Abstract
Objectives. In 2007 neonatal screening (NNS) was expanded to include screening for sickle cell disease (SCD) and beta-thalassaemia. Up until that year no formal recommendations for haemoglobinopathy (carrier) screening existed in the Netherlands. Although it has been subject to debate in the past, preconceptional and prenatal haemoglobinopathy carrier screening are not part of routine healthcare in the Netherlands. This study aimed to explore the decision-making process of the past: why was the introduction of a screening programme for haemoglobinopathy considered to be untimely, and did ethnicity play a role given the history in other countries surrounding the introduction of haemoglobinopathy screening?
Design. A witness seminar was organised, inviting key figures to discuss the decision-making process concerning haemoglobinopathy screening in the Netherlands, thereby adding new perspectives on past events. The transcript was content-analysed.
Results. The subject of haemoglobinopathy screening first appeared in the 1970s. As opposed to a long history of neglect of African-American health in the United States, the heritage of the Second World War influenced the decision-making process in the Netherlands. As a consequence, registration of ethnicity surfaced as an impeding factor. However, overall, official Dutch screening policy was restrained regarding reproductive issues caused by fear of eugenics. In the 1990s haemoglobinopathy screening was found to be ‘not opportune’ due to low prevalence, lack of knowledge and fear of stigmatisation. Currently the registration of ethnicity remains on the political agenda, but still proves to be a sensitive subject.
Discussion. Carrier screening in general never appeared high on the policy agenda. Registration of ethnicity remains sensitive caused by the current political climate. Complexities related to carrier screening are a challenge in Dutch healthcare. Whether carrier screening will be considered a valuable complementary strategy in the Netherlands, depends partly on participation of representatives of high-risk groups in policy making.
Keywords: screening, haemoglobinopathy, sickle cell, ethnicity, reproductive choice, eugenics
Introduction
Haemoglobinopathies (HbP), such as sickle cell disease (SCD) and thalassaemia, are serious autosomal recessive disorders characterised by severe anaemia, variable but severe and debilitating morbidity and a shortened lifespan. In the past, HbPs occurred more frequently in areas where malaria is or was endemic, such as Africa, the Mediterranean area, the Middle East and South-East Asia, but they are now common in most countries worldwide due to increasing migration (Weatherall and Clegg 2001). Globally, approximately 5% of the population is a carrier of a significant variant of haemoglobin disorders with as many as 40% carriers in some regional populations (Modell and Darlison 2008). The prevalence of a positive carrier status of HbP in the Netherlands has been estimated at 4–13.6% depending on ethnic background (Giordano and Harteveld 2006). The birth prevalence of severe hereditary HbP in the Netherlands was 64 infants in 2007 among 182,000 total births (Peters et al. 2009).
Parents who are both carriers of HbP have a one in four chance in each pregnancy of giving birth to a child affected with the disease. Carriers can be identified by simple blood tests [Hb-electrophoresis or High Performance Liquid Chromatography (HPLC)]. Couples could then be informed about their risk, preferably before pregnancy.
The first HbP screening programmes were developed during the 1970s in Mediterranean countries such as Italy, Cyprus and Greece and were aimed at thalassaemia. The United States (US) introduced SCD in neonatal screening (NNS) programmes in some states in the 1970s, but screening became more widespread after publication of the consensus document of the National Institutes of Health (NIH) in 1987 (NIH 1987). In the United Kingdom (UK) the NHS Sickle Cell and Thalassaemia Screening Programme was the first service in the world aiming to link antenatal and NNS. The programme was officially launched in 2001 (NHS Sickle Cell & Thalassaemia Screening Programme 2011).
In 2006 the WHO urged member states to increase the awareness of sickle cell anaemia and to develop systematic and comprehensive programmes for the screening of (the carrier status of) SCD (WHO 2006). HbP programmes based on these recommendations have been developed in several other countries both in and outside Europe and in low, middle and high income countries (WHO 2008).
In the Netherlands, HbP screening did not attract much attention on a national level until 1994 when a report was commissioned by the Consultative Committee Minorities of the Department of Health investigating the possibility of introducing a screening programme for HbP (Rengelink-van der Lee et al. 1994). On the basis of a low prevalence of HbP, fear of stigmatisation of carriers, lack of knowledge amongst both professionals and those at risk the authors concluded that introducing a screening programme was ‘not opportune’ at that time (Schulpen et al. 1998).
Carrier screening in general never appeared high on the policy agenda in the Netherlands. At the moment of performing the Witness Seminar (July 2009) the National Screening Programme in the Netherlands did not include carrier screening of any type of genetic disorder and public discussion on the subject was also absent. The programme does include cascade screening for familial hypercholesterolaemia (FH), screening for neural tube defects in pregnancy (since 2007) as well as trisomy 21 for women above the age of 36 and NNS. In contrast to SCD, cystic fibrosis (CF) was not included in the NNS until 2011. The discussion about CF has mainly been dominated by the suboptimal specificity and the fact that the mutation panel has a different sensitivity for amongst others the Turkish population as compared to western Europeans.
As a result of the discussions on HbP in the 1990s and in the absence of community pressure or sufficient patient demand, no public information on testing for HbP (or the carier status) has been available in the Netherlands before 2007. Until that time testing was only carried out on the basis of unresolved anaemia or established familial risk (van Wijk et al. 2003, de Jonge 2005, Jans and Beentjes 2010).
Information was only available through non-governmental patient organisations. Following a 2005 Health Council report, the NNS programme was expanded in 2007 including screening for SCD (Health Council of the Netherlands 2005). The primary aim of this screening was to identify SCD patients so that early complications of this disease could be prevented. As a result of discussion at the start of the expanded NNS programme it was decided to report on alpha- and beta-thalassaemia as well. For beta-thalassaemia, optimal sensitivity in the laboratory is pursued. Identifying other HbP is not official policy but remains subject of debate. If a case of HbH (thalassaemia) disease or other relevant HbP is diagnosed in the NNS programme, the result is reported to the parents because it is clinically relevant, although unintentionally found. Screening also leads to unsought identification of carriers. Parents may opt out from receiving information on carrier status of their child by ticking a box on the test card. In a survey, 62 out of 3200 parents (1.9%) indicated to have opted out (Lanting et al. 2008). Those parents whose child is found to be a carrier of HbS (sickle cell; other carrier states are not communicated) are invited by their family doctor to be tested to allow for possible reproductive choices in subsequent pregnancies. However, in 2007, only 20 parents of the 806 children with HbS carrier status were referred for genetic counselling, whereas based on a carrier prevalence of 10%, 80 carrier couples were expected (Vansenne et al. 2009).
In 2007 the Health Council of the Netherlands published a report on the subject of preconception care (Health Council of the Netherlands 2007) recommending a pilot study on carrier screening for HbP (and CF); however, to date no governmental advice has been issued to introduce a broad preconceptional screening programme for HbP.
In the US, social activism surrounding race relations and disease dramatically altered the issues of race and health because affected minority groups came together themselves to challenge many mainstream assumptions (Wailoo 2006). Previously the top-down implementation of a screening programme met with opposition; the racial identity of white patients was questioned, social concerns about the interbreeding of races were voiced (Tapper 1999). The community of African Americans viewed screening with suspicion and as part of the long history of interference with self-determination (Wailoo and Pemberton 2006). Indeed, according to Wailoo and Pemberton, ‘(…) every aspect of SCD in the United States speaks to the problem of race and the social condition of African Americans’ (Wailoo and Pemberton 2006). Although screening for SCD has been accepted since the 1980s, screening for carrier status has been the cause of ongoing controversy. Most recently marked by discussion of the testing of recruits to the US Armed Forces and professional athletes (Mitchel 2007, Bonham et al. 2010, Stein 2010).
In the UK, it took considerable effort by the community and health professionals to attract policy interest. They had to overcome barriers of inequality and (institutionalised) discrimination before positive results were achieved by exerting political pressure on the relevant agencies (Anionwu and Atkin 2001). Given the history in the US and the UK, it was questioned whether Dutch policy-makers and (health) professionals were aware of the issues of discrimination and stigmatisation thus causing hesitation about the introduction of a screening programme in the Netherlands. It is not apparent whether elements of the history and discussions in both the US and UK had any (indirect) influence on the Dutch discussion in the 1980s and 1990s. It would be interesting to perform a case study focusing on a country other than either the US or the UK to investigate the agenda setting of a disorder with differential sensitivities in different ethnic groups and with a growing prevalence due to a still increasing group of immigrants and their descendants. This is especially relevant in the light of the fact that the 2007 expansion of the NNS programme has raised discussion of whether or not a carrier screening programme should be introduced in the Netherlands, renewing the discussion which followed the publication of the report in 1994 (Kievits and Adriaanse 2007, Giordano 2008, Cornel et al. 2009, 2011).
While it would be interesting to make a comparison with the history of the US and the UK, to do so would go far beyond the scope of this study. Attention often focuses on the experience of these two countries whereas the Dutch situation is essentially different. Because programmes such as the ones in the US and the UK cannot automatically be transposed to other countries, we prefer to concentrate on the case history of the Netherlands. Although references are made to the history and situation of these two countries, we explicitly did not aim to carry out a comparative study. The aim of this study was to explore why and when the issue of preconception, antenatal or neonatal testing of asymptomatic persons at risk of HbP did or did not receive any attention on the agenda of the Dutch public health authorities. To what extent was this influenced by the political climate at the time and potentially deep-seated concerns about the role of ethnicity and the fear of discrimination?
Methods
Witness seminar
The formulated research questions were investigated using a witness seminar. This method was developed by the Institute of Contemporary British History (ICBH) as a special form of oral history, in which several experts, researchers and policy-makers who each have been associated with a particular set of circumstances or events, are invited to meet in order to explain and debate their recollections of a certain time period and subject (Centre for Contemporary British History 2011). This enables researchers to elaborate on developments in the past and on traditional sources of historical research such as the existing literature. The advantage of using this method to investigate the subject of (the absence of) HbP screening in the Netherlands is that it may generate a better understanding of the original definition of the problem, the collaborative relationships and the controversies. Apart from supporting the current discussion with new background material, this method further enables exploration of possible barriers against the introduction of a broader screening programme for HbP and other ethnicity-related disorders.
Preparations
The Dutch literature, both scientific and grey literature (information produced on all levels in electronic and print formats not controlled by commercial publishing; Grey Literature Network Service 2010), was scrutinised to identify Dutch articles concerned with HbP, both research and otherwise, and to identify those involved in the decision-making process and the discussion about the introduction of a screening programme for HbP.
The search was carried out in the most important Dutch medical journals such as the Dutch Journal of Medicine, General Practitioner & Science, Dutch Journal of Obstetrics and Gynaecology, Journal of Health Science and The Midwives’ Journal. All articles identified were scrutinised for further literature by snow-ball method. Authors, the Ministry of Health archives and non-governmental organisations which were possibly involved in the decision-making process [such as the patient organisation for HbP (OSCAR) and the VSOP (Dutch Genetic Alliance)] were contacted for further documentation, information and correspondence.
Key figures for example clinicians, scientists and policy-makers were contacted and interviewed by telephone.
The collected material was used to identify themes and to construct a discussion guide for the witness seminar (Box 1) which lasted a full day and was divided into three sessions. Key witnesses were invited to attend the seminar.
Box 1.
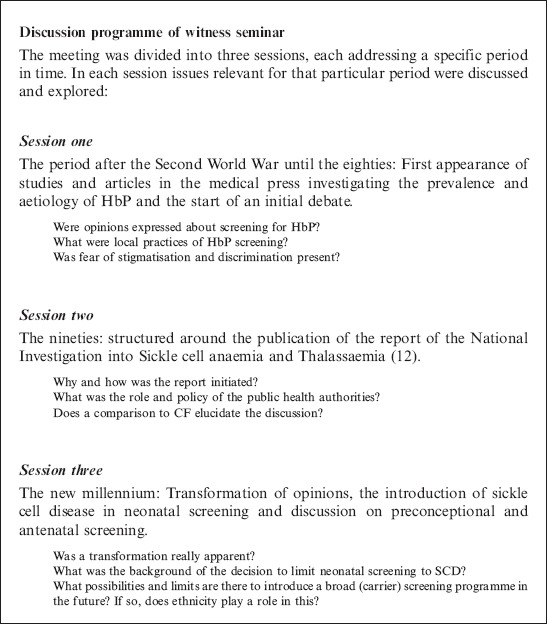
Discussion programme
The study was approved by the Medical Ethics Committee of the VU University Medical Center, Amsterdam.
Study sample
Fourteen witnesses who were involved in the past discussions or decision-making process in the past were invited to participate. They were either active in the field of obstetrics, haematology, paediatrics, genetics, epidemiology, ethics, clinical chemistry, or were working as officials for the Health Inspectorate or the Department of Health. All members of the original advisory committee to the National Investigation into Sickle cell anaemia and Thalassaemia (Rengelink-van der Lee et al. 1994) were contacted and invited to attend the witness seminar (all were able to attend apart from one who was subsequently replaced by a colleague).
The meeting was chaired and facilitated by a medical historian (EH). An introduction to the meeting was presented outlining the historical context of the topic in order to activate the memory of those attending the seminar.
Analysis
Socio-technical analysis
For the analysis of our results we used a study which was carried out to obtain more insight into the process of the potential implementation of a screening programme identifying carriers of CF and HbP before pregnancy (Achterberg et al. 2007). The study was based on the model of co-evolution between technology and society (Rip and Kemp 1998) and helps to identify constraining and enabling factors intrinsic to the introduction of a screening programme for HbP carrier status. For the successful introduction of new technological options, such as screening, attunement between stakeholders on various issues is necessary (Achterberg et al. 2007). These issues range from the technological options necessary to carry out such a screening programme to the necessary facilities and services, the demand particularly by the population at risk, and the political and cultural acceptability of a screening programme for HbP.
We used this model to compare the past to the present situation and to understand why the introduction of a screening programme may or may not have been possible in the past. Including what kind of interactions should be facilitated before the introduction of a screening programme can be possible in the future.
Further analysis
The meeting was fully recorded and the entire discussion was transcribed verbatim and three researchers (SJ, CvE and AMP) were present to take notes.
A full copy of the transcript was sent to all participants for approval and corrections. Such amendments were only allowed to be stylistic or mistakenly remembered facts such as names or dates. The transcript was independently contentanalysed and relevant themes were identified and categorised by four researchers (SJ, CvE, AMP and EH).
The result of the literature search and additional sources of information, such as correspondence and interviews, were used to resolve any disagreements in the discussion.
The central themes identified are discussed in this article and are illustrated with quotations.
Results and discussion
Session one: from the Second World War until the 1980s
Isolated scientists and doctors
Following the Second World War and prior to the 1980s, scientists and doctors began to show an interest in (screening for) HbP. Gradually (research) articles began to appear in the Dutch medical press, originating from a few academic centres in the Netherlands (Huisman et al. 1954, van der Sar 1967, Muntinghe et al. 1971). Initial policy was ad hoc and local, influenced by the interest of specific clinicians. The interest of scientists in the northern city of Groningen, with few inhabitants of immigrant descent, was generated by a professor of paediatrics who had a specific interest in blood group antagonisms. He created a research collaboration with colleagues from the Dutch Caribbean island of Curaçao which originally generated his interest in HbP (Jonxis 1977). This resulted in the thesis of a gynaecologist entitled Haemoglobinopathy Screening in Pregnancy (Landman 1988) which focused primarily on antenatal screening and investigated the influence of HbP on the outcome of pregnancy. An investigation into the HbP status of the child was also included. In the 1960s, the University of Leiden in the western part of the Netherlands attracted a human geneticist, who subsequently developed what would become a well-known reference laboratory for HbP.
Meanwhile an interest in HbP in Amsterdam had also been generated due to a large population of immigrants in the city and the specific interest of doctors specialised in tropical medicine. High risk individuals who attended the local outpatient clinic for tropical medicine, were offered ad hoc (preconception) screening which would inform them of their carrier status. This was carried out as an extra service. Whether this resulted in any further action being taken remains unclear:
We didn't screen just because it was interesting and it wasn't done for research purposes either. It was probably meant to offer people the possibility of [reproductive] choice so that they could take this into account when thinking about their [future] offspring. (Professor of tropical medicine)
However, doctors and scientists had little or no contact with each other and there was no collaboration between universities and their departments on this subject. An explanation for this may be that one department was self-supporting in terms of diagnostic capacity and another centre was isolated because of geographic location. A sense of urgency was not yet perceived by any of the experts:
I think the impact of the research in Groningen has been limited because it was situated in Groningen [and therefore geographically relatively isolated]. The research [on HbP] clearly identified the problems and gave excellent [clinical] advice. But because we were so remotely situated from most of these problems, the results and conclusions weren't picked up by other professionals in the country. No issues of discrimination by colour or anything like that played a part here. (Professor of obstetrics, formerly assigned to the University of Groningen in the north of the Netherlands)
In 1985 a thesis was published in Amsterdam entitled Sickle Cell Disease in the Netherlands (Aluoch 1985) that primarily investigated the prevalence of HbP, haematological and clinical variables and treatment in the Netherlands. The author touches on the subject of HbP screening with a singular closing statement: ‘To omit the performance of Hb electrophoresis in a patient with a Mediterranean or “negroid” background should be considered malpractice’ (Aluoch 1985).
Start of the debate
Before 1980, the population at risk was still relatively small; therefore, HbP received little or no attention. However, due to an increasing influx of migrant workers, the population at risk increased dramatically during the 1980s (Centraal Bureau voor de Statistiek 2010). This resulted in an attempt to supply immigrant minorities with extra support:
… It wasn't until the end of the 80's, now over twenty years ago, that the Ministry of Health had the idea that we should do something coordinated for migrant minorities. We should at least do something about the access to health care for migrant minorities. All the departments within the Ministry of Health put together a budget of three million guilders per year [less than one million English pounds]. They appointed a coordinator, a new job function, which was me. (Civil servant of Department of Health)
In comparison to the US and the UK, it is interesting to note that the initiative for specific health needs for immigrant workers was initiated by the government and not by societal organisations representing the population at risk.
In terms of policy context it is relevant to point out that both in the Dutch Parliament and in society a public debate ensued during the second half of the 1980s when new possibilities for genetic testing and screening became increasingly clear (van El et al. 2010b). A documentary series discussing both the possibility of selecting foetuses and the offer of antenatal testing (Kaizer 1987) was broadcast in 1987 and stirred alarm about the social and ethical consequences of genetic testing. At the end of that same year, the Ministry of Health produced a report on the prevention of congenital anomalies (The Dutch House of Representatives 1987). By including genetic testing as a means to prevent congenital anomalies and to enact ‘responsible parenthood', the suggestion was raised unintentionally that the government would favour a eugenic population policy, stimulating prenatal testing and abortion reminiscent of the Second World War. In the ensuing debate (de Wert and Engel 1988), it was made clear that the goal of genetic counselling should be to inform people, to help them cope with genetic conditions and to support them to choose a course of action appropriate to their individual risk, their family planning, and their ethical and religious standards and then to act in accordance with that decision (Fraser 1974). The general consensus was that governmental prevention policy should refrain from interference with reproductive genetic issues. At this point in time the term ‘prevention’ could no longer be used in context with reproductive genetic screening in the Netherlands. Whereas in other countries, ‘prevention’ is more readily associated with (HbP) screening, ‘offering a reproductive choice’ would be the optimal preferred terminology in the Netherlands (Health Council of The Netherlands 1989). Reproductive choice refers to options that are available to parents when they are both carriers: adoption, remaining childless, change of partner, accept the risk of a possibly affected child, use of donor gametes, or use of prenatal diagnosis and termination of affected pregnancies. Pre-implantation Diagnostics (PID) was not yet available at this time. Concern for the expectations of the at-risk community was not apparent until later on.
In the wake of these debates, governmental policy regarding genetics and reproductive issues became restrained. This probably influenced the 1989 governmental decision led by Christian Democrats and with full support of Parliament not to implement prenatal screening for neural tube defects (The Dutch House of Representatives 1989–1990a, b).
Carrier screening for HbP was not discussed until the second half of the 1980s, when a book entitled Can I Have Your GenePassport? was published exploring the ethical issues related to carrier screening in reproductive healthcare (de Wert and de Wachter 1990). The book resulted from a report that was commissioned by the Department of Health and presented in 1988:
Our first conclusion was that carrier screening could be defended from an ethical point of view given certain conditions. One of the important conditions is the aim of the screening: Is this to maximise prevention or to maximise selective abortion, is it about population eugenics or does it facilitate informed choice. We opted for the last one. (Professor of ethics, author of the book)
The above statement also illustrates the importance of terminology used at the time and how it also generated some discussion: some people preferred to use the term ‘prevention’ whereas ‘informed choice’ was the concept that came to be preferred:
The professor of my department was like a lion in his cage: he was roaring (with me) in the department that prevention [of HbP] should be implemented. But when he had to explain this in public, he was a lot more careful and said that we were the researchers and prevention was the responsibility of the doctors. But they didn't do very much, so the word ‘prevention’ came from us. (Clinical chemist and head of reference laboratory for HbP)
Besides the more general restraint towards screening for reproductive options, another sensitive issue, the registration of ethnicity also became part of the HbP screening debate. Since the 1970s a law on the registration of personal data was in preparation. The law itself was a result of societal commotion starting in 1970 when organisations and journalists stirred up a public protest against the national census. Intellectuals warned against the danger of a government being able to register religion or ethnicity referring to the misuse of personal data resulting in the mass deportation of Jewish citizens in the Second World War. In the proposed law, it was explicitly stated that registration of ethnicity is prohibited (Blessing 2005). This debate continued into the 1980s when the Council of the Sick Fund sent a letter to the State Secretary of Health concerning its statement on the registration of ethnicity:
On November 7 1984 the chairman [of the Council of the Sick Fund] received a telex … in which both national trade unions expressed their opinion of not supporting ethnic registration according to nationality. This accurately reflects the mood at that time. (Former officer of the Health Inspectorate)
Both researchers and other professionals were aware of these issues which influenced their decision-making:
I was involved with the introduction of hepatitis B screening in 1989 and this discussion was very relevant, who do you screen: certain ethnicities at risk or the entire Dutch population? It was said at the time that it is impossible to ask women about their ethnicity, the midwives don't want this, nobody wants this. So we subsequently opted for universal hepatitis screening. (Head of central screening laboratory)
In the US, the debate on sickle cell screening was influenced by suspicions in the Black community that screening was being used to determine racial purity in order to secure white supremacy and specifically black inferiority (Tapper 1999). Scientists, policy-makers and politicians in the Netherlands seemed to have been oblivious to this debate. However, the authors of the aforementioned book Can I Have Your Gene Passport? referred to the situation in the United States:
When we focus on sickle cell disease: the classic example of bad management was the United States. This had much to do with failing technology, miscommunication, confusing the disease with carrier status and in our view specifically because it was introduced in a top-down fashion without any interaction with or involvement of the population at risk. This resulted in our recommendation that (…) you need to create support by involving the population at risk. Without support from the population at risk, invariably you will be accused of racism et cetera. (Professor of ethics)
Session two: the 1990s: introduction of a new law and two reports
This decade was marked by political influence on screening in general and the National Investigation into SCD and Thalassaemia (Rengelink-van der Lee et al. 1994). The strained attitude regarding genetic screening for reproductive issues that was rooted in the 1980s continued in the 1990s. Although this attitude was supported by a broad range of political parties, the leading influence of the Christian Democratic Party (CDA) is relevant to note, because they were part of the government during many years (van El et al. 2010a). Their view on genetics was elaborated in a 1992 report called Genes and Limits (Commission of the Scientific Institute of the Christian Democrat Party 1992) which was motivated by the introduction of new technology and possibilities in reproductive healthcare. In the report both prenatal screening and preconceptional carrier screening were described as undesirable developments because of both the need to protect the foetus and the psychological burden imposed upon people when knowing their future child may have a serious untreatable condition. The report was to influence the general screening debate for years to come:
Genetics is scary, genetics is dirty (…) it [the report of the Christian Democratic Party] was full of a ‘German’ [based on WWII] aversion against eugenics in the past. It is the additional sum of only negative possibilities. The possibility of empowerment did not occur to them. (Professor of ethics)
Shortly after the publication of Genes and Limits the final report of the National Investigation SCD and Thalassaemia was published by Rengelink-van der Lee in 1994 (Rengelink-van der Lee et al. 1994). This report was not initiated by the Department of Health itself but instead, was commissioned by the Consultative Committee Minorities of the Department of Health. The conclusions were based on the prevalence of HbP disease (which was mainly found amongst adopted children born outside the Netherlands) and the results of an anthropological study. The latter concluded that only very limited knowledge amongst both the population at risk and professionals existed. The researchers, who were also concerned about stigmatisation, concluded that introduction of a screening programme for HbP was not ‘opportune’ at this point in time:
Yes, that was the main question [whether the prevalence of HbP in the Netherlands was high enough to warrant the introduction of a screening programme] and the idea of having preconceptional or antenatal screening. We thought that that would be the best scenario but it didn't seem feasible.
Preconceptionally because the knowledge was far too limited and you would have to be able to reach people at a very early stage (…)…and specifically at that time in the 1990s, the knowledge about inheritable diseases was very limited, both with the older immigrants [those who immigrated to the Netherlands a long time ago], e.g., the people from Surinam, as with more recent immigrants. There was no basis on which a screening programme could be introduced. People felt stigmatized which was a very important feeling (…) the fact is that many parents said: I don't want to know if my child is a carrier of a certain disease because I won't be able to find my daughter a suitable wedding partner. (Professor of paediatrics and co-author of report)
The report fuelled the discussion and generated opposition among some professionals who were clearly disappointed with the report's conclusion (Wierenga 1997, van Rhee et al. 1998). Some argued that developing a NNS programme for SCD would be a first step in the right direction (Wierenga and Luteijn 1999) as was alluded to by the report itself.
In the end the result was a negative advice, it can easily be said that this was a heavy blow for us and which determined the future for the next ten years. (Clinical chemist and head of reference laboratory for HbP)
The criteria of Wilson and Jungner (Wilson and Jungner 1968) were not explicitly mentioned in the final report of the National Investigation into SCD and Thalassaemia (Rengelink-van der Lee et al. 1994) nor did they surface in the discussion that followed the report's publication. However, the research that was part of the report investigated the attitude of the target population which was (unintentionally) an elaboration of one of the criteria of Wilson and Jungner, in so far that the test should be acceptable to the target population.
Although the (problematic) registration of ethnicity is not given any attention in this report, it does seem to have influenced the discussion:
And of course even then we weren't allowed to screen on the basis of ethnicity. It was unheard of to pick out all the black kids and submit them to heel prick screening [to investigate for HbP]. That would be ethically impossible […] The Department of Health said to us: well thank you very much, we don't need to implement any policy on this subject. We will put it in a drawer somewhere. (Professor of paediatrics and co-author of report)
A professor of preventative and curative healthcare for children, who was interviewed by telephone, confirms this:
We talked about screening the population at risk but this was such a sensitive subject: it was considered discrimination and was seen as politically very incorrect, even if it was used as positive discrimination.
The constraining influence of the existing political climate is further illustrated when researchers and clinicians were clearly relieved with a change in government (1994):
I believe the change in government in 1994/5 when the Christian Democrats were no longer in office, was very important. I remember this very clearly. We had a very broad discussion with the Department of Health, including the discussion whether or not to screen for Down syndrome which was of course impossible. When the Christian Democrats left office we were all very relieved. (Clinical chemist)
In 1996 a new law was introduced (Population Screening Act; in Dutch: WBO) based on the criteria of Wilson and Jungner, aimed at protecting the general public against the possible hazards of population screening programmes. The sensitivity towards reproductive issues is illustrated by the fact that this law requires a special licence for certain types of screening such as screening for disorders for which no treatment is available, complicating the introduction of prenatal screening (van El et al. 2010b).
Despite a change in the political climate, policy towards HbP carrier screening did not change. Although researchers felt more comfortable in investigating the subjects surrounding screening and the fact that the population at risk doubled in size during the 1990s (Statistics Netherlands 2010), no screening programme for carrier status of HbP has been introduced to date.
Session three: a new millennium: a change in direction
Turning into a new millennium attitudes change and eventually more attention is given to the health needs of the different populations in the Netherlands.
Though at the end of the 1990s attention to the debate on HbP screening decreased, at the turn of the century the debate resurfaced under the influence of an enthusiastic and very driven molecular geneticist (Giordano 1998).
The Netherlands’ population had changed dramatically in the previous decades (Statistics Netherlands 2010); there were now avast variety of immigrants and their descendants from all over the world; for example, not only from Turkey, Morocco, Surinam and the Dutch Antilles, but also from Africa, South-East Asia and the Middle East. Certain ethnic groups had organised themselves (Overlegorgaan Caribische Nederlanders 2010) and SCD and thalassaemia patients had founded their own organisation (OSCAR) in 1989, although the organisation did not become publicly visible until 1998 (OSCAR 2010).
Certain professionals began to realise that by not specifically tailoring healthcare needs to certain groups that this in fact is discriminatory, for example by not offering HbP screening to groups at risk:
I was in London at the conference of the European Society of Human Genetics [during the nineties] where I spoke to Elizabeth Anionwu [see Anionwu and Atkin 2001] … she said you might be worried about discrimination if you start screening for HbP, but the discrimination you create by not screening is probably far greater. (Em. Professor of clinical genetics)
The debate on the registration of ethnicity was renewed and this time it was approached from a different angle; socio-economic influences in health were being investigated as well as possibilities to reduce them (de Walle et al. 1999, Stirbu et al. 2006, Troe et al. 2006). Both education level and ethnicity are analysed to look for possibilities to reduce inequalities in health. In order to use ethnicity and risk factors more constructively and to avoid any negative connotations, some researchers explored the possibility of combining screening for several groups at risk for different disorders (Lakeman et al. 2006). The Netherlands Organisation for Health Care Research and Development funded several projects on HbP screening after 2000 (van den Tweel et al. 2006, Lakeman et al. 2008, Giordano 2009). Results of these projects have contributed to the debate on potential implementation of HbP (carrier) screening.
In 2005 the Health Council of the Netherlands published a report on NNS which stated that the prevalence of certain disorders is changing, influenced by the composition of the population in the Netherlands (The Health Council of The Netherlands 2005). This report resulted in the expansion of the NNS programme when 14 diseases were rapidly added to the programme, one of which was SCD. It was the first time since the previous debate that an official report put the issue of an ethnicity-related disorder on the (public and political) agenda. The possibility of ethnically targeted screening was dismissed in the report, and universal screening proposed instead. The 2007 Health Council report on preconception care (Health Council of The Netherlands 2007) subsequently explicitly mentioned ethnic background as a risk factor, specifically calling attention to HbP, CF and Tay Sachs disease. Comments published in a national newspaper (Cornel et al. 2008) contributed to the debate which led to discussions in Dutch Parliament and official queries at the address of the Minister of Health. In his reply the Minister of Health made the following statement: ‘However the question is if offering screening to certain preselected groups on the basis of ethnicity is desirable and acceptable in our society’ (Klink 2009).
The issue of screening and ethnicity therefore remains difficult to fathom. The Parliamentary discussion is referred to during the witness seminar:
The minister thinks it should be possible to screen by indication but doesn't mention whether pregnancy is seen as an indication. Whether a woman with ancestors from another country wishes to be pregnant is an indication for screening, is not mentioned either. But he does want carrier testing to be carried out in a genetic centre. (Professor of community genetics)
Clearly the registration of ethnicity has returned to the current (political) agenda. In January 2008 the Dutch Society of Clinical Geneticists (VKGN) gave the State Secretary of Health positive advice in response to questions of the department regarding the relevance of the registration of ethnicity related to care in clinical genetics (Knoers and Leschot 2008). However, hospitals and health professionals are reluctant; it is against hospital policy to register the ethnicity of a patient for the benefit of adequate care and the Dutch Federation of Medical Specialists (KNMG) advises restraint in the matter (Baltesen and Rijlaarsdam 2008).
Subsequently a report has been published by the Netherlands Organisation for Health Care Research and Development recommending the registration of ethnicity in the health care sector to facilitate further research in order to reduce differences in health outcomes amongst different ethnic groups in Dutch society (ZonMW 2009).
Despite being back on the agenda, the registration of ethnicity still proves to be a sensitive issue when, although not health-related, a minister lost her post over this debate in 2008 after suggesting ethnic registration as a means to monitor young offenders.
The late 1990s have been politically dominated by a joint Labour and Liberal government, but the new millennium is once again marked by religiously influenced political parties. Recently this has led to a renewed discussion concerning the late termination of pregnancies following prenatal screening whereby specifically religious parties but also the right wing Party for Freedom argue against late terminations of pregnancy, reducing the options available to parents (NRC 2010). The expectation is that the liberal yet right wing government which was installed in October 2010 will not be very forthcoming. Moreover, preconception care in general suffered a setback as the Minister of Health recently denounced any further commitment to this subject (Schippers 2010).
Socio-technical analysis
After having discussed the findings of the witness seminar and the context of discussions on screening for HbP, we will now address the socio-technical analysis. The witness seminar and the preceding literature search have identified the actors and stakeholders involved in the former discussions and decision-making process about whether or not a screening programme for HbP should be introduced in the Netherlands. These individuals can be categorised into four groups: (1) scientists in the field, (2) healthcare professionals, (3) policymakers and (4) the patients and population at risk (Figure 1). We looked at attunement between these stakeholders both in the past and the present on technical options, facilities and services, demand and issues of political and cultural acceptability. Thus more insight was gained into why it was not possible to implement a screening programme in the past and compare this to the current situation.
Figure 1.
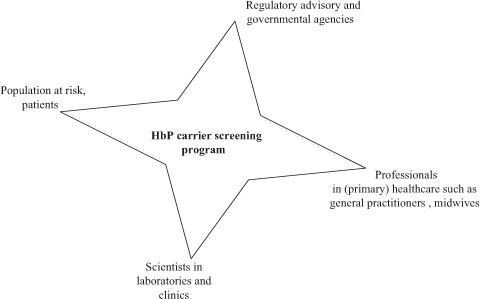
Network of stakeholders and actors involved.
Technological options
In the 1990s, technological options were acceptable as simple and reliable tests were available. HPLC testing for HbP might not yet have been available in all laboratories, however, electrophoresis was a test available to clinicians (Rengelink-van der Lee et al. 1994). Availability and standardisation of testing methods has only improved since this period (Health Council of The Netherlands 2005, Giordano 2006).
Facilities and services
Facilities and services were clearly limited at the time because preconception care was unavailable to the public. Currently preconception services are being offered in most regions; however, this type of care only reaches a very small part of the target population (Hosli et al. 2008). Although preconception services have recently been endorsed by the Dutch Steering Committee on Pregnancy and Childbirth (Steering
Committee Pregnancy and Childbirth 2010), the Minister of Health has refused to financially support further policy development regarding this issue (Schippers 2010).
The National Investigation showed that knowledge amongst professionals was very limited in the early 1990s (Rengelink-van der Lee et al. 1994). Although more attention to the subject has been given since, that is, NNS has been expanded to include SCD (Health Council of The Netherlands 2005), knowledge about these kinds of genetic disorders is still insufficient amongst professionals (Baars et al. 2005, Weinreich et al. 2009).
Besides this, carrier diagnosis is indicated for certain groups of people in case of (unresolved) anaemia as part of the anaemia guideline of both the midwives and the GPs (van Wijk et al. 2003, Jans and Beentjes 2010).
Demand
In the past, limited knowledge amongst the groups at risk also made demand for screening low or non-existent (Rengelink-van der Lee et al. 1994). Recent research has shown that screening is acceptable amongst the groups at risk (Giordano et al. 2006, Weinreich et al. 2009) although cascade screening following a positive carrier status found during the NNS is still limited (Vansenne et al. 2009). After 2000, the patient organisation became more established and expressed the need for implementation of screening services; however, it does not feel as if its voice is being heard (OSCAR, personal communication, 2011).
Political and cultural acceptability
The witness seminar has clearly shown that political and cultural acceptability was non-existent; this was partly caused by the insurmountable discussion on the registration of ethnicity. Moreover, research showed that parents worried about the possible stigmatisation of their affected children (Rengelink-van der Lee et al. 1994). Furthermore, during the 1980s and 1990s the discussion on screening was burdened by the fear of eugenics (van El et al. 2010b). The renewed debate seems to be turning in favour of screening (Cornel et al. 2009) supported by the realisation that ethnicity does play a certain role in healthcare which needs to be investigated further (ZonMW 2009). There is international consensus that a screening programme should be implemented in those countries where HbP is prevalent (WHO 2006), preferably carried out in a primary healthcare setting (Modell and Darlison 2008). Although consensus is still lacking at the national level, health professionals seem willing to carry out screening for HbP (Cornel et al. 2009, Weinreich et al. 2009).
Table 1 gives an overview of the various failing processes of attunement in the past on several dimensions and clarifies which issues have changed in the last decade. This model is helpful in understanding the possibilities and barriers that exist for present-day attunement for implementing HbP carrier screening.
Table 1.
Socio-technical analysis of the past and present with regards to a HbP carrier screening programme.
| Attunement with regard to | Period in 1990s | Present time |
|---|---|---|
| Facilities and services | Insufficient knowledge amongst healthcare professionals. Insufficient collaboration between healthcare professionals. Unclear who should offer and carry out screening programme. No support from a national public health institution. No guideline for anaemia in existence for midwives. No preconception services available. Neonatal screening limited, screening for HbP is not part of the screening. Health authorities decline formulation of recommendations on the basis of report of the National Investigation into Sickle Cell Anaemia and Thalassaemia. |
Well developed testing method for large scale screening. Insufficient knowledge but willingness is increasing amongst healthcare professionals. Carrier diagnosis is part of the anaemia guideline of the midwives and the GPs. Visible public health authority in place which is able to take responsibility for the coordination of screening programmes (RIVM-Centre for Population Screening). Improving collaboration between healthcare professionals under direction of RIVM and professional organisations, but consensus is still insufficient amongst stakeholders. International consensus that a screening programme should be introduced and carried out by primary care professionals. Endorsement of preconception services by the Dutch Steering Committee on Pregnancy and Childbirth. Preconception services available in most regions, although funding postponed by ministry of health. Neonatal screening SCD implemented. |
| Demand | Knowledge amongst population at risk is insufficient. | Knowledge is increasing. OSCAR, patient organisation of HbP, in existence, demanding more (screening) services for group at risk. Uptake of preconception services is low. Screening for SCD in Neonatal screening (2007): Carriers are identified > cascade screening as a result of this screening is limited. |
| Political and cultural acceptability | Parents express worries about finding suitable partners for their daughters therefore screening not acceptable in all cultures. Restrained political attitude on issues of reproductive genetic screening. Disagreements amongst stakeholders. Introduction of law on screening (WBO). |
Renewed debate more in favour of screening. Attitude and culture towards screening in general has changed in the Netherlands, e.g., routine prenatal screening for certain abnormalities implemented (once screening for a certain disorder is implemented > easy to add other conditions). More emancipation of groups at risk. Screening acceptable amongst population at risk. Report by the Health Council of the Netherlands endorses preconception care and identifies certain ethnicities as a risk factor for genetic disorders but this is not seen as a basis on which further policy |
Conclusion
By means of a witness seminar the case history of screening policy in the Netherlands was explored and has demonstrated the influence of the heritage of past events on general healthcare policy, even to this date. Although this method allows researchers to make a further in-depth analysis and explore hidden and sensitive elements of discussions in the past, the method has some weaknesses. The results of the seminar are in part dependent on the participants attending and their recollections which may be hampered by the passing of time. However, by using the group dynamics, participants are invited to respond and add to each other's account, thereby helping to reconstruct past events from different perspectives. While being aware of its limitations, the study is able to address an important issue that has long been neglected by elaborating on traditional and often scarce sources.
Policy makers and healthcare workers have been and still are struggling with the challenge of being able to deliver equitable services for an ethnically diverse population.
As opposed to the US where the history of slavery influenced extremely sensitive discussions surrounding the introduction of (carrier) screening for HbP, healthcare policy in the Netherlands is still burdened by the inheritance of the Second World War when the mass deportations of Jews were supported by a diligent register of the Dutch population which causes ethnic registration still to be problematic until today. In addition, genetic screening for reproductive options proves to be a sensitive subject against the same background. Whereas in the US the National Sickle Cell Disease Control Act was introduced in 1972, followed by the Genetic diseases Act of 1976 and the NIH consensus statement in 1987 (NIH 1987), the discussion in the Netherlands is still ongoing. Difficult and sensitive discussions against a background of collective feelings of guilt and penance have influenced the decision-making process surrounding screening issues, causing extreme apprehension to debate the registration of ethnicity for the benefit of equitable health services. The issue of ethnicity and the fear of possible discrimination proved to be a relevant subject but only played a secondary role in the decision-making process in the end. In the 1990s the restrained political climate regarding genetic screening for reproductive options as illustrated by the Christian Democrat Party and the law on population screening prevented any further development in the HbP screening discussion.
In addition, the Research Report National Investigation into Sickle Cell Anaemia and Thalassaemia made any initiatives towards the introduction of a screening programme by this department superfluous. Although excellent research exploring the possibilities of a screening programme for carrier status of HbP has taken place over the years both in and outside the Netherlands, the results seem to be ‘lost in translation’ (Lenfant 2003). To date, preconceptional and prenatal screening programmes for HbP carrier status have not been introduced in the Netherlands. Screening is still limited to SCD in the NNS programme. The fact that no one, either at governmental level or in the scientific community, convened a meeting at the time to debate and clarify the issues at stake with all professionals and policy-makers involved, exemplifies the lack of attunement at the time.
The discussion in the Netherlands has focused primarily on equality (all persons being equal); however, the realisation that this may limit healthcare equity for some has only surfaced in recent years. Under the influence of increasing immigration to the Netherlands (as elsewhere in Europe), and both departmental and political changes, professional opinion concerning the use of information about ethnicity to benefit public health has begun to change but still remains a sensitive discussion. While a report on the registration of ethnicity by the Health Council of The Netherlands is expected in the near future, a national debate on the introduction of a broader screening programme is urgently required; this should include the voice of the patient organisation which has been insistently calling for further development on this terrain (OSCAR 2010).
Key message
Before 2000, reflecting on the inheritance of the Second World War, reproductive genetic screening and the registration of ethnicity has been politically unacceptable in the Netherlands, causing a barrier for the introduction of HbP carrier screening. It is clear that carrier screening based on ethnicity would only be acceptable under certain conditions, mainly aimed at a guarantee of informed decision-making.
Besides careful analysis of the present situation to resolve existing challenges, future decision-making on an extended screening programme needs to include representatives from groups most at risk of HbP to support the possible implementation of such a programme.
Acknowledgements
The authors wish to thank all the individuals who participated in the witness seminar and S.A. Abbott for his editing and proof reading. This article is the result of a research project of the Centre for Society and Genomics in The Netherlands, funded by the Netherlands Genomics Initiative.
References
- Achterbergh R., et al. Implementation of preconceptional carrier screening for cystic fibrosis and haemoglobinopathies: a sociotechnical analysis. Health Policy. 2007;83(2–3):277–286. doi: 10.1016/j.healthpol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Aluoch J.R. Sickle cell disease in the Netherlands. Origin, prevalence, clinical features and management. University of Amsterdam; 1985. Thesis (PhD) [Google Scholar]
- Anionwu E.N., Atkin K. The politics of sickle cell and thalassaemia. Buckingham, Philadelphia: Open University Press; 2001. [Google Scholar]
- Baars M.J., Henneman L., ten Kate L.P. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genetics in Medicine. 2005;7(9):605–610. doi: 10.1097/01.gim.0000182895.28432.c7. [DOI] [PubMed] [Google Scholar]
- Baltesen F., Rijlaarsdam B. Saving lives with discriminating registration [online] NRC; 2008. Available from: www.nrc.nl [Accessed 14 May]. [Google Scholar]
- Blessing M. The resistance against the census of 1971 [online] Historisch Nieuwsblad. 2005. no. 8. Available from: www.historischnieuwsblad.nl.
- Bonham V.L., Dover G.J., Brody L.C. Screening student athletes for sickle cell trait-a social and clinical experiment. New England Journal of Medicene. 2010;363(11):997–999. doi: 10.1056/NEJMp1007639. [DOI] [PubMed] [Google Scholar]
- Centre for Contemporary British History [CCBH] Oral History Programme [online] 2010. Available from: www.ccbh.ac.uk/witnessseminars.php [Accessed 11 October 2010].
- Genes and limits. The Hague: CDA; 1992. Commission of the Scientific Institute of the Christian Demoratic Party (chaired by Professor E. Bleumink) [Google Scholar]
- Cornel M.C., et al. To screen broader for haemoglobinopathy. Medisch Contact. 2009;45:1874–1877. [Google Scholar]
- Cornel C., Dondorp G., de Wert G. Allow parents the freedom to choose [online] Trouw. 2008. 22 Nov Available from www.trouw.nl.
- Cornel M.C., Lakeman P., Dondorp W. Do not postpone preconceptional carrier screening. Ned Tijdschr Geneeskd. 2011;155:A3205. [PubMed] [Google Scholar]
- de Jonge A. Guidance on preconception care. Utrecht: KNOV; 2005. [Google Scholar]
- de Walle H.E., et al. Effect of mass media campaign to reduce socioeconomic differences in women's awareness and behaviour concerning use of folic acid: cross sectional study. BMJ. 1999;319(7205):291–292. doi: 10.1136/bmj.319.7205.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wert G.M.W.R., de Wachter M.A.W. Can I have your gene-passport? Baarn: Ambo; 1990. [Google Scholar]
- de Wert G.M.W.R., Engel G.L. Genetic counselling as instrument of population eugenics? Some remarks on the report ‘Prevention congenital anomalies’. Medisch Contact. 1988;43:843–845. [Google Scholar]
- Fraser F.C. Genetic counseling. American Journal of Human Genetics. 1974;26(5):636–659. [PMC free article] [PubMed] [Google Scholar]
- Giordano P.C. Haemoglobinopathies in the Netherlands. Diagnostics, epidemiology and prevention. 1998. Thesis (PhD). University of Leiden.
- Giordano P.C. Towards the state of the art neonatal screening for secondary and primary prevention of the hemoglobinopathies in The Netherlands. Validation of technology. Leiden: Leiden University Press; 2006. [Google Scholar]
- Giordano P.C. Doctors should enquire after the ethnicity of their patients [online] News bulletin, Medisch Contact. 2008. Available from: http://medischcontact.artsennet.nl.
- Giordano P.C. Starting neonatal screening for haemoglobinopathies in The Netherlands. Journal of Clinical Pathology. 2009;62(1):18–21. doi: 10.1136/jcp.2008.058826. [DOI] [PubMed] [Google Scholar]
- Giordano P.C., Harteveld C.L. Prevention of hereditary haemoglobinopathies in The Netherlands. Ned Tijdschr Geneeskd. 2006;150(39):2137–2141. [PubMed] [Google Scholar]
- Giordano P.C., et al. Carrier diagnostics and prevention of hemoglobinopathies in early pregnancy in The Netherlands: a pilot study. Prenatal Diagnosis. 2006;26(8):719–724. doi: 10.1002/pd.1490. [DOI] [PubMed] [Google Scholar]
- Grey Literature Network Service. Homepage [online] 2010. Available from: www.greynet.org [Accessed 14 June 2010].
- Health Council of the Netherlands. Heredity: society and science. The Hague: Health Council of the Netherlands; 1989. Publication No. 1989/31. [Google Scholar]
- Health Council of the Netherlands. Neonatal screening. The Hague: Health Council of the Netherlands; 2005. Publication No. 2005/11. [Google Scholar]
- Health Council of the Netherlands. Preconception care: a good beginning. The Hague: Health Council of the Netherlands; 2007. Publication No. 2007/19. [Google Scholar]
- Hosli E.J., et al. Women's motives for not participating in preconception counseling: qualitative study 150. Community Genetics. 2008;11(3):166–170. doi: 10.1159/000113879. [DOI] [PubMed] [Google Scholar]
- Huisman T.H., van der Schaaf P.C., van der Sar A. Studies on hemoglobin abnormality in sickle cell anemia and sickle cell trait. Ned Tijdschr Geneeskd. 1954;98(41):2881–2888. [PubMed] [Google Scholar]
- Jans S., Beentjes M. Anaemia in midwifery practice: guideline. Utrecht: KNOV; 2010. [PubMed] [Google Scholar]
- Jonxis J.H. Hemoglobinopathies in families of foreign workers and other migrant groups. Ned Tijdschr Geneeskd. 1977;121(23):949–954. [PubMed] [Google Scholar]
- Kaizer W. Better than God Documentary. Netherlands: VPRO; 1987. [Google Scholar]
- Kievits F., Adriaanse M.T. Discrimination by inadequate care. Ned Tijdschr Geneeskd. 2007;151:778–779. [Google Scholar]
- Klink A. Answers to the queries from Koser Kaya, member of parliament, on preconceptional carrier screening. The Hague: National Government of the Netherlands; 2009. 2008Z08008/2080906580 Jan. 7. [Google Scholar]
- Knoers V.V.A.M., Leschot N.J. Genetics and ethnicity in letter to State Secretary of health, Wellbeing and Sports. 2008. PG/e 2807914. 22 January.
- Lakeman P., et al. Developing and optimizing a decisional instrument using self-reported ancestry for carrier screening in a multi-ethnic society. Genetics in Medicine. 2006;8(8):502–509. doi: 10.1097/01.gim.0000232461.11153.9a. [DOI] [PubMed] [Google Scholar]
- Lakeman P., et al. Three-month follow-up of Western and non-Western participants in a study on preconceptional ancestry-based carrier couple screening for cystic fibrosis and hemoglobinopathies in the Netherlands. Genetics in Medicine. 2008;10(11):820–830. doi: 10.1097/GIM.0b013e318188d04c. [DOI] [PubMed] [Google Scholar]
- Landman H. Haemoglobinopathies and pregnancy. 1988. Thesis (PhD). University of Groningen.
- Lanting C.I., et al. Evaluation of the neonatal heelprick screening in infants born in 2007 [online] Leiden: TNO Kwaliteit van Leven; 2008. Available from: www.rivm.nl/pns/Images/TNO%20Evaluatie%20hielprik%202008_tcm95-56923.pdf [Accessed 22 July 2011]. [Google Scholar]
- Lenfant C. Shattuck lecture: clinical research to clinical practice: lost in translation? New England Journal of Medicine. 2003;349(9):868–874. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- Mitchell B.L. Sickle cell trait and sudden death- bringing it home. Journal of National Medical Association. 2007;99(3):300–305. [PMC free article] [PubMed] [Google Scholar]
- Modell B., Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organisation. 2008;86(6):480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntinghe O.G., Pietersz R.N., Verhulst J.C. Hemoglobinopathies in immigrants from Surinam. Ned Tijdschr Geneeskd. 1971;115(31):1306–1310. [PubMed] [Google Scholar]
- National Institutes of Health. Newborn screening for sickle cell disease and other hemoglobinopathies. NIH Consensus Statement. 1987;6(9):1–22. April 6–8 [online]. Available from: http://consensus.nih.gov/1987/1987ScreeningSickleHemoglobinopathies061html.htm [Accessed 22 July 2011]. [PubMed] [Google Scholar]
- NHS Sickle Cell & Thalassaemia Screening Programme. Latest News [online] 2011. Available from: http://sct.screening.nhs.uk/ [Accessed 26 May 2011].
- NRC. Christian Union wants to abolish 20 week ultra sound scan [online] 2010. Available from: www.nrc.nl [Accessed 10 February 2010].
- OSCAR. Homepage [online] 2010. Available from: www.oscarnederland.nl [Accessed March 2011].
- Overlegorgaan Caribische Nederlanders [OCaN] Homepage [online] 2010. Available from: www.ocan.nl [Accessed 26 November 2010].
- Peters M., et al. Sickle cell disease in heel injection screening. I. Ned Tijdschr Geneeskd. 2009;153(18):854–857. [PubMed] [Google Scholar]
- Rengelink-van der Lee J.H., van der Most van Spijk M.W., Schulpen T.W. National investigation into Sickle cell anaemia and Thalassaemia: Final Report. Utrecht, The Netherlands: Stichting Gezondheid Derde Wereld Kind; 1994. [Google Scholar]
- Rip A., Kemp R. Technological change. In: Rayner S., Malone E.L., editors. Human choice and climate change. Columbus, OH: Batelle Press; 1998. pp. 327–399. [Google Scholar]
- Schippers E.I. Pregnancy and birth. Ministry of Health, Wellbeing and Sports; 2010. CZ/EKZ 3040205. [Google Scholar]
- Schulpen T.W., et al. Genetic carrier screening for hemoglobinopathies in the Netherlands is not opportune. Ned Tijdschr Geneeskd. 1998;142(18):1019–1022. [PubMed] [Google Scholar]
- Statistics Netherlands [CBS] Population [online] 2010. Available from: www.cbs.nl [Accessed 11 October 2010].
- Steering Committee Pregnancy and Childbirth. A good start: safe health care in pregnancy and childbirth. 2010. Final Report January 6.
- Stein R. Sickle cell testing of athletes stirs discrimination fears. Washington Post. 2010. 20 Sep.
- Stirbu I., et al. Differences in avoidable mortality between migrants and the native Dutch in The Netherlands. BMC Public Health. 2006;6:78. doi: 10.1186/1471-2458-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper M. In the blood. Philadelphia: University of Pennsylvania Press; 1999. [Google Scholar]
- The Dutch House of Representatives (Tweede Kamer der Staten Generaal) Prevention congenital abnormalities. 1987. Report No: TK 20345:2.
- The Dutch House of Representatives (Tweede kamer der Staten Generaal) Population screening neural tube defects. 1989–1990a. Letter from The State Secretary of Welfare, Health and Culture. Parliamentary Documentation. TK 21353:1.
- The Dutch House of Representatives (Tweede kamer der Staten Generaal). Population screening neural tube defects (Report of oral consultation) 1989–1990b. Parliamentary Documentation, TK 21353:2.
- Troe E.J., et al. Ethnic differences in total and cause-specific infant mortality in The Netherlands. Paediatric and Perinatal Epidemiology. 2006;20(2):140–147. doi: 10.1111/j.1365-3016.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- van den Tweel X., et al. Identifying children with sickle cell anaemia in a non-endemic country: age at diagnosis and presenting symptoms. European Journal Pediatrics. 2006;165(8):581–582. doi: 10.1007/s00431-006-0102-7. [DOI] [PubMed] [Google Scholar]
- van der Sar A. Aplastic sickle cell crisis: a report on four cases. Tropical and Geographical Medicine. 1967;19(4):273–285. [PubMed] [Google Scholar]
- van El C.G., et al. Debating about genetic screening criteria. Houten: Prelum Publishers; 2010a. [Google Scholar]
- van El C.G., Pieters T., Cornel M.C. The changing focus of screening criteria in the age of genomics: a brief history from the Netherlands. In: Wieser B., Berger W., editors. Assessing life: on the organisation of genetic testing. Munich, Vienna: Profil verlag; 2010b. pp. 203–224. [Google Scholar]
- van Rhee M.A., Holm J.P., Niermeijer M.F. Genetic carrier screening for hemoglobinopathies: the situation in the Netherlands compared with England. Ned Tijdschr Geneeskd. 1998;142(18):996–997. [PubMed] [Google Scholar]
- Vansenne F., et al. Sickle cell disease in heel injection screening II. Ned Tijdschr Geneeskd. 2009;153(18):858–861. [PubMed] [Google Scholar]
- van Wijk M.A.M., et al. Anaemia: Guideline, M76. Utrecht: NHG; 2003. [Google Scholar]
- Wailoo K., Pemberton P. The troubled dream of genetic medicine. Ethnicity and innovation in tay-sachs, cystic fibrosis and sickle cell disease. Baltimore: The John Hopkins University Press; 2006. [Google Scholar]
- Weatherall D.J., Clegg J.B. Inherited haemoglobin disorders: an increasing global health problem. Bulletin of the World Health Organization. 2001;79(8):704–712. [PMC free article] [PubMed] [Google Scholar]
- Weinreich S.S., et al. Raising awareness of carrier testing for hereditary haemoglobinopathies in high-risk ethnic groups in the Netherlands: a pilot study among the general public and primary care providers. BMC Public Health. 2009;9:338. doi: 10.1186/1471-2458-9-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga K.J. Neonatal screening for sickle-cell disease. Ned Tijdschr Geneeskd. 1997;141(4):184–187. [PubMed] [Google Scholar]
- Wierenga K.J., Luteijn A.J. Heterozygote detection for hemoglobinopathies: situation in the Netherlands as compared to the British. Ned Tijdschr Geneeskd. 1999;143(6):326–327. [PubMed] [Google Scholar]
- Wilson J.M.G., Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. [Google Scholar]
- World Health Organisation (WHO) Sickle cell anaemia. The 59th World Health Assembly; 2006. 117th session; Agenda item 4.8; Resolution EB117.R3. [Google Scholar]
- World Health Organisation (WHO) Management of Hemoglobin disorders. Report of Joint WHO-TIF meeting, Nicosia Cyprus 16–18 November 2007. Geneva: WHO; 2008. [Google Scholar]
- ZonMw (The Netherlands Organisation for Health Research and Development) Ethnicity and Health, ZonMw programme recommendations. 2009. April.


