likely to affect type II skeletal muscle fibers.4 Twist and Eston4 suggested that selective damage of type II muscle fibers was induced because they observed a reduction in the force-velocity relationship in athletes after an acute bout of plyometric exercise. Apart from this indirect suggestion, no other authors to our knowledge have reported the effect of an acute bout of plyometric exercise on the sarcomere structure of specific muscle fiber types.
We hypothesized that plyometric exercise preferentially damages the fast muscle fibers from the unaccustomed rapid stretch of the muscle occurring during the stretch-shortening cycle and the subsequent power requirements for jumping. The purpose of our study was to analyze the skeletal muscle structural and ultrastructural changes induced by an acute bout of plyometric exercise to determine which types of muscle fibers are predominantly damaged.
METHODS
Participants
Eight healthy, sedentary males (age = 22 ± 1 years, height = 179.2 ± 6.4 cm, weight = 78.9 ± 5.9 kg) volunteered to participate in this investigation. Volunteers were informed of the experimental procedures and associated risks before providing written informed consent. This study was approved by the Institutional Review Board for the Protection of Human Participants of Stellenbosch University. All participants completed a health history questionnaire, were healthy, and had no medical contraindications or any muscle or lower limb injury in the previous 6 months. No participants were currently or chronically treated by any corticosteroid-containing medication (including inhaled forms). The participants were asked and reminded to refrain from unaccustomed exercise or vigorous physical activity, to maintain their normal dietary habits, and to not take any anti-inflammatory drugs (eg, nonsteroidal anti-inflammatory drugs) or nutritional supplements (eg, vitamins, protein, or amino acids) during the experimental period. None took any pain or anti-inflammatory medication or performed any vigorous exercise during the study except for the plyometric intervention itself.
Experimental Procedure
Baseline measurements (blood samples, perceived muscle soreness scores, and muscle biopsies) were collected 9 days before the exercise intervention. Blood samples were drawn, and perceived muscle soreness scores were assessed immediately before and 6 hours after the exercise intervention as well as on days 1, 2, and 3; a postintervention muscle sample was collected on day 3.
Plyometric Exercise
Before the exercise intervention, participants completed a brief warm-up, which consisted of 5 minutes of backward and forward running, followed by 5 minutes of general stretching of the leg muscles. They performed 10 sets of 10 maximal squat jumps, separated by 60 seconds of recovery time between sets. This protocol has been used successfully to induce muscle damage in the knee extensor muscles in previous studies.4,5 Before starting the exercise, each participant performed a maximal squat jump, and the maximum height that the top of the head reached was taken as the highest point of the jump. The minimum target height for each jump was then calculated at 95% of this height. The head height reached by the participant during the jump was determined by the researcher, who stood on a chair next to a wall measured in 1-mm increments. The 95% mark was in the researcher's line of sight. On landing, participants were instructed to keep the trunk in an upright position, minimizing hip flexion while adopting a knee joint angle of approximately 90°.4 During the jumps, participants were free to swing their arms to maintain balance and generate momentum with each jump. Between sets, participants were allowed to move around freely, sit down, or perform light stretches. The jump technique was observed by the researchers, and if a participant could not maintain his minimum jump height, trunk position, or 90° knee-joint angle, he was stopped and given a 1-minute rest period before completing that set, thereby completing all 100 jumps. All participants performed failed jumps (range, 2 to 8), but failed jumps were not counted. The participants were allowed to perform short periods (<60 seconds) of static stretching. Such stretching has recently been reported6 in a systematic review to not compromise maximal muscle performance, and evidence that this duration of stretching could possibly provide protection against mechanical injury is poor.7 Nonetheless, many participants felt comfortable with this action between sets. The bout of plyometric exercise lasted approximately 20 minutes.
Blood Sampling
After an equilibration time of 5 minutes in the supine position for each participant, blood samples were drawn from the antecubital vein and creatine kinase (CK) activity measured. Serum CK activity was determined by a pathology laboratory using a 1-step sandwich assay (Access CK-MB assay; Beckman Coulter, Inc, Pinetown, South Africa).
Delayed-Onset Muscle Soreness (DOMS)
Perceived muscle soreness scores were taken in 2 positions (standing and squatting), using a visual pain scale, to indicate soreness of the knee extensors.8 Perceived muscle soreness scores were 0, none; 2, discomfort; 4, annoying; 6, horrible; 8, dreadful; 10, agonizing. A categorical visual pain scale was placed in front of the participant to record the perceived muscle soreness while in the standing and squat positions.
Muscle Biopsy and Sample Preparation
A baseline biopsy was obtained from each participant from a randomly selected leg 9 days before the exercise protocol was performed; a second biopsy was obtained on the third day after the plyometric exercise intervention from the leg that was not biopsied the first time. The biopsied leg was switched to ensure that the structural and ultrastructural damage observed was induced by the exercise intervention and not by the previous biopsy procedure. The muscle biopsies were obtained from the vastus lateralis muscle in a similar position for all participants as described by Kohn et al9 using the suction-assisted technique.
The vastus lateralis muscle was chosen as the site for the muscle biopsy because, among all of the muscles activated10 during the squat jump, it is easiest to access and is routinely biopsied in our laboratory. The biopsy was split into 3 parts: 1 part was frozen in liquid nitrogen for subsequent preparation of a homogenate for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis; 1 part was embedded in tissue freezing medium and frozen in isopentane (cooled in liquid nitrogen) for subsequent cryosectioning and immunofluorescent microscopy; and 1 part (1 × 3 mm) was fixed in 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide for 1 hour, after which samples were dehydrated with graded ethanol (30%, 50%, 70%, 95%, 100%). The dehydrated muscle piece was put in propylene oxide for 30 minutes and embedded into resin (model EPON 812; Electron Microscopy Sciences, Hatfield, PA) with passages of 1:3, 2:2, and 3:1 resin to propylene oxide, then pure resin (24 hours) before inclusion at 50°C for 48 hours. Ultrathin (50- to 70-nm) longitudinal sections were cut with an ultramicrotome (model RM2125 RT; Leica Microsystem Nussloch GmbH, Germany).
SDS-PAGE Gel
Myosin heavy chain isoform content of homogenates of baseline samples was determined using SDS-PAGE according to the method of Kohn et al9 to determine the fiber type percentage. Gels were stained with Coomassie blue R250 and scanned, and the relative percentages of the bands were quantified using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MD).
Immunofluorescence Staining
Cross-sections of the muscle tissue were cut at 10 μm using a cryostat microtome (model CM1100; Leica Microsystem Nussloch GmbH, Wetzlar, Germany) at −22°C and mounted on slides. The next day, the sections were brought to room temperature and rinsed in 0.01-M phosphate buffered saline (PBS) containing 0.25% Triton X-100 (15 minutes) and washed with PBS (3 × 5 minutes). The sections were incubated with Myosin heavy chain II (MHC II; 1:250, A4.74 mouse monoclonal antibody; Developmental Studies Hybridoma Bank, Iowa City, IA) for 1 hour, after which the sections were washed with PBS (3 × 5 minutes), incubated for 1 hour at room temperature with Alexa fluor 488 conjugated secondary antibody (1:250 goat antimouse; Invitrogen, Eugene, OR), washed again with PBS (3 × 5 minutes), incubated for 1 hour at room temperature with dystrophin (1:250 rabbit polyclonal; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), washed again with PBS (3 × 5 min) and incubated for 1 hour at RT with Alexa fluor 594 conjugated secondary antibody (1:250, goat anti-rabbit, Invitrogen, Eugene). The sections were then washed in PBS (3 × 5 min) and mounted with the fluorescent mounting medium (Dako; GLostrup, Denmark). Samples were observed with a direct fluorescence microscope (model DM 5000 CTR; Leica Microsystem Nussloch GmbH) with ×10 and ×20 objectives. The researchers (F.M. and A.W.I.) observed fibers for positivity to MHC II antibody (MHC I, negative; MHC IIx, faintly positive; MHC IIa, strongly positive) to calculate fiber type percentage while also assessing the fibers for loss of dystrophin staining to determine which were damaged. When the loss in dystrophin staining was localized in the sarcolemmas of 2 adjacent fibers and it was impossible to determine with absolute certainty which fiber was damaged, both fibers were considered damaged. For each muscle sample, 3 sections were stained for MHC II and dystrophin, and the best section was used to perform the analysis. A total of 1616 fibers were stained and analyzed.
Transmission Electron Microscopy Analysis
Ultrathin longitudinal sections were placed on the grids and stained with uranyl acetate (5 minutes) followed by lead citrate (5 minutes), as routinely done for electron microscopy analysis. Three grids were prepared for each sample and viewed with a transmission electron microscope (model 1011; JEOL-Jem, Tokyo, Japan). It was possible to observe between 2 and 4 longitudinal fibers in each grid. To ensure that the analyzed fibers were different, ultrathin sections were distanced approximately 20 μm from each other. A total of 54 fibers were analyzed. For each fiber, 4 micrographs were taken to calculate the mitochondria volume percentage (MV%; final magnification of 12 000) and measure the Z-disk widths (final magnification of 30 000). The MV% measurements were made in a random area but far from the sarcolemma. The Z-disk measurements were made at the region of filament overlap, where 10 randomly chosen straight Z-disks were outlined and the mean widths measured. Following the criteria for fiber typing of Prince et al,11 the fibers with a high MV% and wide Z-disk have been classified as slow-twitch oxidative (SO or type I fiber); the fibers with a high MV% and thin Z-disk have been classified as fast-twitch oxidative-glycolytic (FOG or type IIa fiber); whereas the fibers with a low MV% and thin Z-disk have been classified as fast-twitch glycolytic (FG or type IIx fiber).11,12 Sarcomere damage was easily noted and classified according to severity of Z-disk streaming.
Statistical Analysis
We analyzed changes in DOMS (in standing and squatting positions) and in CK activity over time using 1-way, repeated-measures analysis of variance (1 × 6). Significant differences were further evaluated by the post hoc Bonferroni test. Unpaired t tests were used to determine if the proportion of fibers damaged (no damage versus damage) had different MV% values or different Z-disk widths. Statistical analyses were performed using PASW (version 18; SPSS Inc, Chicago, IL). All data are presented as mean ± standard deviation, and the level of statistical significance was set at P < .05.
RESULTS
Serum CK Activity and DOMS
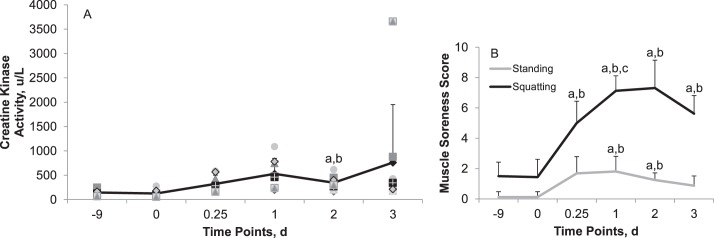
Plasma CK activity increased after the plyometric exercise intervention (Figure 1A). The mean elevation was highest at day 1 and then tended to return to baseline values.
Figure 1.
Changes in, A, plasma creatine kinase (CK) activity and, B, delayed-onset muscle soreness (DOMS) over time: 9 days (−9) and immediately before (0) the plyometric exercise intervention and 6 hours (0.25) and 1, 2, and 3 days after the exercise intervention. Symbols represent individual participant's values. Perceived muscle soreness scores: 0, none; 2, discomfort; 4, annoying; 6, horrible; 8, dreadful; 10, agonizing. a Different from −9 (P < .05). b Different from time 0 (P < .05). c Different from time 6 hours (P < 0.05). High interparticipant variability existed in plasma CK response after exertional muscle damage (see 3 days after exercise intervention), which could be attributed to genetic factors.13
The magnitude of muscle soreness perceived after the exercise intervention was experienced as higher in the squatting position than in the standing position (Figure 1B). In the standing position, DOMS peaked 1 day after exercise, whereas in the squatting position, DOMS peaked 2 days after exercise.
Immunofluorescence
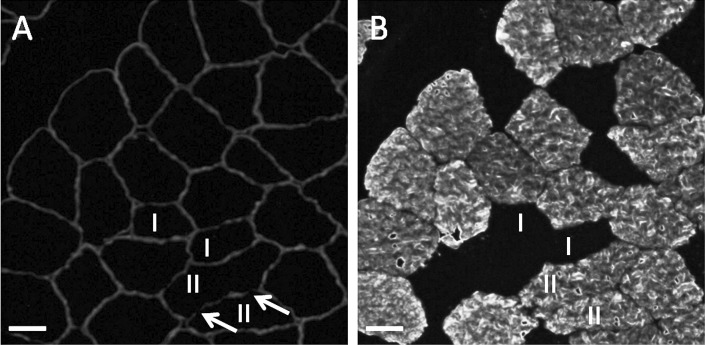
Loss of dystrophin staining was used to quantify muscle damage (Figure 2). Of 1616 total muscle fibers analyzed, only 155 (9.6 %) were damaged. Of the 155, 117 were fast twitch, representing 7.2% of all fibers. However, the fiber type distribution was unequal: 56 type IIx, 1060 type IIa, and 500 type I. The proportions of damaged fibers within the subgroups were also different, with most of the damage to the fast-twitch fibers: 14.3% of the type IIx fibers, 10.3% of IIa, and only 7.6% of type I.
Figure 2.
Immunofluorescence of cross-sections of muscle fibers from a muscle biopsy taken 3 days after the plyometric exercise intervention. A, Double immunostaining with antidystrophin and, B, anti-myosin heavy chain II. Arrows show the loss in dystrophin staining, II indicates MHC-II positive fibers, and I, MHC-I fibers. Scale bars = 30 μm.
Transmission Electron Microscopy
Each participant's postexercise muscle biopsy was used; 54 fibers were analyzed for MV% and the Z-disk width (Figure 3). A population of fibers was characterized by both high MV% values and wide Z disks. These fibers, according to the criteria described in the Methods section, were classified as SO fibers. All the remaining fibers had a large range of MV% values and narrow Z disks. A cutoff of 3.5 MV% was used to separate the FG and FOG fibers. We chose this value because it was the lower boundary for the MV% of SO fibers. Between 5 and 11 fibers per participant were evaluated; following these criteria, the mean fiber types quantified morphologically were 23.2% ± 28.7% FG, 49.2% ± 23.4% FOG, and 27.6% ± 18.8% SO. A similar percentage distribution was observed with the SDS-PAGE analysis (15.0 ± 3.6% type IIx, 51.1% ± 3.4% type IIa, 33.9% ± 3.6% type I) and the immunofluorescence analysis (5.3% ± 4.7% type IIx, 61.8% ± 15.2% type IIa, 32.9% ± 13.5% type I). The SO fibers identified using morphologic criteria had thicker Z lines (100.0 ± 6.4 nm) than the FOG and FG fibers (76.7 ± 6.2 nm and 77.7 ± 5.9 nm, respectively), whereas the FG fibers had lower MV% values (1.8% ± 0.7%) than the FOG and SO fibers (3.9% ± 1.0% and 4.3 ± 1.1%, respectively).
Figure 3.
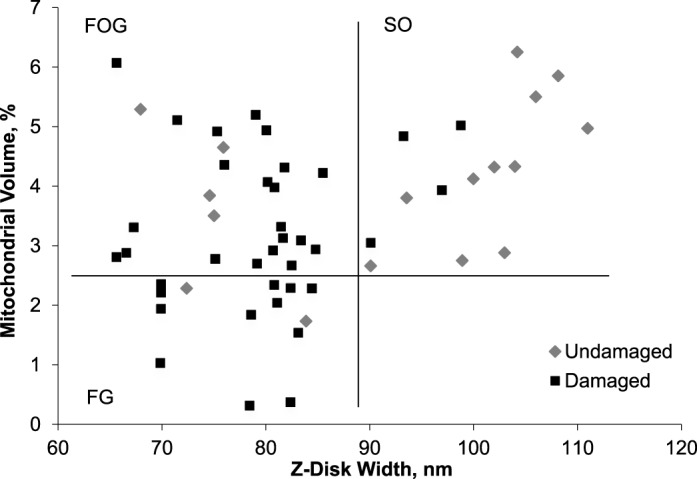
Mitochondrial volume percentage versus Z-disk width of each fiber postplyometric exercise (n = 54). Horizontal (mitochondrial volume % = 2.5) and vertical (90 nm) lines indicate the cutoff among the fast-twitch glycolytic (FG), fast-twitch oxidative-glycolic (FOG), and slow-twitch oxidative (SO) fibers. The quadrants thus formed separate fiber types (FG, FOG, and SO). ⋄ indicates undamaged fiber; ▪, damaged fiber.
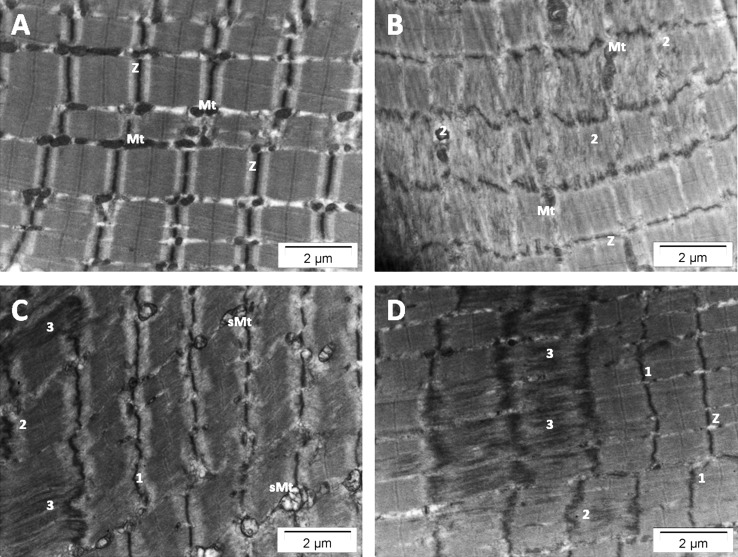
Fibers from the baseline biopsy did not show any ultrastructural damage in sarcomere organization. However, structural disorganization of several sarcomeres was observed in 37 of the 54 postexercise fibers analyzed. The sarcomere damage was focused in the region of filament overlap. Disruption of the Z-disk architecture was present, with streaming of the Z disk, and sometimes the structure appeared to be completely absent (Figure 4). In the former case, undamaged mitochondria were observed in the damaged area; only in the latter case was it impossible to visualize mitochondria in the area. In 2 FOG fibers only, swollen mitochondria were observed near the sarcomere with streamed Z disks. The damaged fibers had lower MV% values (3.2% ± 1.4%) and thinner Z disks (79.3 ± 1.4 nm) than the undamaged fibers (4.0 ± 1.3 MV% and 92.4 ± 14.4 nm, respectively; P < .02 and < .001, respectively). After the plyometric exercise intervention, 85.7% and 84.0%, respectively, of FG and FOG fibers had sarcomere damage, whereas only 26.7% of SO fibers were damaged.
Figure 4.
Electron micrographs of longitudinal skeletal muscle from a biopsy taken 3 days after the plyometric exercise intervention. A, Normal sarcomeres with regular alignment and regular banding patterns (Z, Z-disk). B, Damaged fiber showing Z-disk streaming with normal mitochondria (Mt). C, Damaged sarcomeres with swollen mitochondria (sMt). D, Disrupted sarcomeres showing complete absence of the Z disk and mitochondria. 1 = wavy appearance of the Z disk, 2 = mild Z-disk streaming, 3 = disintegration of the Z disk.
DISCUSSION
This study provides direct evidence that an acute bout of plyometric exercise preferentially affects type II fibers. Strengthening this conclusion is the fact that the evidence was obtained using 2 separate methods.
Until now, different indirect approaches have been used to provide evidence that plyometric exercise mainly affects type II fibers. Several authors2,14 investigated fiber-specific adaptations to longitudinal plyometric-training protocols, arguing that the adapted fibers would be those that had been damaged. These investigators reached the conclusion that the enhanced functional performance after plyometric training could be explained by changes in the contractile apparatus of all muscle fiber types, independent of fiber-specific characteristics. In contrast, we found that mainly fast-twitch fiber damage occurred, regardless of whether the profile was oxidative or glycolytic. A possible explanation for the previous findings is that the duration of exposure over weeks masked any acute damage or early adaptations to the fast-twitch fibers. Assessing performance up to 72 hours after an acute bout of plyometric exercise, Twist and Eston4 observed a reduction in the force-velocity relationship, which specifically suggested that selective damage of type II muscle fibers was induced. However, the conclusion could not be supported by any morphologic data because they did not biopsy muscle. Using a similar jumping protocol, we present morphologic evidence of loss in dystrophin staining with fluorescent microscopy and clearly evident Z-disk streaming with transmission electron microscopy. With both techniques, the fiber-specific damage was proven, not by statistical association analysis but by fiber identification of the same samples.
Transmission electron microscopy analysis demonstrated different severities of muscle damage, mainly in the fast-twitch muscle fibers at the site of the Z disks, including mild Z-disk streaming with a wavy appearance; extensive Z-disk streaming, where the Z disk had a wavy and broadened appearance; and finally smearing, where the Z-disk material was dispersed into adjacent sarcomeres. The same types of muscle damage have been described by Friden and Lieber.15 These authors hypothesized that high-tension eccentric contraction would result in Z-disk–to–Z-disk intermediate filament breakage and that this protein disruption further exposes disrupted Z proteins and globular proteins to active lysosomal enzymes, causing additional degradation.15
Morphologic studies that investigated which fiber type was more susceptible to damage after other forms of eccentric exercise in humans are limited, but fast-twitch fibers are preferentially damaged.16,17 For the first time in 1983, selective damage to fast fibers during eccentric (bicycle) training was demonstrated,17 with a damage ratio of 3:1 between type II and type I fibers. In a 1986 subsequent study, Jones et al16 confirmed the earlier observation using other muscle groups (biceps and gastrocnemius) and exercise tasks not related to daily life activities, such as backward walking.
The possible mechanisms whereby fast-twitch fibers are selectively damaged could be due to structural differences between fast-twitch and slow-twitch muscle fibers. Fast-twitch fibers are characterized by narrower and weaker Z disks; therefore, during muscle lengthening with contraction, it is thought that the stronger sarcomeres pull the weaker ones apart, resulting in Z-line streaming.5 Another proposed mechanism of preferential fast-twitch muscle damage when exercises are repeated a sufficient number of times is that, during the early stages of eccentric muscle action, these specific fibers are fatigued and thereafter cannot generate adenosine triphosphate (ATP), resulting in a state of rigor that leads to mechanical disruption.15 Neither of these proposed mechanisms can be excluded or categorically favored. Our results show that the damaged fibers have a significantly lower MV% and narrower Z disks, suggesting that both characteristics play fundamental roles in the damage. In fact, Z-disk streaming was present in fibers with a small Z-disk width, and sometimes it was impossible to visualize mitochondria in the damaged area, suggesting a kind of hypoxia, which was confirmed in other damaged areas by the swollen mitochondria observed near streamed Z lines.
This ultrastructural damage was less severe than that reported by Lauritzen et al18 after 70 maximal eccentric biceps contractions using a dynamometer. The severe ultrastructural damage they observed in a very high percentage of fibers included hypercontracted myofibrils and necrotic fiber segments. In contrast, after plyometric exercise with required performance of 95% of maximum jump height, ultrastructural damage was limited to between 2 and 20 damaged sarcomeres per field of view. Moreover, the immunofluorescent data showed that only 9.6% of the fibers appeared with a damaged sarcolemma, which can explain the more modest leak of CK into circulation compared with the values observed in other studies that used isolated, exclusively eccentric exercise. Furthermore, during plyometric jumping, the active muscle groups can withstand loads in excess of 5 times the athlete's body weight19 (well beyond the force that could be voluntarily produced), and this activation occurs in less than 60 milliseconds (voluntary action speed = 120 milliseconds).20 When we take this into consideration, the plyometric exercise should seem more severe in comparison with maximal eccentric contraction. However, because more muscle fibers are recruited with ballistic exercise21 such as plyometric jumping than with an isolated eccentric model, it is also possible that less damage is observed with plyometric jumping.
Perceived muscle soreness is not considered a valid method for quantifying muscle damage because the magnitude of ultrastructural alterations and damage may not necessarily determine the magnitude of muscle pain and vice versa.22 Nevertheless, the current data present a similar time course for CK and perceived DOMS. Both peaked 1 day after the acute bout of plyometric exercise and then slowly returned to normal values.
A limitation of the biopsy procedure that should be taken into account is the limited amount of muscle tissue extracted during a biopsy, which does not allow whole-muscle damage to be assessed and could therefore result in overestimation or underestimation. However, the smaller amount of muscle damage compared with that observed after eccentric exercise of the elbow flexor and extensor muscle groups may be partly due to the difference in the frequency of eccentric contractions by those muscles during daily activities or a difference in muscle architecture.23
CONCLUSIONS
Our study shows that plyometric exercise mainly affects fast-twitch fibers, damaging the sarcolemma as well as the sarcomere at the site of the Z disk (microtrauma). Although the severity and extent of the damage was much less severe than seen with other highly controlled eccentric exercise protocols, plyometric jumping is currently used as a popular training method.
Athletic trainers should refrain from prescribing high-volume plyometric exercise bouts within quick succession or after other forms of high-intensity exercise that are known to stress mainly fast-twitch muscle fibers. Athletes unaccustomed to this form of exercise need sufficient time to regenerate damaged fibers. In fact, repeated microtrauma may result in fibrosis and chronic inflammation24 that may further exacerbate the fibrotic response and accumulation of unremodeled scar tissue25; this will, in turn, lead to reduced biomechanical strength.
ACKNOWLEDGMENTS
This research was supported by the Medical Research Council of South Africa and the National Research Foundation. Filippo Macaluso, PhD, is supported by a Claude Leon Trust Postdoctoral Fellowship and Ashwin W. Isaacs, MSc, by a scholarship awarded by the National Research Foundation.
REFERENCES
- 1.de Villarreal ES, Kellis E, Kraemer WJ, Izquierdo M. Determining variables of plyometric training for improving vertical jump height performance: a meta-analysis. J Strength Cond Res. 2009;23(2):495–506. doi: 10.1519/JSC.0b013e318196b7c6. [DOI] [PubMed] [Google Scholar]
- 2.Markovic G, Mikulic P. Neuro-musculoskeletal and performance adaptations to lower-extremity plyometric training. Sports Med. 2010;40(10):859–895. doi: 10.2165/11318370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Vissing K, Brink M, Lonbro S, et al. Muscle adaptations to plyometric vs. resistance training in untrained young men. J Strength Cond Res. 2008;22(6):1799–1810. doi: 10.1519/JSC.0b013e318185f673. [DOI] [PubMed] [Google Scholar]
- 4.Twist C, Eston R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur J Appl Physiol. 2005;94(5–6):652–658. doi: 10.1007/s00421-005-1357-9. [DOI] [PubMed] [Google Scholar]
- 5.Marginson V, Rowlands AV, Gleeson NP, Eston RG. Comparison of the symptoms of exercise-induced muscle damage after an initial and repeated bout of plyometric exercise in men and boys. J Appl Physiol. 2005;99(3):1174–1181. doi: 10.1152/japplphysiol.01193.2004. [DOI] [PubMed] [Google Scholar]
- 6.Kay AD, Blazevich AJ. Effect of acute static stretch on maximal muscle performance: a systematic review. Med Sci Sports Exerc. 2012;44(1):154–164. doi: 10.1249/MSS.0b013e318225cb27. [DOI] [PubMed] [Google Scholar]
- 7.McHugh MP, Cosgrave CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports. 2010;20(2):169–181. doi: 10.1111/j.1600-0838.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith LL, Brunetz MH, Chenier TC, et al. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res Q Exerc Sport. 1993;64(1):103–107. doi: 10.1080/02701367.1993.10608784. [DOI] [PubMed] [Google Scholar]
- 9.Kohn TA, Essen-Gustavsson B, Myburgh KH. Do skeletal muscle phenotypic characteristics of Xhosa and Caucasian endurance runners differ when matched for training and racing distances? J Appl Physiol. 2007;103(3):932–940. doi: 10.1152/japplphysiol.01221.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chimera NJ, Swanik KA, Swanik CB, Straub SJ. Effects of plyometric training on muscle-activation strategies and performance in female athletes. J Athl Train. 2004;39(1):24–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Prince FP, Hikida RS, Hagerman FC, Staron RS, Allen WH. A morphometric analysis of human muscle fibers with relation to fiber types and adaptations to exercise. J Neurol Sci. 1981;49(2):165–179. doi: 10.1016/0022-510x(81)90076-9. [DOI] [PubMed] [Google Scholar]
- 12.Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J. 2007;30(1):73–79. doi: 10.1183/09031936.00146906. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson PM, Hoffman EP, Zambraski E, et al. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol. 2005;99(2):564–569. doi: 10.1152/japplphysiol.00130.2005. [DOI] [PubMed] [Google Scholar]
- 14.Malisoux L, Francaux M, Nielens H, Theisen D. Stretch-shortening cycle exercises: an effective training paradigm to enhance power output of human single muscle fibers. J Appl Physiol. 2006;100(3):771–779. doi: 10.1152/japplphysiol.01027.2005. [DOI] [PubMed] [Google Scholar]
- 15.Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171(3):321–326. doi: 10.1046/j.1365-201x.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones DA, Newham DJ, Round JM, Tolfree SE. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 18.Lauritzen F, Paulsen G, Raastad T, Bergersen LH, Owe SG. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J Appl Physiol. 2009;107(6):1923–1934. doi: 10.1152/japplphysiol.00148.2009. [DOI] [PubMed] [Google Scholar]
- 19.McMahon TA, Greene PR. The influence of track compliance on running. J Biomech. 1979;12(12):893–904. doi: 10.1016/0021-9290(79)90057-5. [DOI] [PubMed] [Google Scholar]
- 20.Toft E, Sinkjaer T, Andreassen S. Mechanical and electromyographic responses to stretch of the human anterior tibial muscle at different levels of contraction. Exp Brain Res. 1989;74(1):213–219. doi: 10.1007/BF00248294. [DOI] [PubMed] [Google Scholar]
- 21.Newton RU, Murphy AJ, Humphries BJ, Wilson GJ, Kraemer WJ, Hakkinen K. Influence of load and stretch shortening cycle on the kinematics, kinetics and muscle activation that occurs during explosive upper-body movements. Eur J Appl Physiol Occup Physiol. 1997;75(4):333–342. doi: 10.1007/s004210050169. [DOI] [PubMed] [Google Scholar]
- 22.Nurenberg P, Giddings CJ, Stray-Gundersen J, Fleckenstein JL, Gonyea WJ, Peshock RM. MR imaging-guided muscle biopsy for correlation of increased signal intensity with ultrastructural change and delayed-onset muscle soreness after exercise. Radiology. 1992;184(3):865–869. doi: 10.1148/radiology.184.3.1509081. [DOI] [PubMed] [Google Scholar]
- 23.Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K. Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol. 2011;111(2):211–223. doi: 10.1007/s00421-010-1648-7. [DOI] [PubMed] [Google Scholar]
- 24.Stauber WT, Knack KK, Miller GR, Grimmett JG. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve. 1996;19(4):423–430. doi: 10.1002/mus.880190402. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaou PK, Macdonald BL, Glisson RR, Seaber AV, Garrett WE., Jr Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987;15(1):9–14. doi: 10.1177/036354658701500102. [DOI] [PubMed] [Google Scholar]