Abstract
Background: International guidelines for medical research involving human subjects maintain the primacy of informed consent while recognizing cultural diversity. Methods: This article draws on empirical data obtained from interviews with physician-researchers in teaching hospitals of Lahore, Pakistan, to identify social and cultural factors that affect the consent process for participants in research. Results: This article presents variable findings with regards to communication, comprehension, and decision making. While some physicians consider that social factors such as lack of education, a patriarchal family system, and skepticism about research can make patients dependent on either the physician-researcher or the family, others believe that patients do make independent decisions. Conclusions: In light of the findings, the article ends with a recommendation for communication and decision making that is sensitive to the local sociocultural environment while at the same time meeting the ethical imperative of respect for persons.
Keywords: decision making, consent process, developing world [bio]ethics, empirical ethics
There is a need for the establishment of an overarching bioethics that acknowledges the importance of local norms while reflecting a global perspective (Hellsten 2008; Hongladarom 2004; Macer 2006; Widdows 2007). International guidelines for medical research involving human subjects (e.g., the Declaration of Helsinki), though emphasizing individuals’ voluntary participation in research, allow for consultation with family and community members (World Medical Association [WMA] 2008). The Nuffield Council on Bioethics (NCOB) and the UNESCO Universal Declaration on Bioethics and Human Rights (UDBHR) also require researchers “to be sensitive to cultural difference” (NCOB 2002, 51–52) and give “due regard” to “cultural diversity and pluralism,” without “infring[ing] upon human dignity, human rights and fundamental freedoms” (UNESCO 2005, article 12). The adoption of the UNESCO declaration reiterates that bioethical concerns are international and multicultural, and requires that the principles of UDBHR be adapted to accommodate varying types of patients, cultures, and traditions (Have 2006). It is therefore necessary to know how perspectives on issues in bioethics are shaped in the local sociocultural environment. To be attentive to the normative while respecting the local requires “empirical ethics research that is at once descriptive and normative” (Emerson et al. 2009, 102).
The primacy of informed consent in research involving human participants is well established (Emanuel et al. 2000). It reflects the ethical principle of respect for autonomy, which is one aspect of the principle of respect for persons. A patient's voluntary decision to consent should be predicated on communication and comprehension of information about the research, but this may not always be possible. Practical and ethical challenges to communication and decision making may be related to patients’ unfamiliarity with and difficulty in understanding scientific concepts (see Appelbaum 2010; Dawson and Kass 2005; Marshall 2008; Moodley et al. 2005), because of either illiteracy (Ezeome and Marshall 2009; Khan 2008; Lynoe et al. 2001; Muthuswamy 2005) or lack of equivalent terminology for scientific terms in local languages (Dawson and Kass 2005; Molyneux et al. 2004; Mystakidou et al. 2009). Researchers may also think it unnecessary to disclose all information to patients regarding research (Khan 2008; Newton and Appiah-Poku 2007). After the information has been communicated, patients go through a decision-making process in which some patients decide independently while other patients depend primarily on the family or physician-researchers (DeCosta et al. 2004; Gitanjali et al. 2003; Jafarey 2006; Marshall 2006; Marshall 2008; Molyneux et al. 2004; Mumtaz and Salway 2009; Shaibu 2007).
There is a need for data to inform the development of strategies that are sensitive to the local sociocultural environment while at the same time meeting the ethical imperative of respect for persons. The purpose of this study was to identify the sociocultural factors that, from the perspective of physician-researchers, affect comprehension and decision making by research participants, in teaching hospitals in Lahore, Pakistan, and to understand how physician-researchers manage these issues.
METHODOLOGY
The empirical fieldwork for this article was carried out as part of a doctoral project in which the author was responsible for interviewing and observing the participants. Ethical clearance was obtained from the Oxford University Medical Science Division Research Ethics Committee, the National Bioethics Commission of Pakistan, and institutional review boards at the relevant fieldwork sites. After ethical clearance was granted, the heads of departments or principal investigators of trials from which participants would be recruited were approached. The initial participants were used to recruit other participants through the use of snowball sampling, and recruitment continued until the data showed no new themes (Guest et al. 2006; Sankar and Jones 2008).
Participants
Semistructured interviews with 33 physician-researchers from six tertiary care hospitals were conducted after obtaining their consent. Of these 33 participants, 18 were men and 15 were women. Eleven physician-researchers were involved in international collaborative research and 18 in local research, while four had conducted both local and international research.
The interviews lasted between 45 minutes and 1 1/2 hours and were digitally recorded; they were later transcribed and then translated (by the author). Interviews followed a topic guide (Figure 1). The topic guide was developed through a review of the literature on research ethics and discussions with colleagues.
Figure 1.

Topic guide.
Coding and Data Analysis
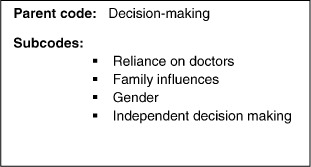
A preliminary coding scheme was developed by the author and reviewed by two advisors. On review, the preliminary coding scheme was reduced to two primary themes, each of which contained several subthemes. Rereading the reports revealed further themes and the data were reanalyzed, using NVivo 8, a software program for the analysis of qualitative data. In this article, findings related to the two primary themes, (I) communication and comprehension and (II) decision making, are presented (Figures 2 and 3).
Figure 2.

Results.
Figure 3.
Results.
RESULTS
Communication and Comprehension of Information
Variations in Understanding Research
A common theme among all participants was that providing information about research to a population that has varied levels of understanding is a challenge:
You cannot explain everything to everybody. Informed part varies from patient to patient. They may have understood it at their level. It is difficult; you have to adjust [information] accordingly. I think there just comes a point, if you see that the patient does not understand it, perhaps some of the investigators would say “this is the best [treatment] and you should do it and I would advise you to do it.” It is still left to the patient to decide, but it becomes more of a persuasion, but this is because their understanding is so [poor]. (Dr. 1, Male)
Lack of comprehension, they reflected, surrounds not only research trials but also concepts of health and disease. According to the physician-researcher participants, patients invariably look at the negative aspects:
The first thing is that they should agree and go along with us and before that they should understand as to what are they going to do and what are we going to do. So in that they have confusion. If we tell them all about the research, convey everything even then they are unable to understand as to what it is that we will be doing with them. (Dr.2, Male)
When we come across an illiterate person or very poor guy at times we do explain to [them] but at times, they are not interested in that [explanation] or they don't understand what we say. (Dr. 16, Male)
In the participants’ experience, if patients are educated and understand English, then it is easy to talk to them, and if they are familiar with science, then their understanding is better. If preliminary clinical data from the trial in question were available, the physician-researchers participating in this study would inform patients that the trial is not a “laboratory experiment” and that other patients have used this medicine and benefited; however, participants also reported that patients were skeptical. Most patients, especially the uneducated, would “rely on us [the physician-researcher] for making a decision regarding participation.” On the other hand, many educated patients would say that they do not want to be guinea pigs: “You are involved in experimenting on us, we don't need experiments.” To avoid these problems, some participants would not “waste” time in explaining everything about the trial to patients. When convinced that the patient will benefit, participants would simply say (to patients) that this trial is “good and will benefit you;” they emphasized that this was not based on bad-faith intentions, but rather that it was necessary to expedite consent:
We should give the facts especially if patients are educated. But if I feel the patient needs to be convinced because the understanding level is not so much. I think in that scenario, maybe, I would try to convince the patient that it'll be beneficial for the patient, not from my study's point of view let me get that clear. (Dr. 22, Female)
A few (six) participants, however, reported encountering illiterate patients who pick up clues from conversations between the physician-researcher and the relative accompanying these patients. Given adequate time and effective communication, patients do understand:
When you have time, perhaps it is easy to make your patient understand—again like I said we need to communicate well. I have seen completely illiterate people really understand the concept of research really well … I think a general mistrust of research is something that is more prevalent in people who are more aware as opposed to people who are, as opposed to people who are possibly still relating to the concept of doctors as, you know, their healers. They would believe whatever you'll tell them. (Dr. 19, Female)
Patients Are Overwhelmed
According to participants, knowledge of (allopathic) medicine is generally lacking among their patients. Therefore, giving detailed information overwhelms patients and creates confusion:
The more you talk in detail to the people, the more actually they get confused about it because they do not have the background knowledge of medicine, necessary to sift through the information. I tell them bit by bit. (Dr.6, Female)
Keeping this in mind, most participants said that they provide “tailor-made” information regarding research, especially if patients are skeptical of it:
We feel if told in very straightforward words that you are in a trial and this is test drug or test something that they may out reject it outright. But that does not happen if people take consent in an indirect manner rather than being very direct. (Dr.17, Male)
Communication Skills
According to participants, a relationship of trust is integral to the doctor–patient relationship. To communicate effectively, physician-researchers would empathize and make patients feel comfortable:
It is just that they have to feel comfortable with [the fact] that I am not trying to give them something which is going to hurt them it just might help them, you know. So that concept has to be very clear and it has to be conveyed to the patient that this is not any guinea pig surgery or any guinea pig procedure that we are trying to do. (Dr.30, Male)
In order to gauge patients’ understanding of the trial and whether early interaction with them is useful, one physician-researcher reported:
[In our research] First we explained in simple words and afterward had an interactive session and tried to see if people understood so after that once we did that in which I think may be sixty to seventy percent of the people understood what we were talking about. (Dr.23, Female)
Sometimes information has to be provided in a language that is spoken by the patient, but not the physician-researcher. Communication is then facilitated by engaging a family member or hospital staff as a translator. In the former situation, the relative is closely involved in decision making:
If they [patients] are Punjabi speaking and if you talk in Urdu [even then] they will not understand. The question does not even arise that any of them would understand any English. And if you feel that they are not able to grasp, then you ask the patient that if they have an attendant. Mostly, they do have someone who is educated or at least has some ability in understanding [Urdu]. (Dr.22, Female)
Time Constraints
According to the participants, it takes time to communicate adequately to patients the benefits and risks of research. Patients do not ask questions at the initial consultation but return later with queries. It is not necessary to be present in person: just to be at the other end of a phone, as long as the physician-researcher is accessible. Most participants stated that “accessibility was a major issue” in a busy general hospital where the physician-researchers were also conducting outdoor (outpatient) clinics, and on average seeing 30 patients. They were looking after indoor patients (inpatients) and were part of the on-call team too. Participants reported that physicians work in conditions of paucity—financial as well as temporal. A direct consequence of lack of time is the inability to have detailed discussions with patients regarding participation in research:
There is not enough time, we are not able to give them proper time, it makes [me] feel guilty, and that is because we are overburdened. We try [our best] and provide them, with a copy of consent form that go and read it and then come back with your decision. (Dr.29, Male)
In these circumstances, one physician-researcher reported that he would call research patients on his “off duty” days, to ensure that he had time to discuss the research. At one institution, three physician-researchers were involved only in research and were able to have detailed discussion with patients. Three other physician-researchers addressed time constraints by dividing their day into two parts—in the morning they would do “hospital work” and in the afternoon “research work”:
If I feel that, the patient will benefit from the trial and I plan to enroll [them] after three months for example then I talk to them early about the trial. This way when enrollment time approaches they [are] prepared [mentally]. [I] usually explain by either drawing on paper or using stories. I take 40–45 minutes discussing with the patient [and spouse] so that there is no ambiguity regarding the drug and options available to the patient. (Dr.14, Male)
Decision Making
All participants were of the opinion that most patients require help from either them or their families in deciding whether to participate in research. However, trends are changing and there are instances when patients decide independently.
Reliance on Doctors
According to participants, patients’ reliance on them is common; most patients ask what they (physician-researchers) would do in similar circumstances. There is trust between the patient and physician-researcher that is reflected in the patient requesting, and at times implicitly authorizing, the doctor to decide:
Yes, it happens like this, they ask us. Sometimes you could tell they have not really understood [the research], and as they are my patients, trust develops and they would then say “doctor sahib we will do as you say.” (Dr.25, Female)
If the relationship between researcher and subject is strong and the subject is confident [in the ability of the physician-researcher], then I don't think so there is any problem. You see I know them and they know me—I have treated them and their family for [a long time], so they know what type of person I am. (Dr.23, Female)
An important concern for the participants in such circumstances was to avoid making “wrong decisions” because it could cause irreparable damage to the patient's health. Therefore, some of the respondents do a risk–benefit analysis before advising:
[This is] a difficult situation because they trust me and rely [on me]. You are all in self (by yourself). I may place myself in their [patient's] place—I then go back to the drug and read up on the information provided by the company so as not to miss any point regarding the side effects. So I do not go blindly into it. (Dr.2, Male)
Some participants, however, refrain from advising and only give the information regarding the trial and let the patient decide:
I only tell them that these are the benefits and risks. Our job is only to tell them and I do not tell them what to do. I say go and discuss with your family and then come and tell me. The decision is theirs … if I make them join then it is possible that they will not come again. (Dr.27, Male)
Family Influences
According to the participants, decision making is a “family thing” in “our” culture. Invariably, patients are accompanied by a member (or members) of their family, especially elderly patients and women (rarely are women unaccompanied). The person (or persons) accompanying the patient starts asking questions, and throughout the consultation, patients contribute very little. Participants reported that patients are often dependent on others in deciding and have long consultations, either with their physician or with family members:
Our people in general are very dependent on each other. Individuals taking a decision in any frame of life is difficult for them, men or women, they take combined, joint decisions, believe in joint families, you know, so that's the way it's done and you have to face it. (Dr.6, Female)
Participants reported that in Pakistan the family plays an important role; it is common practice to follow the “elders” of the family. The general trend is to reach a decision after “talking to my family” and many times it is the decision of the head of the family that prevails:
Our society is a lot different from outside [the West]. Even for routine procedures they say “let me discuss this with my family” and sometimes it gets frustrating because we know what is right for the patient but they come back and say “my father does not agree.” It is very frustrating. So decision making in our setup, in our culture is a lot different. It's not made by individuals, it's made by families. (Dr.24, Male)
The “dependency” on family has its advantages too, according to some participants. In circumstances where the patient is disturbed or under stress, it is easier to talk to the relative accompanying them. A caring member of the family is helpful in these situations:
If an untoward result is received then it is easy to talk to the caring son [or daughter]. I think ninety-five percent [of relatives] are caring and considerate. I also would not like to know if something is bad [regarding my test results]. (Dr.26, Male)
Other participants also shared the view that “reliance on family” is beneficial, especially when patients are undecided about whether or not to enroll in research:
Sometimes patients are double minded whether they should [enroll in a trial] or not. Then they consult their relatives who are close to them … the benefit is that discussion helps clear their mind. (Dr.27, Male)
Gender
In the participants’ experience, the head of the family, who is usually male, makes decisions regarding women; sometimes, the mother-in-law makes such decisions. The reason is that in Pakistani culture men usually have the decision-making power:
Many times, it happens because this is our culture. Women are unable to take decision. They will rely on the men folk, even if they [men] are much younger but they will like tell you that “no, the man will come and decide” and in fact sometimes one feels terrible because of this gender bias because myself being a woman but you forget your own feministic views and end up asking the same questions. (Dr.6, Female)
The degree of dependence, and often the education status of women, dictate the degree of their involvement in decision making. Although men have the dominant role in making decision, their decisions are based on “care and concern”:
You see, although I've said that the men have a dominant role in making a decision, but generally their decision is in favor of their women rather than for any lucrative purposes or any other advantages risking their women. The other thing is that in our culture, generally, conversation is between the two males. So they may have a say but that say is communicated through their male partners. (Dr.17, Male)
Many women would want to consent to research after bahmi mushawarrat (mutual consultation). Most married women are accompanied by their husbands and would want to consult them before enrolling. According to the physician-researchers participating in this study, this “gave them confidence.”
However, this “dependency” has some disadvantages, reflects Dr.14, Male:
Well you know, like I have told you that I have come across situations where the patient, she wanted to take part [in the trial] but the husband did not. He thought we were experimenting so she did not [enroll]. Of course, it should be her [decision]. Unfortunately, it is just like that you know.
He went on to say:
[Women] do understand that they should come to a doctor but their [women's] family members put them under a lot of stress. The pressure comes from family and especially from the husband that she should first see [a] hakeem [traditional healer] or [perform] dum darood [religious incantation for healing] you know and by the time they come to us it [disease] is really advanced. Again it also depends from which background [socioeconomic] the patient comes, okay, but still a very minor percentage who in spite of being educated, they will follow the similar norms and cultural practices.
Another participant added:
It is very rare that it is women's choice and equally rare that in certain families it is a woman who is the dominant person. If a woman is that dominating then male has to keep quiet, you know once in a while. (Dr.23, Female)
Independent Decision Making
Although participants reported that most patients rely on the family, some patients do make an independent decision about whether to enroll or not.
I gave this [patient] a lot of my time and explained all the benefits that he will get and I was sure that the trial will benefit him but then after two days he calls me and says he does not wish to enroll. What can I do, it's his decision. (Dr.2, Male)
Similarly, there were instances in the participants’ experience where women made independent decisions. These were usually, though not always, educated women, who realized the benefits of a particular research study and wanted to enroll:
As far as I have come across [women] do decide themselves they do not need to, you know, get sort of a formal permission [from the husband]. They are capable of taking decisions and husbands go along with their decisions. (Dr.22, Female)
DISCUSSION
The capacity to understand and communicate, along with the ability to reason, is necessary for giving consent (Beauchamp and Childress 2001; Buchannan and Brock 1989). This study shows that physician-researchers’ experience with patients’ understanding varies. Communicating with educated patients is easier, especially if they understand scientific concepts. Although consultations with patients are in Urdu or the local language, the teaching and training of medicine in Pakistan is in English, and the absence of equivalent medical or scientific terminology in the local languages poses practical challenges. This is similar to the experiences of researchers in other settings (Dawson and Kass 2005; Marshall 2006; Molyneux et al. 2004; Mystakidou et al. 2009).
Patients’ ability to understand research is further compromised by the short time frame in which information is provided; absence of background knowledge of their disease and its treatment (Dawson and Kass 2005; Marshall 2008); and scientific concepts such as “experiment”—which to most patients meant something scientists do in a laboratory on “guinea pigs.” Lack of education itself is not a hindrance to understanding (Gitanjali et al. 2003; Luna 1993; Newton and Appiah-Poku 2007), because illiterate people can be intelligent, but participants noted that communicating with uneducated patients is difficult and time-consuming.
The inability to read (Khan 2008) and the inability to understand medical terminology add to the problem. Under such circumstances, patients require help and advice. How this is done is important. It is recommended that information is given in simple, uncomplicated language over a period of time (Dein and Bhui 2005; Sanchez et al. 2001).
Preliminary data from earlier phases (I and II) of a trial may provide some basis for assessing the potential for individual benefit (Brock 2008, 608). If the physician-researchers had such information about a trial and were confident as to the benefits to patients, they tried to be realistic about the type and amount of information to be given to the patients—the participants’ moral values had an important bearing on their decision. The fact that too much information can overwhelm patients and create confusion requires that a limit be drawn on the amount of information that is provided (Helgesson et al. 2005; Newton and Appiah-Poku 2007; Sreenivasan 2003). What is considered optimal information is not a settled issue. According to Lynoe and Hoyer (2005), in Europe and North America the pendulum has swung from minimal toward too much information—both strategies result in suboptimal information practices.
In the participants’ experience, most patients rely on them for decision making. Most physician-researchers reported helping patients decide; as morally reflective agents they try to act in the best interest of the patient, who is the weaker party in the relationship (Weijer and Miller 2003). Instances where patients rely on their attending physician (and family) to decide on their behalf are reported from other Asian countries as well (DelPozo and Fins 2008; Sirinivasan and Loff 2006; Yousaf et al. 2007). In Pakistani society, doctors are placed in a position of privilege; they are respected, and the suffix sahib—a term used to show deference—is reflexively attached to doctor—“doctor sahib.” Doctors are considered instruments of healing, a status sanctioned by religion, and play a pivotal role in health-related decisions. This degree of trust has also been reported by others (Jafarey and Farooqui 2005; Molyneux et al. 2004; Nabulsi et al. 2011).
In this study, participants reported variation in patients’ dependency on family for decision making. The normative requirement is for competent individuals to decide autonomously and voluntarily. However, autonomy comes in varying degrees, because persons are enmeshed in social bonds. In Pakistan, it is in relation to and with other members of the family that persons usually define themselves. Dependence on family members for making decisions including medical decisions is not unique to Pakistan (see Akabayashi and Slingsby 2006; Chen and Fan 2010; Dein and Bhui 2005; Ezeome and Marshall 2009; Shaibu 2007; Turoldo 2010; Xiaomei 2011).
The study shows that in the participants’ experience, this dependency can be useful—where it coincides with the best interest of the patient. Sometimes, however, it generates ethical challenges, especially where there is a conflict between participants’ moral values and the patriarchal system. Some physician-researchers reported struggling with tensions between a patient's interest on one side and the family's decision on the other; sometimes the family's decisions were not in the patient's interests. For example, patients could not enroll in a potentially beneficial trial, declined necessary treatment, or underwent ineffective treatment from alternate sources, not because it was their (autonomous) decision but because their—especially women's—ability to decide independently was constrained. Similar situations are reported in India and Kenya where female participants refused participation because they were not in a position to make independent decisions (Gitanjali et al. 2003; Marshall 2008). A study from Karachi (in southern Pakistan) reports that in the case of conflict over research participation between the family and research participants, 74% of the respondents felt that if the research participants are men, then their opinion has primacy, whereas only 53% felt the same in case of women (Jafarey 2006).
Is this form of heteronymous decision making (i.e., decisions imposed by others), where the rights and best interests of the participants may not be considered, ethically tenable? Under the rubric of respect for persons, practices that transgress a patient's right to promote her well-being (and dignity) are unethical (Fitchett et al. 2011; NCOB 2002; UNESCO 2005). On this view, the uncritical acceptance of heteronymous decision making, which may compromise a patient's best interest (and rights), is unethical. During consultations, while engaging with the family, it is essential for physician-researchers to encourage patients to express their values and involve them in decision making. They then become active participants, rather than passive recipients of decisions made by others (Hill et al. 2008). This will help to “engender equality, enable trust, and foster solidarity: these are normative aspirations” (Emerson et al. 2009, 102).
There are, however, advantages of this dependency on family. For example, when patients are overwhelmed by illness, the capacity to comprehend and understand and to reach an autonomous decision is reduced (O'Neill 2002). In these circumstances, patients delegate the decision making to their surrogates. Such “mutual consultation” can help “clear their mind” and “gives them confidence;” this is predicated on families having ties of affection and care (Mumtaz and Salway 2009). Patients entrust others with decision making because they value “interdependence, solidarity and trust” (Turoldo 2010:549). It is their (autonomous) decision to do so. On this view, respect for persons requires respecting what they value.
There are several limitations to this study. The author was of the same profession and ethnicity as the research participants, and this may have influenced their responses. On one hand, it may have put them at ease; on the other, it may have put them on guard about being tested for their ethical practices. Moreover, the findings are based on the experiences of physician-researchers from hospitals in one city and reflect what physician-researchers reported but not necessarily what they do in practice.
Although the preliminary coding scheme was reviewed by the author's advisors, the interviews, coding, and analysis were conducted by the author alone; as such, the findings reflect just one researcher's view of the data. Other themes related to informed consent, such as the reasons patients have for consenting to research and whether consent ought to be verbal or written, were not reported here due to space constraints. In addition, patients’ perspectives regarding informed consent were not reported here.
In conclusion, if respect for persons is the ethical goal, it is necessary to reflect on the local moral worlds. Under the rubric of respect for persons, patients’ values ought to be respected; therefore, patients’ reliance (emotional and otherwise) on the family should be valued. However, the same principle urges us to be attentive to the patient's interests, which may be violated by “heteronymous decision making.”
How then to uphold respect for persons and be sensitive to the local context? This article recommends an approach that is simultaneously practical and sensitive to the local sociocultural environment: Physician-researchers ought to involve patients in decisions concerning them without undermining the importance of the family. This can be accomplished by talking to the patient in conjunction with the family—giving the patient a chance to voice her values and the family the confidence that they are a part of the decision-making process too. However, in so doing, it is imperative that the patient's well-being is not jeopardized by considerations of or for the family. Since time is an issue for physician-researchers, it is proposed that other medical staff in these teaching hospitals (e.g., house physicians, clinical psychologists) may be engaged for this deliberative process.
Acknowledgments
I acknowledge with thanks the guidance and helpful comments of Professor Michael Parker, Dr. Alison Shaw, and Dr. Mohammad Anwar. I thank my research participants, for without them this project would not have come to fruition. This article has benefited substantially from the encouraging and thoughtful comments of the associate editor and two anonymous reviewers of AJOB Primary Research, for which I thank them. This paper is part of my D.Phil, research funded by the Wellcome Trust Biomédical Ethics grant (WT 086307) and was presented at the 5th Postgraduate Bioethics Conference, London (2011). It has benefited from the insightful comments of my colleagues. I am also grateful to Peter Pritchard for his help with the review of the initial draft and Tahir, Hasan, Hamza, and Hussein for their support.
REFERENCES
- Akabayashi A., Slingsby B. Informed consent revisited: Japan and the US. American Journal of Bioethics. 2006;6:9–14. doi: 10.1080/15265160500394549. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. S. Understanding understanding: An important step toward improving informed consent to research. AJOB Primary Research. 2010;1(2):1–3. [Google Scholar]
- Appelbaum P. S. Understanding understanding: An important step toward improving informed consent to research. AJOB Primary Research. 2010;1(2):1–3. [Google Scholar]
- Beauchamp T. L., Childress J. F. Principles of biomedical ethics. New York: Oxford University Press; 2001. [Google Scholar]
- Brock D. Philosophical justification of informed consent in research. In: Emanuel E. G., Crouch R. A., Lie R. K., Miller F. G., Wendler D., editors. The Oxford textbook of clinical research ethics. New York: Oxford University Press; 2008. pp. 606–611. [Google Scholar]
- Buchannan A., Brock D. Deciding for others—The ethics for surrogate decision making. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Buchannan A., Brock D. Deciding for others—The ethics for surrogate decision making. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Chen X., Fan R. The family and harmonious medical decision-making: Cherishing an appropriate Confucian moral balance. Journal of Medicine and Philosophy. 2010;35:573–586. doi: 10.1093/jmp/jhq046. [DOI] [PubMed] [Google Scholar]
- Dawson L., Kass N. E. Views of US researchers about informed consent in international collaborative research. Social Science & Medicine. 2005;61(6):1211–1222. doi: 10.1016/j.socscimed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- DeCosta A., S'Souza N., Krishnan S., et al. Community based trials and informed consent in rural India. Journal of Medical Ethics. 2004;30:318–323. doi: 10.1136/jme.2002.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dein S., Bhui K. Issues concerning informed consent for medical research among non-westernized ethnic minority patients in the UK. Journal of the Royal Society of Medicine. 2005;98:354–356. doi: 10.1258/jrsm.98.8.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelPozo P., Fins J. Islam and informed consent. Cambridge Quarterly of Healthcare Ethics. 2008;17:273–279. doi: 10.1017/S096318010808033X. [DOI] [PubMed] [Google Scholar]
- Emanuel E. J., Wendler D., Grady C. What makes clinical research ethical? Journal of the American Medical Association. 2000;283(20):2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- Emerson C. I., Upshur R. E. G., Daar A. S. Empirical bioethics research in the developing world: When the “is” is close to an “ought”. American Journal of Bioethics. 2009;9:101–103. doi: 10.1080/15265160902874445. [DOI] [PubMed] [Google Scholar]
- Ezeome E., Marshall P. Informed consent practices in Nigeria. Developing World Bioethics. 2009;3:138–148. doi: 10.1111/j.1471-8847.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- Fitchett J. R., Ferran E., Footer K., et al. Health and human rights: An area of neglect in the core curriculum? Journal of Medical Ethics. 2011;37(4):258–260. doi: 10.1136/jme.2010.037556. [DOI] [PubMed] [Google Scholar]
- Gitanjali B., Raveendran R., Pandian D. G., et al. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference? Journal of Post Graduate Medicine. 2003;49(2):109–113. [PubMed] [Google Scholar]
- Guest G., Bunce A., Johnson L. How many interviews are enough? Field Methods. 2006;18(1):59–82. [Google Scholar]
- Have H. T. Activities of UNESCO in the area of ethics. Kennedy Institute of Health Care Ethics. 2006;16:333–350. doi: 10.1353/ken.2006.0024. [DOI] [PubMed] [Google Scholar]
- Helgesson G., Ludviggsson J., Gustafsson Stolt U. How to handle informed consent in longitudinal studies when participants have a limited understanding of the study. Journal of Medical Ethics. 2005;31:670–673. doi: 10.1136/jme.2004.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten S. Global bioethics: Utopia or reality? Developing World Bioethics. 2008;8:70–81. doi: 10.1111/j.1471-8847.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Hill Z., Tawiah-Agyemang C., Odei-Danso S., et al. Informed consent in Ghana: What do the participants really understand? Journal of Medical Ethics. 2008;34:48–53. doi: 10.1136/jme.2006.019059. [DOI] [PubMed] [Google Scholar]
- Hongladarom S. Asian bioethics revisited: What is it? And is there such a thing? Eubios Journal of Asian and International Bioethics. 2004;14:194–197. [Google Scholar]
- Jafarey A. M. Informed consent: Views from Karachi. Eastern Mediterranean Health Journal. 2006;12:S50–S55. [PubMed] [Google Scholar]
- Jafarey A., Farooqui A. Informed consent in the Pakistani milieu. Journal of Medical Ethics. 2005;31:93–96. doi: 10.1136/jme.2002.002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R. I. Informed consent and some of its problems in Pakistan. Journal of Pakistan Medical Association. 2008;58:82–84. [PubMed] [Google Scholar]
- Luna F. Paternalism and the argument from illiteracy. Bioethics. 1993;9:284–290. doi: 10.1111/j.1467-8519.1995.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Lynoe N., Hyder S. M. Z., Chowdhury A. M. R., et al. Obtaining informed consent in Bangladesh. New England Journal of Medicine. 2001;344(6):460–461. doi: 10.1056/NEJM200102083440617. [DOI] [PubMed] [Google Scholar]
- Lynoe N., Hoyer K. Quantitative aspects of informed consent: considering the dose response curve when estimating quantity of information. Journal of Medical Ethics. 2005;31:736–738. doi: 10.1136/jme.2005.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macer D. The globalization of ethics and balancing cultural diversity with universal bioethics. In: Cam P., Ibana R. A., Van Duc Pham, editors. Philosophical perspectives on globalization. Seoul: Korean National Commission for UNESCO; Asia Pacific Philosophy Network for Democracy (APPEND); 2006. [Google Scholar]
- Marshall P. Informed consent in international health research. Journal of Empirical Research on Human Research Ethics. 2006;1:25–42. doi: 10.1525/jer.2006.1.1.25. [DOI] [PubMed] [Google Scholar]
- Marshall P. A. Cultural competence and informed consent in international health research. Cambridge Quarterly of Health Care Ethics. 2008;17:206–214. doi: 10.1017/S0963180108080237. [DOI] [PubMed] [Google Scholar]
- Molyneux C., Peshu N., Marsh K., et al. Understanding of informed consent in a low-income setting: Three case studies from the Kenyan coast. Social Science & Medicine. 2004;59:2547–2559. doi: 10.1016/j.socscimed.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Moodley K., Pather M., Myer L. Informed consent and participants perception of influenza vaccine trials in South Africa. Journal of Medical Ethics. 2005;31:727–732. doi: 10.1136/jme.2004.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz Z., Salway S. Understanding gendered influences on women's reproductive health in Pakistan: Moving beyond the autonomy paradigm. Social Science & Medicine. 2009;68(7):1349–1356. doi: 10.1016/j.socscimed.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Muthuswamy V. Ethical issues in HIV/AIDS research. Indian Journal of Medical Research. 2005;121:601–610. [PubMed] [Google Scholar]
- Mystakidou K., Panagiotou I., Katsaragakis S., et al. Ethical and practical challenges in implementing informed consent in HIV/AIDS clinical trials in developing or resource-limited countries. Journal of Social Aspects of HIV/AIDS. 2009;6:46–57. doi: 10.1080/17290376.2009.9724930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabulsi M. Y., Khalil M. Y., Makhoul J. Parental attitudes towards and perceptions of their children's participation in clinical research: A developing-country perspective. Journal of Medical Ethics. 2011;37:420–423. doi: 10.1136/jme.2010.035899. [DOI] [PubMed] [Google Scholar]
- Nuffield Council on Bioethics. Research-developing-countries-ethical-framework. 2002. Available at: http://www.nuffieldbioethics.org (accessed January 16, 2011)
- Newton S. K., Appiah-Poku J. The perspective of researchers on obtaining informed consent in developing countries. Developing World Bioethics. 2007;7(1):19–24. doi: 10.1111/j.1471-8847.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- O'Neill O. Public health or clinical ethics: thinking beyond borders. Ethics and International Affairs. 2002;16:35–45. doi: 10.1111/j.1747-7093.2002.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Sanchez S., Salalzar G., Tijero M., et al. Informed consent procedures: Responsibilities of researchers in developing countries. Bioethics. 2001;15(5/6):398–412. doi: 10.1111/1467-8519.00250. [DOI] [PubMed] [Google Scholar]
- Sankar P., Jones N. L. Semi-structured interviews in bioethics research. In: Siminoff L. J., Siminoff L. A., editors. Advances in bioethics: Empirical methods for bioethics: A primer. Oxford: Elsevier; 2008. p. 11. [Google Scholar]
- Shaibu S. Ethical and cultural considerations in informed consent in Botswana. Nursing Ethics. 2007;14(4):503–509. doi: 10.1177/0969733007077884. [DOI] [PubMed] [Google Scholar]
- Sirinivasan S., Loff B. Medical research in India. Lancet. 2006;367:1962–1964. doi: 10.1016/S0140-6736(06)68861-2. [DOI] [PubMed] [Google Scholar]
- Sreenivasan G. Does inform consent to research require comprehension? Lancet. 2003;362:2016–2018. doi: 10.1016/S0140-6736(03)15025-8. [DOI] [PubMed] [Google Scholar]
- Turoldo F. Relational autonomy and muticulturalism. Cambridge Quarterly of Health Care Ethics. 2010;19:542–549. doi: 10.1017/S0963180110000496. [DOI] [PubMed] [Google Scholar]
- UNESCO. Universal declaration on bioethics and human rights. 2005. Available at: http://unesdoc.unesco.org/images/0014/001461/146180E.pdf(accessed January 16, 2011. [PubMed]
- Weijer C., Miller P. Therapeutic obligation in clinical research. Hastings Center Report. 2003;33(3):3. [PubMed] [Google Scholar]
- Widdows H. Global bioethics moral neo-colonialism? An investigation of the issue in the context of bioethics. Bioethics. 2007;21(6):305–315. doi: 10.1111/j.1467-8519.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki. 2008. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html(accessed January 16, 2011)
-
Xiaomei Z. Diversified and in harmony, but not identical (
 ): Harmonising international guidelines with cultural values and national traditions. Asian Bioethics Review. 2011;3:31–35. [Google Scholar]
): Harmonising international guidelines with cultural values and national traditions. Asian Bioethics Review. 2011;3:31–35. [Google Scholar] - Yousaf R., Fauzi A., How S. H., et al. Awareness, knowledge and attitude towards informed consent among doctors in two different cultures in Asia: across sectional study in Malaysia and Kashmir, India. Singapore Medical Journal. 2007;48(6):559–565. [PubMed] [Google Scholar]


