Abstract
HIV-associated neurocognitive disorders (HAND) remain highly prevalent in the era of cART, but there are no validated psychological interventions aimed at improving cognitive outcomes. This study sought to determine the potential benefit of semantic cueing on category fluency deficits, which are prevalent in HIV and impact daily functioning. Eighty-six HIV-infected individuals and 87 demographically matched seronegative participants were administered a standard (i.e., uncued) and a cued category fluency task. Results revealed significant improvements in cued versus uncued performance in HIV, particularly for persons with lower levels of education. The cueing benefit observed may inform rehabilitation efforts aimed at ameliorating HAND.
Keywords: infectious disease, verbal fluency, semantic memory, cognitive rehabilitation, executive functions, cognitive neuropsychology
Despite significant advances in the management of HIV infection in the era of combined antiretroviral therapies (cART), the prevalence of HIV-associated neurocognitive disorders (HAND) remains high {1} and greatly impacts health outcomes {2}. However, there are no empirically validated cognitive remediation strategies to treat HAND. In fact, there are relatively few theoretically driven studies examining ways to improve specific cognitive functions in HIV-infected individuals {cf. 3, 4} on which broader rehabilitation efforts may be based. Research examining cognitive and behavioral strategies derived from cognitive theory and aimed at remediating HAND is needed, and may help to improve everyday activities and overall quality of life among persons living with HIV infection. This study therefore sought to examine a theory-driven approach to enhance cognition using a verbal fluency paradigm. Verbal fluency deficits are a viable target for this preliminary work because it is a common feature of HAND {e.g., 5} and is a unique predictor of everyday functioning activities, including medication and financial management {6, 7}.
Approximately half of HIV-infected individuals are impaired on verbal fluency tasks {5}, which require rapid, self-initiated search and retrieval from lexico-semantic memory stores in order to generate as many words as possible within 60 seconds that begin with a particular letter or belong to a specific semantic category. HIV infection is generally associated with mild-to-moderate deficits in both letter and category fluency, which tend to worsen with disease progression {8}. One influential neuropsychological theory suggests that optimal verbal fluency performance depends on two dissociable components, namely, clustering and switching {9, 10}. Specifically, clustering refers to the generation of words within specific semantic subcategories and is thought to be an automatic retrieval process mediated by temporal systems and semantic memory stores {e.g., 11}. Switching describes the ability to disengage from one lexico-semantic cluster in order to search for, engage, and retrieve words from another relevant cluster {10} and is viewed as a more controlled executive (i.e., strategic) aspect of verbal fluency associated with frontal systems {e.g., 12, 13}. Within this framework, HIV-associated fluency deficits appear to be primarily reflective of deficits in switching, but not clustering {14, 15, 16 17}, which is consistent with underlying HIV-associated frontostriatal neuropathogenesis. Of clinical relevance, deficits in verbal fluency switching are uniquely predictive of self-reported declines in instrumental activities of daily living among older HIV-infected adults {17}. Moreover, HIV-associated switching impairment is exacerbated when the demands to switch are explicit and rapid {14}, such as in an alternating category fluency paradigm. Both implicit and explicit switching abilities in HIV infection have also been associated with well-validated tests of executive functions {14, 17}, providing further evidence that HIV-associated fluency impairment appears to be driven by the strategic components of the task (e.g., cognitive flexibility).
This pattern of verbal fluency switching deficits may be amenable to improvement through provision of semantic cues, which would presumably reduce the executive demands of the task. Cued fluency interventions have been successful in improving verbal fluency performance in other populations with frontal systems dysfunction and corresponding deficits in switching. For example, Randolph et al. {18} found that individuals with Parkinson’s and Huntington’s disease benefitted significantly from cueing, whereas individuals with Alzheimer’s disease did not. Consistent with this notion, Drane et al. {19} reported that epileptic individuals with frontal lobe seizure onset benefited significantly more from cueing than those with temporal lobe seizure onset, even while controlling for baseline performance on the uncued semantic fluency task. Considering this literature and the growing need for ways to improve cognition in HIV, the primary aim of this study was to determine whether fluency performance in HIV-infected individuals may be improved with semantic cueing aids.
Method
Participants
Participants included 86 HIV-infected individuals and 87 seronegative comparison subjects (total N = 173) who were recruited from the San Diego community and local HIV clinics. All subjects were enrolled within the last three years (date range May, 2008-February 2011) in a NIMH-funded R01 (MH073419) examining the combined effects of HIV and aging on prospective memory at the San Diego HIV Neurobehavioral Research Program. Although the groups did not differ in age (or other demographics), all participants were ≤40 or ≥50 years of age due to the design of the parent study. Given this restriction, age group (i.e., ≤40 or ≥50 years) was considered in our statistical models to ensure that the observed findings were not an artifact of the parent study design. HIV serostatus was confirmed by enzyme linked immunosorbent assays (ELISA) and a Western Blot confirmatory test.
Participants were excluded if they met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV 4th ed.) criteria {20} for current psychiatric conditions (i.e., Major Depressive or Generalized Anxiety Disorders) or recent substance abuse or dependence (i.e., within six months of evaluation) as determined by the Composite International Diagnostic Interview (CIDI version 2.1) {21}. Individuals were also excluded if they tested positive for illicit drugs (other than marijuana) on a urine toxicology screen administered on the day of testing. Additional exclusion criteria included histories of severe psychiatric conditions (e.g., schizophrenia), neurological diseases (e.g., seizure disorders, closed head injuries with loss of consciousness greater than 15 minutes, active CNS opportunistic infections), or medical conditions (e.g., advanced liver disease) known to affect cognition, or an estimated verbal IQ score of less than 70 based on the Wechsler Test of Adult Reading (WTAR) {22}.
Demographic, psychiatric, substance use, medical, and HIV disease characteristics are presented in Table 1. The study groups were comparable in terms of age, education, sex, and ethnicity (all ps > 0.10). While the HIV-infected individuals had a slightly greater proportion of individuals with diagnoses of lifetime Major Depressive Disorder and hepatitis C virus (all ps < 0.05), the groups did not differ in proportions of lifetime Generalized Anxiety Disorder or lifetime Substance Use Disorders (both ps > 0.10).
Table 1.
Demographic, Psychiatric, Substance Use, Medical, HIV Disease, and Cognitive Characteristics of the Study Groups.
| Variable | HIV − (n=87) |
HIV + (n=86) |
p-value |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 43.4 (14.5) | 46.2 (13.4) | 0.202 |
| Education (years) | 14.1 (2.2) | 13.6 (2.7) | 0.247 |
| Sex (% Male) | 71.3% | 81.4% | 0.117 |
| Ethnicity (% Caucasian) | 58.6% | 55.8% | 0.709 |
| Estimated Verbal IQ a | 102.8 (9.1) | 99.8 (10.8) | 0.122 |
| NP Impairment (% impaired) | 19.5% | 34.9% | 0.023 |
| Psychiatric & Substance Use Characteristics | |||
| Total Mood Disturbance (POMS)b | 36.0 (22.0, 56.0) | 40.0 (23.0, 58.5) | 0.313 |
| Major Depressive Disorder (% lifetime) | 32.2% | 46.5% | 0.054 |
| Generalized Anxiety Disorder (% lifetime) | 3.5% | 9.3% | 0.115 |
| Substance Use Disorder (% lifetime) | 64.4% | 62.8% | 0.829 |
| Medical Characteristics | |||
| Hepatitis C Virus (%) | 10.3% | 23.5% | 0.021 |
| HIV Disease Characteristics | |||
| AIDS (%) | - | 45.3% | - |
| cART (%) | - | 88.4% | - |
| Plasma Viral Load (% detectable) | - | 22.6% | - |
| Nadir CD4 Count (cells/µL)b | - | 220.0 (119.5, 326.8) | - |
| Current CD4 Count (cells/µL)b | - | 568.0 (382.0, 775.0) | - |
| Cognitive Variables | |||
| Letter Fluency: B (raw) | 16.2 (4.7) | 14.1 (4.9) | 0.003 |
| Category Fluency: Clothing (raw) | 19.1 (4.7) | 16.8 (4.2) | <0.001 |
| Category Switchingc: Total Correct | 17.3 (2.9) | 15.2 (2.7) | <0.001 |
| Category Switchingc: Accurate Switches | 15.9 (3.8) | 14.0 (3.1) | <0.001 |
| BNT (Total Correct) | 53.4 (6.1) | 52.7 (6.6) | 0.493 |
Note. Data represent means and standard deviations unless otherwise noted;
Based on the Wechsler Test of Adult Reading;
Median (interquartile range).
Animals and musical instruments;
HIV = Human immunodeficiency virus. POMS = Profile of Mood Disturbance. AIDS = Acquired immune deficiency syndrome. cART = Combined antiretroviral therapy. CD4 = Cluster of differentiation 4. BNT = Boston Naming Test.
Materials and Procedure
The procedures involved in this study were approved by the human subjects institutional review board at the University of California, San Diego. Each participant provided written, informed consent and was administered a standard measure of semantic fluency (i.e., Uncued Category Fluency) and a modified cued semantic fluency task (i.e., Cued Category Fluency) as part of a comprehensive neuropsychological, psychiatric, and medical evaluation. Categories for the semantic fluency tasks included “home items” and “supermarket items”. In order to control for the use of different categories and any administration order effects, the semantic fluency categories (i.e., home items and supermarket items) and task format (i.e., Uncued versus Cued) were counterbalanced across subjects and the order of fluency task administration was randomized. The standard, Uncued Category Fluency task required participants to generate as many words as possible within 60 seconds that belonged to a particular category (i.e., home items or supermarket items). For the modified, Cued Category Fluency Task, participants were allowed 60 seconds total as in the standard fluency task, although it was broken into four 15-second blocks, each of which were preceded by a specific cue (i.e., a subordinate category) that was presented visually to the participant. The cues for home items included (1) office, (2) kitchen, (3) bedroom, and (4) bathroom, and the cues for supermarket items were (1) fruits and vegetables, (2) meat and seafood, (3) cleaning products, and (4) things that people drink.
Alongside the cued and uncued category fluency tasks, participants were also administered non-experimental verbal fluency tasks including letter (i.e., the letter “b”), and category (i.e., clothing) fluency. Participants were also administered a category fluency switching measure, where participants were asked to alternate between two semantic categories (i.e., animals and musical instruments) while generating as many words as possible within 60 seconds (e.g., dog, flute, cat, piano). Performance on the letter and category fluency tasks was indexed by the total number of correct responses, and performance on the category switching paradigm was determined by the total number of correct words generated (i.e., sum of total correct responses from both target categories) and total number of accurate switches (i.e., sum of correct across-category switches). Participants were also administered WTAR and the Boston Naming Test (BNT) {23} as indicators of semantic knowledge.
The remainder of the neuropsychological test battery included measures designed to assess cognitive domains commonly affected by HIV infection in accordance with Frascati guidelines {24}, including attention, executive functions, speed of information processing, motor skills, and learning and memory {see 6 for more detail}. Blind clinical ratings of impairment and demographically adjusted T-scores were determined for each cognitive domain using standard methods {see 24, 25}. The clinical ratings ranged from one (i.e., above average, T ≥ 55) to nine (i.e., severely impaired, T ≤ 20), where scores of 5 or higher (i.e., T < 40) indicated cognitive impairment {see 26}. A significantly greater proportion of HIV-infected group demonstrated overall neuropsychological impairment relative to the seronegative participants (p < 0.05; see Table 1).
Data Analyses
Data were first screened for significant outliers (i.e., data points > 3.5 SDs from the overall group mean) and evaluated for normality (i.e., Shapiro-Wilk W test, p < 0.05). While some of the variables of interest were non-normally distributed, the findings did not differ when non-parametric statistics were used. Thus, a parametric statistical approach was used throughout the analyses for consistency and ease of interpretation. The level of significance used for these analyses was designated as 0.05, two-tailed. First, a repeated measures analysis of variance (ANOVA) was conducted to examine the primary study hypotheses, with HIV serostatus as the between subjects factor and cueing (i.e., Cued and Uncued Category Fluency total scores) as the within subjects factor. Age group (i.e., ≤40 or ≥50 years) was also considered in the statistical models to ensure that the observed findings were not an artifact of the parent study design {16}. Follow-up independent- and paired-samples t-tests were conducted in order to explore between- and within-group differences, respectively, and effect sizes were calculated using Hedge’s g statistics.
In order to explore factors that may be associated with the ability to benefit from cueing in the HIV infected group, we first conducted a median split on a calculated difference score variable (i.e., “Benefit Score”), which has been used as an indicator of cueing benefit in prior studies examining cued versus uncued fluency paradigms {e.g., 18}. The Benefit Score was calculated by subtracting the participant’s overall score (i.e., total words recalled) on the Uncued Category Fluency test from his/her overall score on the Cued Category Fluency measure (i.e., Cued – Uncued). The median split derived from the Benefit Score (median = 1.0, range = −13 to 20) was then used to classify each HIV-infected individual into one of two groups; viz., “Cuing Benefit” or “No Benefit”. This approach yielded 49 participants in the Cuing Benefit group (mean = 5.57, S.D. = 4.3) and 37 participants in the No Benefit group (mean = −4.14, S.D. = 3.2). Of note, we used the categorical Benefit/No Benefit variable because we were primarily interested in determining factors that may be attributed to the ability to benefit from cueing itself in the HIV group, rather than the degree of cueing benefit. However, the findings did not differ when the Benefit Score was examined as a continuous variable. Next, analyses exploring the relationship between the presence and magnitude of cueing benefit and potentially contributing demographic, psychiatric and substance use, medical, HIV disease and treatment characteristics and cognitive variables were confined to these two groups (i.e., the HIV-infected participant group only) to control for Type I error, and examined using independent samples t-tests (for continuous variables) or chi-squared tests (for categorical variables).
Results
Means and standard deviations for the study measures are presented in Table 1. Analyses revealed poorer performance within the HIV-infected group on both Letter (p = 0.003) and Category (p < 0.001) fluency relative to their seronegative counterparts. The HIV-infected group also generated fewer words overall on the Category Switching paradigm (p < 0.001) and made fewer accurate switches (p < 0.001). The groups did not differ in oral word reading (i.e., WTAR) or confrontation object naming (i.e., Boston Naming Test) (both ps > 0.10).
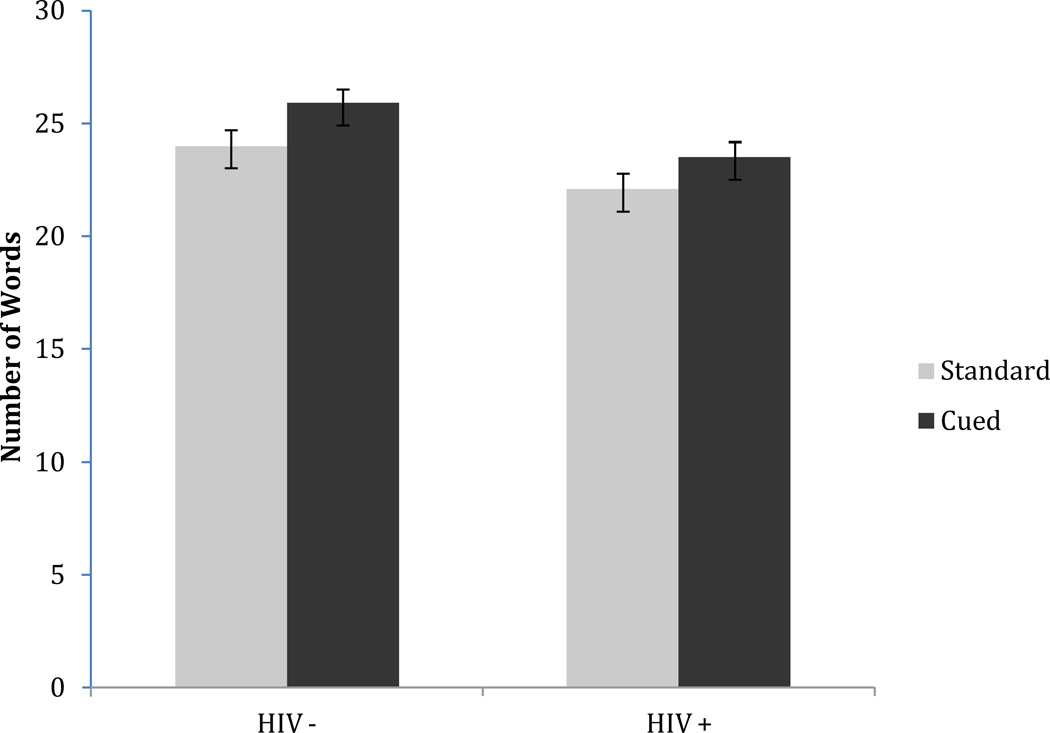
Figure 1 displays the relationship between HIV status and the total words generated on the Cued and Uncued Category Fluency tasks. Significant main effects were observed for HIV-status [F(1,170) = 7.19; p = 0.008] and Fluency condition [F(1, 170) = 12.98; p < 0.001], although there was no significant interaction (p > 0.10) and no main effect of age group (p > 0.10). Follow-up analyses revealed that both the HIV-infected group (p = 0.002, g = 0.22) and their seronegative counterparts (p = 0.040, g = 0.32) generated more words on the cued fluency measure. In addition, the HIV-infected subjects generated slightly fewer words on the Uncued Category Fluency measure (p = 0.067, g = −0.28), and significantly fewer words on the Cued Fluency task relative to their seronegative counterparts (p = 0.007, g = −0.41).
Figure 1.
Significant main effects of HIV serostatus (i.e., HIV+ and HIV−) and standard versus cued fluency output (ps < 0.05).
The Cuing Benefit and No Benefit groups within the HIV-infected sample were comparable on age, ethnicity, and gender (ps > 0.10) factors; interestingly, however, the Cuing Benefit group had obtained significantly fewer years of education (M = 13.1, SD = 2.8) as compared to the No Benefit sample (M = 14.4, SD = 2.5; p = 0.026; g = 0.41). The Cuing Benefit and No Benefit groups did not differ in any other potentially confounding medical, psychiatric, substance use, or general cognitive factors (all ps > 0.10). On the non-experimental fluency tasks, those that benefitted from cueing performed significantly worse on the letter fluency task relative to those that did not benefit (p = 0.042, g = 0.41), although the two groups demonstrated comparable performance on the remainder of the tasks (category and category fluency switching, both ps > 0.10).
Discussion
Consistent with prior research on verbal fluency in HIV infection {8, 14, 15, 16, 17}, we found that serostatus was associated with mild to moderate verbal fluency impairment. Importantly, findings from this study suggest that HIV-associated category fluency deficits may be amenable to improvement with cueing. Specifically, we observed that performance in the HIV-infected group improved significantly when provided with structured cueing that reduced the executive demands of the task (g = 0.22). Of note, a similar degree of improvement from cueing was also observed in the seronegative comparison sample. Yet, when the executive demands were minimized (i.e., on the cued fluency task), performance of the HIV-infected group became nearly identical (mean number of words generated = 23.5, SD = 6.29) to that of their seronegative counterparts on the uncued fluency task (mean number of words generated = 24.0, SD = 6.80; p > 0.10, g = 0.06). Such findings are consistent with the cueing benefit observed in individuals with other conditions with category fluency deficits driven primarily by dysfunction in the executive (i.e., strategic) aspects of the task {e.g., 17, 18}. These results offer some hope that HIV-associated cognitive impairment and the potential subsequent functional decline may be alleviated to some degree with the use of cognitive strategies aimed at reducing the executive demands required for their everyday activities.
Results of this study also provide novel insight with regard to the potential contributing factors associated with benefit from cueing in HIV-infected individuals. Specifically, HIV-infected individuals who benefitted from cueing had significantly fewer years of education relative to those who did not benefit. No other significant demographic, psychiatric, or neuromedical associations with cueing were observed, suggesting that the education effect is unlikely to be an artifact of any of those factors. This finding echoes previous research showing that individuals with intractable frontal lobe epilepsy and limited formal education benefitted more from cueing than the better educated temporal lobe participants {18}. Interestingly, while research has found that individuals with higher years of education may be more likely to benefit from cognitive strategies when undergoing cognitive rehabilitation {e.g., 28}, our findings run contrary to these data, and suggest that some individuals with lower education may indeed experience benefit from cognitive strategies in the context of targeted cognitive rehabilitation.
Of the cognitive variables examined (i.e., the clinical fluency measures and the BNT), only letter fluency performance was significantly associated with cueing benefit within the HIV group. Specifically, HIV infected individuals who benefitted from cueing performed moderately more poorly on the letter fluency task relative to those who did not benefit (g = −0.41). As letter fluency performance is thought to rely heavily on executive processes, this finding provides further support that HIV-associated executive dysfunction may be alleviated to some degree with strategies such as cueing whereby the executive demands are minimized. Arguing against the executive dysfunction hypothesis is the comparable performance between the groups observed on the category switching measure, which was unexpected given our prior research {i.e., 14} showing that category fluency switching is heavily reliant on executive processes. Thus, it is also possible that the letter fluency was simply a result of Type I error. Nonetheless, future research examining the specific executive processes that may dissociate the abilities required for optimal performance on these tasks may aid in the specification of HIV associated executive dysfunction and help in the development of specific cognitive strategies targeted towards the weaker executive abilities in this population.
Several limitations of this study are worth noting. First, due to the design of the parent study, our sample did not include individuals between the ages of 40 and 50 years. However, age was included in the statistical analyses to account for this potential confound. Moreover, significant associations were lacking between age and cueing benefit, though it is possible that some HIV infected individuals were already functioning. Second, these findings are limited to the exemplar noun categories used in this study (i.e., home and supermarket items), raising questions about the generalizability to other categories (e.g., proper nouns) as well as other fluency paradigms, such as letter and action (i.e., verb) fluency {29}. Lastly, the generalizability of these findings may be limited by the exclusion of individuals with current DSM-IV axis I disorders and our relatively immunologically healthy HIV sample.
In conclusion, results from this study provide further support for verbal fluency impairment in HIV infection and novel insight into the potential benefit of cognitive strategies such as cueing in the remediation of this deficit, particularly for HIV-infected individuals with fewer years of education. While a direct examination between cueing benefit and improvement in daily functioning was not conducted in this study, preliminary research in HIV infection has suggested that other cognitive interventions (e.g., spaced retrieval strategies) may have important functional implications (e.g., may improve medication adherence) {3}. Future research should investigate this possibility, and similar paradigms could be included in cognitive intervention studies attempting to alleviate HIV associated cognitive impairment. For example, one could examine the potential benefits of cueing (e.g., providing structured subcategories) during performance on script generation tasks, which has been shown to be impaired in individuals with HIV-associated neurocognitive disorders and is highly relevant to everyday functioning {30}. Moreover, one could also examine the potential benefits of categorization techniques, which involve cueing strategies and have been successfully implemented through cognitive intervention programs in other clinical populations (e.g., mild cognitive disorder) {31}. Categorization training involves teaching individuals to organize (i.e., categorize) large amounts of related information (e.g., a list of medications, important tasks, or groceries) into relevant or meaningful subcategories which then act as cues for retrieval. To our knowledge, categorization techniques have not been formally examined in HIV-infected individuals, although may be a valuable technique to examine in future cognitive intervention studies, as it can be easily implemented and rehearsed in the clinical setting (e.g., asking participants to recall lists of nouns) and can also be generalized to tasks related to activities of everyday life that are highly relevant to the HIV-infected population (e.g., recalling a list of medications, a shopping list, or a “to do” list). For example, if an individual needed to remember his/her of current medications to recall at a future medical appointment, he/she could mentally organize each medication into specific and meaningful subcategories (e.g., morning/afternoon/evening medications, HIV/psychiatric/other medical illness medications), thereby providing internal cues (i.e., subcategories) that may facilitate accurate recall of the medication list. These strategies may be further individually modified if specific HIV-associated cognitive deficits (e.g., susceptibility to intrusions) appear to be interfering with accurate recall. Finally, future research examining the longitudinal effects of cognitive cueing intervention procedures is necessary to formally examine the sustainability of cueing improvement following such interventions. If effective at ameliorating HIV associated cognitive deficits, such interventions may provide valuable insight into the treatment of HIV associated neurocognitive disorders, and may subsequently improve the ability of affected individuals to carry out essential activities of daily living (e.g., medication adherence).
Acknowledgements
The San Diego HIV Neurobehavioral Research Program (HNRP) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Roald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S., Tanya Wolfson, M.A.
This research was supported by National Institutes of Health grants R01-MH073419 and T32-DA031098 to Dr. Woods, P30-MH62512 to Dr. Grant, and K23 NSO49100 to Dr. Drane (which included the development of several cued and uncued category fluency paradigms). The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Marizela Cameron, P. Katie Riggs, and Katie Doyle for their help with study management.
Footnotes
The authors state no conflict of interest.
References
- 1.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: A population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neundorfer MM, Camp CJ, Lee MM, et al. Compensating for cognitive deficits in persons aged 50 and over with HIV/AIDS: A pilot study of cognitive intervention. Journal of HIV/AIDS and Social Services. 2004;3:79–97. [Google Scholar]
- 4.Weber E, Woods SP, Kellogg E, et al. Self-generation enhances verbal recall in individuals infected with HIV. J Int Neuropsychol Soc. doi: 10.1017/S135561771100124X. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 6.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 7.Woods SP, Morgan EE, Dawson M, et al. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. J Clin Exp Neuropsychol. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]
- 8.Iudicello JE, Woods SP, Parsons TD, et al. Verbal fluency in HIV infection: A meta-analytic review. J Int Neuropsychol Soc. 2007;13:183–189. doi: 10.1017/S1355617707070221. [DOI] [PubMed] [Google Scholar]
- 9.Gruenewald PJ, Lockhead GR. The free recall of category examples. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:225–240. [Google Scholar]
- 10.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 11.Tröster AI, Fields JA, Testa JA, et al. Cortical and subcortical influences on clustering and switching in the performance of verbal fluency tasks. Neuropsychologia. 1998;4:295–304. doi: 10.1016/s0028-3932(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 12.Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia. 1993;31:17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- 13.Ho AK, Sahakian BJ, Robbins TW, et al. Verbal fluency in Huntington’s disease: A longitudinal analysis of phonemic and semantic clustering and switching. Neuropsychologia. 2002;40:1277–1284. doi: 10.1016/s0028-3932(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 14.Iudicello JE, Woods SP, Weber E, et al. Cognitive mechanisms of switching in HIV-associated catgory fluency deficits. J Clin Exp Neuropsychol. 2008;30:797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millikin CP, Trepanier LL, Rourke SB. Verbal fluency component analysis in adults with HIV/AIDS. J Clin Exp Neuropsychol. 2004;26:933–942. doi: 10.1080/13803390490510842. [DOI] [PubMed] [Google Scholar]
- 16.Woods SP, Conover E, Rippeth JD, et al. Qualitative aspects of verbal fluency in HIV-associated dementia: a deficit in rule-guided lexical-semantic search processes? Neuropsychologia. 2004;42:801–809. doi: 10.1016/j.neuropsychologia.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Iudicello JE, Woods SP, Deutsch R, et al. Combined effects of HIV and aging on semantic verbal fluency: A view of the cortical hypothesis through the lens of clustering and switching. J Clin Exp Neuropsychol. doi: 10.1080/13803395.2011.651103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph C, Braun AR, Goldberg TE, et al. Semantic fluency in Alzheimer’s, Parkinson’s, and Huntington’s disease: Dissociation of storage and retrieval failures. Neuropsychology. 1993;7:82–88. [Google Scholar]
- 19.Drane DL, Lee GP, Cech H, et al. Structured cueing on a semantic fluency task differentiates patients with temporal versus frontal lobe seizure onset. Epilepsy and Behavior. 2006;9:339–344. doi: 10.1016/j.yebeh.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.World Health Organization. Composite international diagnostic interview. Version 2.1. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 22.Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Author; 2001. [Google Scholar]
- 23.Goodglass H, Kaplan E, Barresi B. The assessment of aphasia and related disorders. 3rd edition. Philadelphia: Lippincott, Williams, & Wilkins; 2001. [Google Scholar]
- 24.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton RK, Kirson D, Velin R, et al. The utility of clinical ratings for detecting early cognitive change in HIV infection. In: Grant I, Martin A, editors. The Neuropsychology of HIV Infection. New York: Oxford University Press; 1994. pp. 188–206. [Google Scholar]
- 26.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Neuropsychologia. 2004;43:1144–1151. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 27.Heaton RK, Miller SW, Taylor MJ, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 28.Sherer M, Sander AM, Nick TG, et al. Early cognitive status and productivity outcome after traumatic brain inury: Findings from the TBI model systems. Arch Phys Med Rehabil. 2002;83:183–192. doi: 10.1053/apmr.2002.28802. [DOI] [PubMed] [Google Scholar]
- 29.Woods SP, Carey CL, Tröster AI, et al. Action (verb) generation in HIV-1 infection. Neuropsychologia. 2005;31:17–28. doi: 10.1016/j.neuropsychologia.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Scott JC, Woods SP, Vigil O, et al. Script generation of activities of daily living in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2011;17:740–745. doi: 10.1017/S135561771100052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]