Abstract
Background We assessed the influence of season of birth on duration of breastfeeding and other feeding patterns in three population-based birth cohort studies in the city of Pelotas, Southern Brazil.
Methods In 1982, 1993 and 2004, all hospital-born children in the city were enrolled in three cohort studies (n = 5914, 5249 and 4287, respectively). Children and their mothers were periodically visited in the first 2 years of life, to collect information on the duration of breastfeeding and the ages at which different types of foods were introduced on a regular basis. Two independent variables were studied: month of birth and mean environmental temperature in the first month of life. Survival analyses and chi-squared tests were used to evaluate the associations. Temperature-based slope indices of inequality were also calculated.
Results Duration of breastfeeding was lower among children born from April to June (months preceding winter) and spending their first month of life in colder temperatures. The influence of season of birth on breastfeeding patterns and the introduction of cow's milk differed according to maternal education, with the strongest effects among children belonging to less educated mothers. Early introduction of fruits (1982 and 1993 cohorts) and vegetables (1982 cohort) were also associated with lower environmental temperature in the first month of life, but not with trimester of birth.
Conclusion Colder temperatures adversely affect duration of breastfeeding and feeding patterns in infancy, especially among the poorest. This finding should be considered in breastfeeding promotion programmes.
Keywords: Breastfeeding, climate, cohort studies, temperature, supplementary feeding
Introduction
Breastfeeding is universally recognized as the ideal way of feeding infants. Its benefits in terms of child nutritional status, cognitive and physical development, and in reducing morbidity and mortality—particularly due to diarrhoea, ear infections, respiratory tract infections and meningitis—are well known.1–4 Recent evidence from long-term cohorts suggests that breastfeeding may also help reduce the incidence of some chronic diseases and related conditions in adults.5,6
Based on current knowledge about the benefits of breastfeeding and the adverse effects of early introduction of supplementary foods, the World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life, at which age complementary feeding should be provided along with continued breastfeeding.1–3 Achieving this is still problematic in most countries, and many women are likely to introduce foods or fluids before this age.1,7,8
Many variables can affect the duration of breastfeeding including maternal education, economic conditions, social and cultural characteristics and health policies on promotion, protection and support for breastfeeding.7,8 Environmental conditions have been related to a number of health conditions, including morbidity and mortality, in studies from low- and middle-income countries.9,10 Few studies are available on the seasonality of breastfeeding, and most were carried out in tropical settings where there are two seasons—the wet and dry seasons—and where the prevalence of maternal undernutrition is high.11–15 A Swedish study also reported an association.16 This was the only study we were able to locate in a setting with four distinct seasons.
Due to the paucity of data on infant feeding patterns by season of birth in settings with four well-defined seasons related with environmental temperature (spring, summer, autumn and winter), we tested this association in three population-based birth cohort studies launched in 1982, 1993 and 2004, in the city of Pelotas, Southern Brazil.
Methods
Pelotas is a Southern Brazilian city with a population of approximately 340 000. The weather is subtropical with four well-defined seasons (Table 1). In 1982, 1993 and 2004, all hospital-born children in the city were enrolled in three cohort studies (n = 5914, 5249 and 4287, respectively) using similar methods.17–19 Hospital births account for over 98% of all births in the city.
Table 1.
Description of the variables in the three Pelotas birth cohort studies
| Pelotas Cohort |
|||
|---|---|---|---|
| 1982 | 1993 | 2004 | |
| Cohort total | 5914 | 5249 | 4287 |
| Median of maternal education (years) | 5.5 | 6.2 | 8.0 |
| Low maternal BMI (<18.5 kg/m2, %) | 7.8 | 8.9 | 4.9 |
| Median duration (months) of | |||
| Any breastfeeding | 2.9 | 3.0a | 6.2 |
| Exclusive breastfeeding | –b | 0.1a | 1.2 |
| Predominant breastfeeding | 2.0 | 1.9a | 2.5 |
| Median age (months) at the introduction of | |||
| Cow's milk | 3.1 | 2.0a | 3.1 |
| Formula | 2.2 | 1.6a | 1.6 |
| Fruits | 3.3 | 3.5a | 4.1 |
| Vegetables/legumes | 3.7 | 4.0a | 5.0 |
| Mean environmental temperature according to trimester of birthc | |||
| January–March | 22.2 (2.2) | 23.1 (2.0) | 22.5 (1.9) |
| April–June | 15.9 (4.0) | 16.1 (4.5) | 16.2 (4.3) |
| July–September | 14.7 (3.6) | 12.6 (3.6) | 14.3 (3.6) |
| October–December | 19.1 (3.5) | 20.2 (3.0) | 19.2 (2.9) |
| Mean environmental temperature in the 1st month of lifec | |||
| Coldest quintile | 12.8 (0.6) | 11.7 (0.7) | 13.2 (0.6) |
| Quintiles 2–4 | 18.1 (2.4) | 18.0 (3.1) | 17.9 (2.7) |
| Hottest quintile | 23.1 (0.4) | 23.0 (0.8) | 23.0 (0.6) |
aWeighted results for a sample of all low birthweight children and a systematic sample of 20% of the remaining children.
bNot available but assumed to be less than 1 week based on key informants.
cMean and standard deviation.
Mothers were interviewed soon after delivery about socio-demographic characteristics and health conditions during gestation and delivery. All newborns were weighed using calibrated paediatric scales.
In the 1982 cohort, all children born between January and April were followed up at the beginning of 1983, and in 1984 the entire cohort was visited again (see Supplementary Table available at IJE online). In the 1993 cohort, a sample of births was followed up at ages 6 and 12 months, including all low-birthweight children (<2500 g) and a systematic sample of ∼20% of the remaining children; results were weighted so as to reproduce the proportion of low birthweight in the perinatal sample. In 2005, all children born in 2004 were followed at age 3 and 12 months.
The follow-up interviews were carried out in the homes, and mothers reported if the child was still breastfeeding, the duration of breastfeeding and the ages at which different types of foods were introduced on a regular basis. Specific questions asked about introduction of cow's milk, formula, fruits and vegetables, and other solids or semi-solid foods. Breastfeeding patterns were classified as: exclusive breastfeeding—breastfed children who were not fed any other fluids or foods; predominant breastfeeding—breastfed children who were also fed other fluids, such as water or tea, but who were not fed solid or semi-solid foods; and partial breastfeeding—children who were fed breast milk complemented with other types of milk, such as cow's milk or formula, or with solid or semi-solid foods. In 1982, information was collected when the child was aged around 11 months for those interviewed in 1983 (one-third of the cohort) and at the mean age of 19 months for the rest of the sample, who were only visited in 1984. For the other two cohorts, information was collected at the 12-month visit.
Since the importance of exclusive breastfeeding became evident only in the late 1980s,20,21 specific questions regarding the ingestion of tea or water were not included in the 1982 cohort, and for this reason, information on exclusive breastfeeding is not available for this cohort, but key informants report that virtually all children received fluids from the very first day of life. Exclusive breastfeeding was also of short duration in the 1993 cohort, but increased somewhat by 2004 (Table 1). For these reasons, it was not possible to study variability in exclusive breastfeeding in the three cohorts, and predominant breastfeeding duration was investigated.
Predominant and any breastfeeding were evaluated as continuous variables, and also as the prevalence of early interruption of breastfeeding (≤ 1 month). Ages at the introduction of different foods were evaluated as dichotomous variables, considering 1 month as cutoff for early introduction of cow's milk and formula, and 3 months for fruits and vegetables or legumes.
Two variables were used to define season of birth: calendar month of birth, grouped in quarters: January–March (summer), April–June, (autumn) July–September (winter), October–December (spring); and mean environmental temperature, obtained from the register of the Meteorological Research and Weather Forecast Center from the Federal University of Pelotas (www.cppmet.ufpel.edu.br). Four models based on quintiles of mean environmental temperature were tested: on the actual day of birth, mean temperature during the first month of life, during the first 3 months, and during the first 6 months of life. Mean environmental temperature in the first month of life showed strongest associations with the outcomes, and was selected as the independent variable. As the results for quintiles 2–4 of environmental temperature were very similar for prevalence analysis, they were collapsed into one category, whereas for survival analyses quintiles 2–5 were combined.
We used the chi-squared tests to measure associations between exposures and the outcomes, including tests for heterogeneity (trimester of birth) and for trend (mean temperature). Statistical analysis was carried out using Stata 11.0 (Stata Corp., College Station, Texas, USA) to calculate the percentage of breastfed children and of introduction of foods at different ages and to estimate the median duration of these variables. Survival analyses (Kaplan–Meier test) were also used to compare breastfeeding duration in months. Due to an a priori assumption of a possible interaction between temperature and socio-economic conditions, tests for interaction with maternal education were carried out. Interactions between the cohorts (1982, 1993 and 2004) and explanatory variables were also carried out; because these interactions were significant, pooled results across all cohorts are not presented.
Slope indices of inequality (SIIs), based on a linear regression of levels of the outcome variables on temperature quintiles (coded 1–5) were also calculated. These can be interpreted as the absolute difference in prevalence of the outcome among children at the two extremes of the temperature range.22
The Federal University of Pelotas Ethical Committee approved all phases of the three Pelotas birth cohort studies. Informed consent was collected at each follow-up.
Results
In the 1982 cohort, 79% of all cohort children were located in 1983 and 87% in 1984 (mean ages 11.3 and 19.4 months, respectively). In the 1993 and 2004 cohorts, follow-up rates were >93% in all visits. Refusals to participate in the perinatal studies were always <1%. Follow-up rates in these follow-up visits were high in the three cohorts for all groups of birthweight and socio-economic conditions.23
When compared with the 1982 and 1993 cohorts, the median duration of any breastfeeding—reflecting full weaning—was 3 months longer in the 2004 cohort (Table 1). Duration of predominant breastfeeding and the age of introduction of fruits and vegetables also showed a steady increase. In contrast, the age at the introduction of cow’s milk decreased between 1982 and 1993, but increased again in 2004. The age at introduction of formula decreased between 1982 and 1993, and remained stable thereafter. Maternal education increased steadily between 1982 and 2004.
As expected, weather conditions varied according to trimester of birth: the hottest trimester was January–March and the coldest July–September (Table 1). There were few differences between the cohort years, but 1993 was slightly colder.
There was evidence of interaction between maternal education and environmental temperature for three outcomes: duration of any breastfeeding (P = 0.04 in 1982, 0.11 in 1993 and 0.02 in 2004), duration of predominant breastfeeding (P = 0.09 in 1982, 0.13 in 1993 and 0.02 in 2004) and age of introduction of cow's milk (P = 0.10, 0.19 and 0.15, respectively). For this reason, all analyses for these three outcomes were stratified by maternal education, coded as 0–4 years or >4 years.
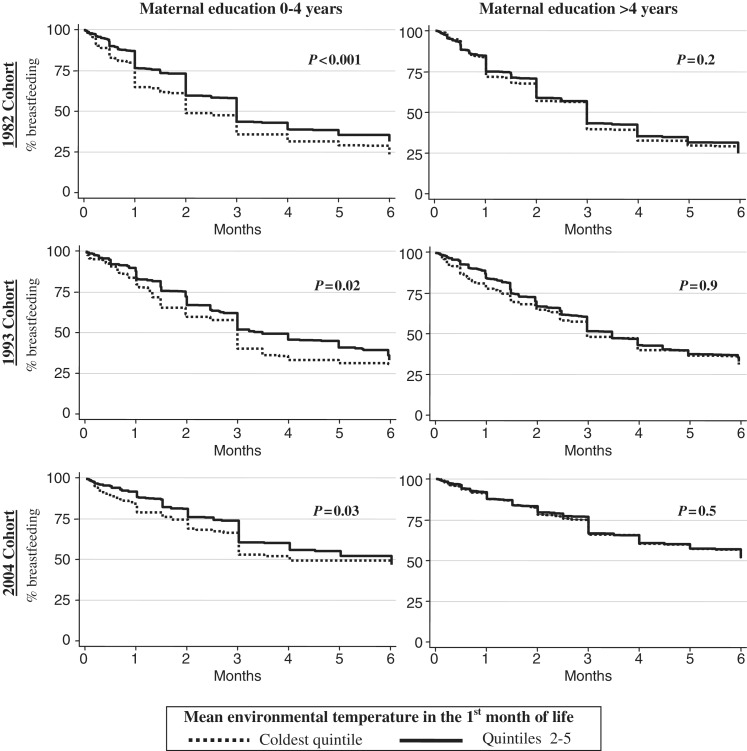
Figure 1 shows that infants with low maternal education and whose first month of life was in the coldest quintile of environmental temperature were completely weaned earlier than those in the warmest quintile (P < 0.05 in all the cohorts). This association was not evident in the higher maternal education groups in any cohort. A similar pattern was found with predominant breastfeeding, but the differences in the low maternal education group showed a P < 0.05 only for the 1982 cohort (data not shown). The higher P-levels in the low education group were not due to smaller sample sizes as there were many more mothers in the more educated category.
Figure 1.
Duration of any breastfeeding according to mean environmental temperature in the first month of life. Stratified by maternal education. Pelotas birth cohort studies
Table 2 presents prevalence of early interruption of breastfeeding (≤1 month) according to season of birth and temperature quintiles. In the 1982 cohort, early interruption of any breastfeeding was higher among those born in April–June compared with those born in other trimesters, but the association was restricted to the low maternal education group. In the 1993 and 2004 cohorts, the prevalence of early interruption of partial breastfeeding was also higher in the same months, but the differences were smaller and P-levels were higher; also, there was no evidence of interaction with maternal education.
Table 2.
Prevalence of early interruption of breastfeeding (≤ 1 month) according to season of birth, stratified by maternal education; Pelotas birth cohort studies
| 1982 Birth Cohort |
1993 Birth Cohort |
2004 Birth Cohort |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Maternal education |
Maternal education |
Maternal education |
|||||||
| 0–4 years | >4 years | 0–4 years | >4 years | 0–4 years | >4 years | ||||
| All % | n (%) | n (%) | All % | n (%) | n (%) | All % | n (%) | n (%) | |
| Any breastfeeding ≤ 1 month | |||||||||
| Trimester of birtha | P = 0.007 | P < 0.001 | P = 0.4 | P = 0.04 | P = 0.1 | P = 0.2 | P = 0.2 | P = 0.5 | P = 0.2 |
| Jan–Mar | 30.5 | 413 (28.3) | 878 (31.4) | 19.1 | 83 (20.5) | 251 (18.7) | 14.1 | 167 (17.4) | 856 (13.6) |
| Apr–Jun | 34.9 | 447 (40.9) | 895 (31.8) | 25.1 | 91 (29.1) | 260 (23.7) | 16.5 | 176 (19.3) | 897 (15.9) |
| Jul–Sep | 32.6 | 451 (33.0) | 945 (32.5) | 21.0 | 100 (26.0) | 243 (18.9) | 15.3 | 158 (13.9) | 872 (15.7) |
| Oct–Dec | 29.0 | 417 (29.5) | 881 (28.8) | 16.4 | 103 (15.5) | 255 (16.9) | 13.1 | 122 (14.8) | 804 (13.1) |
| Temperature 1st monthb,c | P < 0.001 | P < 0.001 | P = 0.003 | P = 0.08 | P = 0.06 | P = 0.2 | P = 0.1 | P = 0.04 | P = 0.5 |
| Coldest quintile | 38.1 | 343 (43.4) | 725 (35.6) | 25.5 | 74 (28.1) | 186 (24.5) | 16.1 | 117 (24.8) | 707 (14.9) |
| Quintiles 2–4 | 31.1 | 1041 (31.9) | 2153 (30.7) | 18.8 | 230 (21.0) | 601 (17.9) | 14.7 | 391 (14.6) | 2039 (14.9) |
| Hottest quintile | 27.8 | 344 (26.5) | 721 (28.3) | 19.1 | 73 (16.8) | 223 (19.9) | 13.7 | 115 (14.8) | 683 (13.6) |
| SII (95% CI)d | −21.1 (−32.7, −9.5) | −9.1 (−13.9, −4.4) | −14.1 (−19.9, −8.6) | −5.0 (−21.5, 11.4) | −12.4 (−32.2, 7.4) | −1.6 (−4.1, 0.9) | |||
| Predominant breastfeeding ≤ 1 month | |||||||||
| Trimester of birtha | P = 0.1 | P = 0.003 | P = 0.7 | P = 0.2 | P = 0.1 | P = 0.3 | P = 0.6 | P = 0.6 | P = 0.5 |
| Jan–Mar | 37.5 | 405 (37.8) | 864 (37.3) | 35.6 | 84 (34.4) | 267 (36.0) | 26.1 | 161 (31.7) | 837 (25.1) |
| Apr–Jun | 41.3 | 430 (49.1) | 866 (37.3) | 42.8 | 92 (51.6) | 265 (39.7) | 28.2 | 172 (31.4) | 875 (27.5) |
| Jul–Sep | 39.7 | 431 (41.1) | 915 (39.1) | 36.6 | 104 (44.9) | 250 (33.2) | 26.5 | 154 (25.3) | 846 (26.8) |
| Oct–Dec | 37.1 | 391 (38.1) | 836 (36.7) | 39.3 | 104 (37.2) | 262 (40.2) | 25.7 | 122 (31.2) | 788 (24.9) |
| Temperature 1st monthb,c | P < 0.001 | P < 0.001 | P = 0.02 | P = 0.4 | P = 0.2 | P = 0.8 | P = 0.7 | P = 0.1 | P = 0.8 |
| Coldest quintile | 44.7 | 327 (51.1) | 698 (41.8) | 41.4 | 76 (47.0) | 187 (38.7) | 27 | 114 (38.6) | 686 (25.2) |
| Quintiles 2–4 | 38.0 | 1001 (40.5) | 2080 (36.8) | 38.2 | 234 (39.5) | 619 (37.7) | 26.7 | 384 (27.3) | 1994 (26.5) |
| Hottest quintile | 36.0 | 329 (35.9) | 703 (36.0) | 37.6 | 75 (37.1) | 238 (37.8) | 26.2 | 111 (29.7) | 666 (25.8) |
| SII (95% CI)d | −18.9 (−30.4, −7.5) | −7.2 (−15.2, 0.7) | −12.3 (−22.0, −2.7) | −1.0 (−3.1, 1.1) | −11.1 (−37.2, 15.0) | −0.8 (−4.6, 3.0) | |||
aχ2 test for heterogeneity.
bχ2 test for trend.
cMean environmental temperature in the first month of life.
dTemperature-based slope indices of inequality.
Results for environmental temperature in the first month of life were more clear cut. Temperature was inversely associated with early interruption of partial breastfeeding in the 1982 cohort. This trend was more evident among those with lower maternal education, and similar patterns are evident in the 1993 (P = 0.06) and 2004 (P = 0.04) cohorts. The SIIs show that differences between the temperature extremes were consistently higher among infants born to mothers with low education in every cohort, although not all associations showed a P < 0.05.
In the 1982 cohort, early interruption of predominant breastfeeding (Table 2) and early introduction of cow’s milk (Table 3) were also higher among those born in April–June and those who lived their first month of life in the coldest temperatures, and again the differences were more evident in the lower maternal education group. In the 1993 and 2004 cohorts, the patterns were similar, but differences were somewhat smaller as shown by the SIIs, and P-levels increased.
Table 3.
Prevalence of early introduction of cow's milk (≤ 1 month) according to season of birth, stratified by maternal education; Pelotas birth cohort studies
| 1982 Birth Cohort |
1993 Birth Cohort |
2004 Birth Cohort |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Maternal education |
Maternal education |
Maternal education |
|||||||
| 0–4 years | >4 years | 0–4 years | >4 years | 0–4 years | >4 years | ||||
| All % | n (%) | n (%) | All % | n (%) | n (%) | All % | n (%) | n (%) | |
| Trimester of birtha | P = 0.001 | P = 0.001 | P = 0.02 | P = 0.6 | P = 0.3 | P = 0.5 | P = 0.5 | P = 0.4 | P = 0.3 |
| Jan–Mar | 17.8 | 371 (17.8) | 807 (17.8) | 25.1 | 83 (33.7) | 266 (22.4) | 14.8 | 159 (21.4) | 830 (13.6) |
| Apr–Jun | 24.1 | 411 (29.7) | 839 (21.3) | 29.0 | 90 (37.8) | 265 (26.0) | 16.2 | 171 (21.1) | 870 (15.2) |
| Jul–Sep | 23.3 | 430 (22.3) | 912 (23.8) | 25.5 | 103 (29.1) | 250 (24.0) | 14.7 | 153 (17.7) | 844 (14.2) |
| Oct–Dec | 22.0 | 387 (20.4) | 833 (22.8) | 27.6 | 103 (26.0) | 258 (28.3) | 13.9 | 120 (16.7) | 783 (12.1) |
| Temperature 1st monthb,c | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.08 | P = 0.07 | P = 0.4 | P = 0.6 | P = 0.2 | P = 0.9 |
| Coldest quintile | 27.6 | 321 (31.2) | 692 (26.0) | 29.8 | 76 (36.4) | 186 (27.1) | 14.8 | 114 (25.4) | 682 (13.2) |
| Quintiles 2–4 | 21.1 | 972 (21.1) | 2024 (21.2) | 25.1 | 229 (27.5) | 615 (24.2) | 15.3 | 379 (20.3) | 983 (14.4) |
| Hottest quintile | 18.3 | 306 (19.0) | 675 (18.1) | 23.5 | 74 (24.5) | 238 (23.2) | 13.9 | 110 (20.9) | 662 (12.8) |
| SII (CI 95%)d | −15.4 (−30.4, −0.3) | −9.9 (−13.1, −6.7) | −14.9 (−26.0, −3.8) | −4.7 (−8.5, −0.9) | −5.6 (−16.5, 5.2) | −0.5 (−5.5, 4.5) | |||
aχ2 test for heterogeneity.
bχ2 test for trend.
cMean environmental temperature in the first month of life.
dTemperature-based slope indices of inequality.
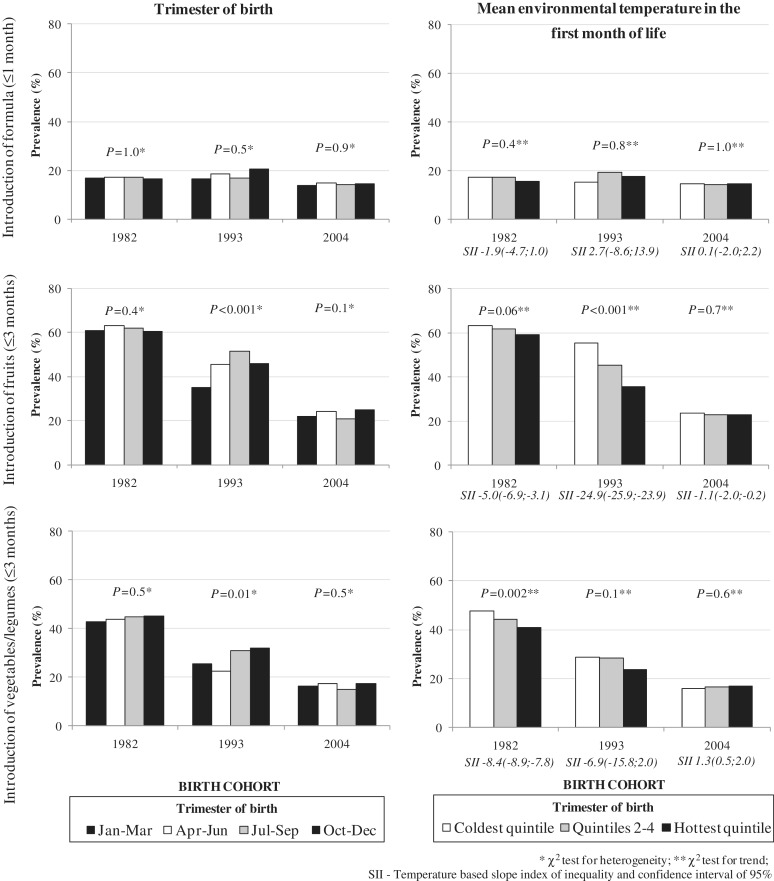
Figure 2 shows results for early introduction of formula, fruits and vegetables or legumes; these are not stratified by maternal education because there was no evidence of any interactions (P > 0.2), so they are showed separately from early introduction of cow's milk results. Formula introduction did not show any associations with season or temperature. Early introduction of fruits was most common in children born in the third quarter in 1993—in contrast to the results in Tables 2 and 3 which tended to be highest in the second quarter. No association between season and introduction of fruits was evident in the 1982 or 2004 cohorts. In 1982 (P = 0.06) and 1993 (P = 0.001), but not in 2004, early introduction of fruits was most common in infants in the lowest temperature quintile. Analyses of the introduction of vegetables or legumes were inconsistent. There was an inverse association with temperature in 1982, and in 1993 early introduction was highest in the fourth quarter and lowest in the second.
Figure 2.
Prevalence of early introduction of formula (≤ 1 month), fruits (≤ 3 months) and vegetables/legumes (≤ 3 months) according to season of birth. Pelotas birth cohort studies
All the analyses were repeated adjusting for possible confounding factors (sex, skin colour, family income, maternal education and type of delivery), but this adjustment did not lead to any substantial changes in the results.
Discussion
We evaluated the impact of season of birth and ambient temperature during the perinatal period on the duration of breastfeeding and feeding patterns in the first months of life. Three main conclusions emerged. First, duration of breastfeeding was lower among children born from April to June (the months preceding winter) and living their first month of life in colder temperatures. Secondly, the influence of season of birth on breastfeeding patterns and the introduction of cow's milk differed according to maternal education, with the strongest effects among children belonging to less educated mothers. Third and finally, in the 1982 and 1993 cohorts, early introduction of fruits and vegetables was associated with lower environmental temperature in the first month of life, but not with trimester of birth.
Previous studies in low-income countries have shown that environmental conditions in the perinatal period are involved—directly or indirectly—with the duration of breastfeeding and maternal milk composition. Four African studies, two from Gambia,11,15 one from Senegal14 and one from Tanzania,12 have shown that season of birth influences breastfeeding patterns. In addition to high prevalence of maternal undernutrition, these countries have tropical climates: a dry season (when the harvest occurs) and a rainy season (associated with food scarcity).10 So, birth during or shortly after the hungry season (July-December) resulted in excess mortality during the first year of life and is associated with decrease in breastfeeding, as well as lower maternal caloric intake, increased maternal morbidity and lower body weight in both the mother and the child.24 Two Gambian studies evaluated maternal milk composition: one examined vitamin C content25 and the other assessed levels of IgA.26 These studies found that levels of both substances—known for their protective immunological properties—were higher in the dry season and significantly decreased during the rainy months that are associated with food scarcity. An earlier study from Egypt13 had investigated breastfeeding patterns in two cross-sectional surveys in the hot and cool seasons, showing that the prevalence of exclusive breastfeeding in children aged 6–11 months was substantially higher in the hot than in the cool season—this study was performed before the older age of 6 months for exclusive breastfeeding was recommended by international organizations. We found a single study from high-income countries also reported on this topic.16 In a case–control study from Sweden, both diabetic cases and healthy controls born during the summer were exclusively breastfed for a mean period of 2.2 months. Corresponding figures for children born during winter were 2.8 months (P < 0.04), spring 2.5 months (n.s.) and autumn 2.7 months (P < 0.05).
It is difficult to compare the African studies with the present results, because maternal undernutrition is highly prevalent in these settings, and variations in breastfeeding are likely driven by nutritional factors rather than by environmental temperature. The Egyptian results, however, are in line with our findings of a direct association between breastfeeding duration and temperature. Results from the Swedish study, however, are in the opposite direction.
Since maternal undernutrition in the urban setting of Pelotas is rare, and food availability is uniform throughout the year, other mechanisms may play a role. Reduced liquid intake is a possibility, but because few if any homes in Pelotas have central heating, temperature itself may affect feeding practices. Neuroendocrine mechanisms associated with seasonal temperature differentials are well documented in animals,27–29 and although no specific studies on breast milk production in humans were located, the tendency to store fat in the winter may conceivably be related to lower milk output.
Night-time temperatures in the Pelotas winter are consistently <10°C and sometimes drop below freezing, and temperatures inside most homes are not much above outside temperatures (www.cppmet.ufpel.edu.br). Breastfeeding often takes place on more than one occasion during the night, and mothers may be reluctant to get out of bed to feed in a cold home. Even for mothers who are still breastfeeding, giving a bottle of milk to the child at bedtime can prevent having to wake up and get out of bed more than once. Another potential explanation is that children have more frequent and more severe respiratory infections during the winter,9,30 and nasal obstruction can prevent them from suckling properly. Mastitis is also more frequently diagnosed in colder months.31
Maternal education is a key socio-economic indicator that influences infant morbidity and mortality through multiple pathways.32,33 The association between maternal education and breastfeeding duration varies from setting to setting. In rich countries, highly educated mothers are more likely to start breastfeeding and to continue for longer periods; in poor countries, the opposite trend is often observed.8,34,35 In Pelotas, a middle-income setting, more educated mothers are more likely to breastfeed for up to 6–9 months than less educated mothers, but after 9 months breastfeeding is more common among the latter.36
Our finding that lower maternal education modified the effect of temperature on breastfeeding patterns is consistent with previous studies from the 1982 cohort on hospitalizations due to asthma and pneumonia in the first years of life, in which the excess risk for infants born in the coldest months was restricted to those from poor families.9
Effect modification by socio-economic status—in this case assessed through maternal education, which is a very close proxy in the case of Pelotas—may help elucidate possible causal pathways. Poor families are less likely to have any type of heating in their homes, than are rich families. Type of building materials also varies strongly according to socio-economic position; in 1986, 48% of low education mothers lived in brick houses, compared with 80% of those with > 4 years of schooling. The proportion of poor families living in brick houses showed a notable improvement in the last 20 years, whereas the increase among the wealthiest was modest (72% and 86% in 2006, respectively). As a consequence, the postulated effects of low temperature on willingness to breastfeed during the night would be consistent with a greater effect on poor than on rich mothers. Availability of heating and improved quality of homes may also explain why seasonality and temperature effects were greater in 1982 than in 2004, and why the difference between different social groups decreased over time, as housing for the poor improved. An additional explanation for why effects of temperature seem to have decreased over time is that such programmes have been able to reach virtually all mothers36 and partly offset negative influences on breastfeeding practices—although much still remains to be done. Cultural factors are another possibility for our findings,8 as lower maternal education could lead to a stronger belief in the need for supplementation in coldest temperatures, so other foods may be included as a strategy for keeping the babies warm. Future qualitative researches (such as interviews with poor mothers who suspended breastfeeding or introduced other foods in the first months of life) may help to understand the underlying processes and to investigate the reasons for these decisions.
Effect modification by maternal education may also partly explain the comparability between our results and those from Egypt13 (be more similar to the Pelotas poor) and Sweden16 (more similar to the Pelotas rich). It would not explain, however, why in Sweden higher temperatures were associated with shorter breastfeeding, because in the Pelotas rich no association was detected.
Our cohorts have the advantage of being population based, and of having employed consistent methods over a 22-year span. Studying outcomes in the three cohorts reduces the likelihood of chance findings, and increases external validity. A common limitation of births cohorts, especially in low- and middle-income countries, is attrition bias,37 but it is unlikely to have affected our findings, as follow-up rates for the Pelotas cohorts were very high for all groups of sex, family income or birthweight.23
Additional studies from low- and middle-income settings with four well-defined seasons are necessary to confirm our findings. Although it may be difficult if not impossible to change the seasonality of births—despite the fact that children born in cold months appear to have several negative outcomes in addition to shorter breastfeeding9,38—information on potential pathways linking low temperatures to suboptimal feeding practices will contribute to improving promotion programmes.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
This article is based on data from the study ‘Pelotas birth cohort studies’ conducted by Postgraduate Program in Epidemiology at the Universidade Federal de Pelotas. The birth cohort studies are currently supported by the Wellcome Trust Initiative entitled Major Awards for Latin America on Health Consequences of Population Change. Previous phases of the studies were supported by the International Development Research Center, the World Health Organization, Overseas Development Administration, European Union, National Support Program for Centers of Excellence (PRONEX), the Brazilian National Research Council (CNPq) and Brazilian Ministry of Health.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in the three Pelotas birth cohort studies, and the Pelotas teams, including research scientists, interviewers, workers and volunteers.
Conflict of interest: None declared.
KEY MESSAGES.
Durations of predominant and any breastfeeding were lower among children born from April to June (the months preceding winter) and living their first month of life in colder temperatures.
Early introduction of cow's milk was also higher among those born in April–June and those who lived their first month of life in the coldest temperatures.
The influence of season of birth on breastfeeding patterns and the introduction of cow's milk differed according to maternal education, with the strongest effects among children belonging to less educated mothers.
Early introduction of fruits (1982 and 1993 cohorts) and vegetables (1982 cohort) were associated with lower environmental temperature in the first month of life, but not with trimester of birth.
References
- 1.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Kakuma R. The Optimal Duration of Exclusive Breastfeeding: a Systematic Review. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 3.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database. Syst Rev. 2002:CD003517. doi: 10.1002/14651858.CD003517. [DOI] [PubMed] [Google Scholar]
- 4.Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(Suppl. 3):S15. doi: 10.1186/1471-2458-11-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fall CH, Borja JB, Osmond C, et al. Infant-feeding patterns and cardiovascular risk factors in young adulthood: data from five cohorts in low- and middle-income countries. Int J Epidemiol. 2011;40:47–62. doi: 10.1093/ije/dyq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horta BL, Bahl R, Martines JC, Victora CG. Evidence on the Long-term Effects of Breastfeeding: Systematic Review and Meta-analyses. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 7.Bhutta ZA, Chopra M, Axelson H, et al. Countdown to 2015 decade report (2000-10): taking stock of maternal, newborn, and child survival. Lancet. 2010;375:2032–44. doi: 10.1016/S0140-6736(10)60678-2. [DOI] [PubMed] [Google Scholar]
- 8.Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38:259–68. doi: 10.1111/j.1552-6909.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez DA, Victora CG, Goncalves H. The effects of season at time of birth on asthma and pneumonia in childhood and adulthood in a birth cohort in southern Brazil. Cad Saude Publica. 2008;24:1089–102. doi: 10.1590/s0102-311x2008000500016. [DOI] [PubMed] [Google Scholar]
- 10.Moore SE, Fulford AJ, Streatfield PK, Persson LA, Prentice AM. Comparative analysis of patterns of survival by season of birth in rural Bangladeshi and Gambian populations. Int J Epidemiol. 2004;33:137–43. doi: 10.1093/ije/dyh007. [DOI] [PubMed] [Google Scholar]
- 11.Prentice A, Prentice AM, Cole TJ, Whitehead RG. Determinants of variations in breast milk protective factor concentrations of rural Gambian mothers. Arch Dis Child. 1983;58:518–22. doi: 10.1136/adc.58.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellen DW. Weaning, complementary feeding, and maternal decision making in a rural east African pastoral population. J Hum Lact. 2001;17:233–44. doi: 10.1177/089033440101700307. [DOI] [PubMed] [Google Scholar]
- 13.Serdula MK, Seward J, Marks JS, Staehling N, Galal O, Trowbridge FL. Seasonal differences in breast-feeding in rural Egypt. Am J Clin Nutr. 1986;44:405–09. doi: 10.1093/ajcn/44.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Simondon KB, Simondon F. Mothers prolong breastfeeding of undernourished children in rural Senegal. Int J Epidemiol. 1998;27:490–94. doi: 10.1093/ije/27.3.490. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead RG, Rowland MG, Hutton M, Prentice AM, Muller E, Paul A. Factors influencing lactation performance in rural Gambian mothers. Lancet. 1978;2:178–81. doi: 10.1016/s0140-6736(78)91920-7. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsson U, Ludvigsson J. Seasonal variation of birth month and breastfeeding in children with diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14:43–46. doi: 10.1515/jpem.2001.14.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Santos IS, Barros AJ, Matijasevich A, Domingues MR, Barros FC, Victora CG. Cohort Profile: The 2004 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 2010;40:1461–68. doi: 10.1093/ije/dyq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Victora CG, Barros FC. Cohort profile: The 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2006;35:237–42. doi: 10.1093/ije/dyi290. [DOI] [PubMed] [Google Scholar]
- 19.Victora CG, Hallal PC, Araujo CL, Menezes AM, Wells JC, Barros FC. Cohort profile: The 1993 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2008;37:704–09. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 20.Rea MF. A review of breastfeeding in Brazil and how the country has reached ten months' breastfeeding duration. Cad Saude Publica. 2003;19(Suppl. 1):S37–45. doi: 10.1590/s0102-311x2003000700005. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization/United Nations Children's Fund. Innocenti Declaration on the Protection, Promotion and Support of Breastfeeding. Florença: World Health Organization/United Nations Children's Fund; 1990. [Google Scholar]
- 22.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med. 1997;44:757–71. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 23.Barros AJ, Santos IS, Matijasevich A, et al. Methods used in the 1982, 1993, and 2004 birth cohort studies from Pelotas, Rio Grande do Sul State, Brazil, and a description of the socioeconomic conditions of participants' families. Cad Saude Publica. 2008;24(Suppl. 3):S371–80. doi: 10.1590/s0102-311x2008001500002. [DOI] [PubMed] [Google Scholar]
- 24.Huffman SL, Chowdhury A, Chakraborty J, Simpson NK. Breast-feeding patterns in rural Bangladesh. Am J Clin Nutr. 1980;33:144–54. doi: 10.1093/ajcn/33.1.144. [DOI] [PubMed] [Google Scholar]
- 25.Bates CJ, Prentice AM, Prentice A, Paul AA, Whitehead RG. Seasonal variations in ascorbic acid status and breast milk ascorbic acid levels in rural Gambian women in relation to dietary intake. Trans R Soc Trop Med Hyg. 1982;76:341–47. doi: 10.1016/0035-9203(82)90185-7. [DOI] [PubMed] [Google Scholar]
- 26.Weaver LT, Arthur HM, Bunn JE, Thomas JE. Human milk IgA concentrations during the first year of lactation. Arch Dis Child. 1998;78:235–39. doi: 10.1136/adc.78.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chemineau P, Guillaume D, Migaud M, Thiery JC, Pellicer-Rubio MT, Malpaux B. Seasonality of reproduction in mammals: intimate regulatory mechanisms and practical implications. Reprod Domest Anim. 2008;43(Suppl. 2):40–47. doi: 10.1111/j.1439-0531.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 28.Ebling FJ, Barrett P. The regulation of seasonal changes in food intake and body weight. J Neuroendocrinol. 2008;20:827–33. doi: 10.1111/j.1365-2826.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- 29.Dragovich D. Effect of low winter temperatures on milk production of dairy cows grazed on farms in a warm temperate climate. Int J Biometeor. 1980;24:167–73. [Google Scholar]
- 30.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118–26. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 31.Evans K, Evans R, Simmer K. Effect of the method of breast feeding on breast engorgement, mastitis and infantile colic. Acta Paediatr. 1995;84:849–52. doi: 10.1111/j.1651-2227.1995.tb13777.x. [DOI] [PubMed] [Google Scholar]
- 32.Cleland JG, Van Ginneken JK. Maternal education and child survival in developing countries: the search for pathways of influence. Soc Sci Med. 1988;27:1357–68. doi: 10.1016/0277-9536(88)90201-8. [DOI] [PubMed] [Google Scholar]
- 33.Gakidou E, Cowling K, Lozano R, Murray CJ. Increased educational attainment and its effect on child mortality in 175 countries between 1970 and 2009: a systematic analysis. Lancet. 2010;376:959–74. doi: 10.1016/S0140-6736(10)61257-3. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Contemporary patterns of breast-feeding. Report on the WHO Collaborative Study on Breast-Feeding. Geneva, Switzerland: World Health Organization; 1981. [Google Scholar]
- 35.Brion MJ, Lawlor DA, Matijasevich A, et al. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol. 2011;40:670–80. doi: 10.1093/ije/dyr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victora CG, Matijasevich A, Santos IS, Barros AJ, Horta BL, Barros FC. Breastfeeding and feeding patterns in three birth cohorts in Southern Brazil: trends and differentials. Cad Saude Publica. 2008;24(Suppl. 3):S409–16. doi: 10.1590/s0102-311x2008001500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golding J, Birmingham K. Enrollment and response rates in a longitudinal birth cohort. Paediatr Perinat Epidemiol. 2009;23(Suppl. 1):73–85. doi: 10.1111/j.1365-3016.2008.01001.x. [DOI] [PubMed] [Google Scholar]
- 38.Basta NO, James PW, Craft AW, McNally RJ. Season of birth and diagnosis for childhood cancer in Northern England, 1968-2005. Paediatr Perinat Epidemiol. 2010;24:309–18. doi: 10.1111/j.1365-3016.2010.01112.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.