Abstract
The chemokine superfamily consists of a large number of ligands and receptors. At first glance, this family appears redundant and their ligand-receptor relationships promiscuous, making its study challenging. However, analyzing this family from the evolutionary perspective greatly simplifies understanding both the organization and function of this apparently complex system. In particular, the functions of a subgroup of chemokines (designated homeostatic chemokines) have played pivotal roles in advancing our understanding of the organization and function of the cellular networks that shape the immune system. Here, we update the full scope of the human and mouse chemokine superfamilies, their relationships, and summarize several important roles that homeostatic chemokines play in the immune system.
Introduction
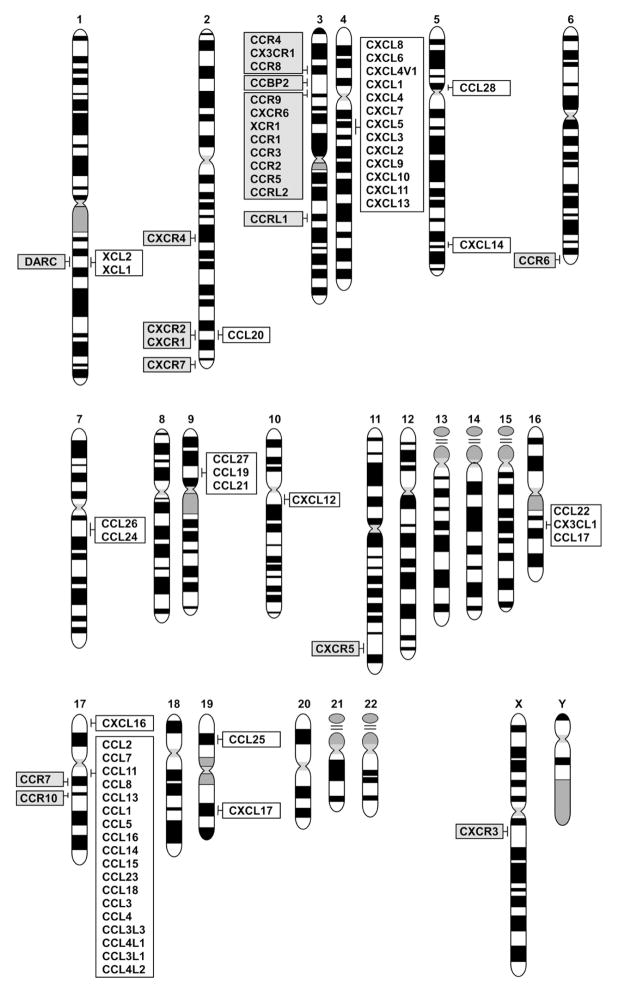
Since the identification of the chemokines CXCL8 (IL-8) and CCL2 (MCP-1) in the late 1980s, the chemokine superfamily has expanded rapidly (Rollins, 1997; Yoshie et al., 2001; Zlotnik and Yoshie, 2000; Zlotnik et al., 2006). An initial wave of chemokine discovery occurred in the early 1990s, when some chemokines that attracted neutrophils and monocytes were discovered. Their identification was facilitated by the abundance of their transcripts in activated cells that participate in inflammatory responses. Their receptors were soon identified and were found to be a subgroup of class A G protein-coupled receptors (GPCRs) (Vassilatis et al., 2003). However, the initial ligand-receptor relationships that were described were found to be promiscuous: a single chemokine binds to several chemokine receptors, while a single chemokine receptor has multiple chemokine ligands. Moreover, the chemokine genes were found to map to two discrete chromosomal sites, thus forming two large gene clusters, one for CXC chemokines (in human, 4q13.3) and another for CC chemokines (in human, 17q12)(Fig 1). Such unorthodox features of the chemokine system along with their innate immunity roles attracted little attention from immunologists during these early years. Most of these chemokines are now classified as inflammatory, because they play pivotal roles in controlling leukocyte recruitment during inflammatory responses.
Figure 1.
Chromosomal map of the human chemokine and chemokine receptor genes. The full set of human chromosomes is shown with the locations of the chemokine and chemokine receptor genes indicated as follows: chemokines (white) and chemokine receptors (gray).
However, a ‘second round’ of chemokine identification started in the mid 1990s, facilitated by rapidly expanding EST (expressed sequence tag) databases. In contrast to the inflammatory chemokines, the expression of these chemokines is constitutive and restricted to specific cells or organs. Moreover, in contrast to inflammatory chemokines that mainly attract macrophages or neutrophils, they are chemotactic for subsets of lymphocytes or dendritic cells, cells that mediate acquired immunity. Indeed, many were found to play pivotal roles in immune responses and in defining the cellular organization of organs of the immune system. Their receptors also revealed far less promiscuous interactions. In fact, many of them exhibit highly restricted ligand-receptor relationships. In both the human and mouse genomes, the genes encoding homeostatic chemokines are located outside the familiar CXC or CC chemokine clusters (Fig 1). These findings have led to a paradigm shift in our concept of chemokine function. The functional characterization of these ‘second round’ chemokines led to major advances in our understanding of the function and organization of the immune system. It became apparent that many homeostatic chemokines play major roles in the organization of the immune system and are indeed ‘master regulators’ of the movement and localization of lymphocyte and dendritic cell subsets in the body (Moser et al., 2004; Zlotnik et al., 2006). In this review, our objectives include updating the classification and nomenclature of the chemokine superfamily, including the clarification of some nomenclature issues concerning human and mouse chemokines. We also summarize recent developments concerning functions of this interesting gene family in the immune system and finally, we comment on its potential to yield therapeutics or biomarkers of human disease.
Chemokine nomenclature updated
In our previous review (Zlotnik and Yoshie, 2000), we proposed a systematic nomenclature of the chemokines that originated at the Keystone symposium on Chemokines and Chemotactic Receptors in 1999. This nomenclature system was quickly accepted and has helped establish a standard chemokine terminology that has greatly facilitated their study. However, because of the rapid and divergent evolution of the chemokine system, species like humans and mice exhibit differences in their chemokine ligands (Zlotnik et al., 2006). The originally described chemokine relationships between human and mouse were based on sequence similarities and phylogenic analyses. Consequently, there were some ambiguities in the assignment of species counterparts. We now have more powerful tools, including the human and mouse genomes, to improve on these analyses. The genomic organization of the human and mouse chemokine genes provide insights into the evolutionary forces that shape this superfamily and also confirm that in some cases, a given ligand exists in one species but not the other (Nomiyama et al., 2010; Zlotnik et al., 2006). Given these advances, we need to update the human and mouse chemokine nomenclature systems.
Since 2000 (Zlotnik and Yoshie, 2000), in-depth analyses of the mouse and human genomes have identified all the chemokine genes. Table 1 contains the complete families of human and mouse chemokines. The chemokine molecular signature includes four conserved cysteine residues that form two disulfide bonds pairing the first with the third and the second with the fourth cysteines. Based on the arrangement of the N-terminal two cysteine residues, chemokines are grouped into four subfamilies: CXC, CC, (X)C, and CX3C. In the CXC chemokines, one amino acid separates the first two cysteines, while in CC chemokines, these two cysteines are adjacent. A single member of the CX3C subfamily, CX3CL1 or fractalkine, has three amino acids between the two cysteines, while the first and third cysteines are missing in the (X)C subfamily. Recently, another possible type of chemokine (CX) has been reported in Zebrafish (Nomiyama et al., 2008), which lack one of the two N-terminus cysteines but retain the third and fourth ones; however, there is no evidence that this latter kind of chemokine exists in mammals.
Table 1.
Human and mouse chemokines
| Chemokine | Other Names (human) | Category | Gene symbol | Other names (mouse) | Receptor | ||
|---|---|---|---|---|---|---|---|
| Human | Mouse | Agonistic | Antagonistic | ||||
| CXC subfamily | |||||||
| CXCL1 | GROα, MGSA | I, ELR | CXCL1 | Cxcl3 | Gm1960 | CXCR2 | |
| CXCL2 | GROβ | I, ELR | CXCL2 | Cxcl2 | MIP-2 | CXCR2 | |
| CXCL3 | GROγ | I, ELR | CXCL3 | Cxcl1 | KC | CXCR2 | |
| CXCL4 | PF4 | Pt, non-ELR | PF4 | – | CXCR3-B | ||
| CXCL4L1 | PF4V1 | Pt, non-ELR | PF4V1 | Pf4 | CXCR3-B | ||
| CXCL5 | ENA78 | I, ELR | CXCL5 | – | CXCR2 | ||
| CXCL6 | GCP2 | I, ELR | CXCL6 | Cxcl5 | LIX | CXCR1, CXCR2 | |
| CXCL7 | NAP-2 | Pt, I, ELR | PPBP | Ppbp | CXCR1, CXCR2 | ||
| CXCL8 | IL-8 | I, ELR | IL-8 | – | CXCR1, CXCR2 | ||
| CXCL9 | MIG | I, non-ELR | CXCL9 | Cxcl9 | CXCR3 | CCR3 | |
| CXCL10 | IP-10 | I, non-ELR | CXCL10 | Cxcl10 | CXCR3 | CCR3 | |
| CXCL11 | I-TAC | I, non-ELR | CXCL11 | Cxcl11 | CXCR3, CXCR7 | CCR3, CCR5 | |
| CXCL12 | SDF-1 | H, non-ELR | CXCL12 | Cxcl12 | CXCR4, CXCR7 | ||
| CXCL13 | BLC, BCA-1 | H, non-ELR | CXCL13 | Cxcl13 | CXCR5, CXCR3 | ||
| CXCL14 | BRAK | H, non-ELR | CXCL14 | Cxcl14 | Unknown | ||
| – | – | U, non-ELR | – | Cxcl15 | Lungkine Weche | Unknown | |
| CXCL16 | SR-PSOX | I | CXCL16 | Cxcl16 | CXCR6 | ||
| CXCL17 | DMC | U | CXCL17 | Cxcl17 | Unknown | ||
| CC subfamily | |||||||
| CCL1 | I-309 | I | CCL1 | Ccl1 | TCA-3 | CCR8 | |
| CCL2 | MCP-1 | I | CCL2 | Ccl2 | JE | CCR2 | |
| CCL3 | MIP-1α, LD78α | I | CCL3 | Ccl3 | CCR1, CCR5 | ||
| CCL3L1 | LD78β | I | CCL3L1 | – | CCR1, CCR3, CCR5 | ||
| CCL3L3 | LD78β | I | CCL3L3 | – | |||
| CCL4 | MIP-1β | I | CCL4 | Ccl4 | CCR5 | ||
| CCL4L1 | AT744.2 | I | CCL4L1 | – | |||
| CCL4L2 | – | I | CCL4L2 | – | |||
| CCL5 | RANTES | I, Pt | CCL5 | Ccl5 | CCR1, CCR3, CCR5 | ||
| CCL7 | MCP-3 | I | CCL7 | Ccl7 | MARC | CCR1, CCR2, CCR3 | CCR5 |
| CCL8 | MCP-2 | I | CCL8 | – | CCR1, CCR2, CCR5 | ||
| – | – | I | Ccl8 | CCR8 (mouse) | |||
| CCL11 | Eotaxin | D | CCL11 | Ccl11 | CCR3, CCR5 | CXCR3, CCR2 | |
| – | – | I | – | Ccl12 | MCP-5 | ||
| CCL13 | MCP-4 | I | CCL13 | – | CCR2, CCR3 | ||
| CCL14 | HCC-1 | P | CCL14 | – | CCR1, CCR3, CCR5 | ||
| CCL15 | HCC-2 Leukotactin-1 |
P | CCL15 | Ccl9 | CCF18, MIP-1γ | CCR1, CCR3 | |
| CCL16 | LEC, HCC-4 | U | CCL16 | – | CCR1, CCR2, CCR5 CCR8, H4 |
||
| CCL17 | TARC | D | CCL17 | Ccl17 | ABCD-2 | CCR4 | |
| CCL18 | PARC, DC-CK1 | H | CCL18 | – | PITPNM3 | CCR3 | |
| CCL19 | MIP-3β, ELC | H | CCL19 | Ccl19 | CCR7 | ||
| CCL20 | MIP-3α, LARC | D | CCL20 | Ccl20 | CCR6 | ||
| CCL21 | SLC, 6Ckine | H | CCL21 | Ccl21a | CCR7 | CXCR3 (mouse) | |
| – | – | H | – | Ccl21b | CCR7 | ||
| – | – | H | – | Ccl21c | CCR7 | ||
| CCL22 | MDC | D | CCL22 | Ccl22 | ABCD-1 | CCR4 | |
| CCL23 | MPIF-1 | P | CCL23 | Ccl6 | C10 | CCR1, FPRL-1 | |
| CCL24 | Eotaxin-2 MPIF-2 |
H | CCL24 | Ccl24 | CCR3 | ||
| CCL25 | TECK | H | CCL25 | Ccl25 | CCR9 | ||
| CCL26 | Eotaxin-3 | I | CCL26 | (Ccl26-ps) | CCR3, CX3CR1 | CCR1, CCR2, CCR5 | |
| CCL27 | CTACK, ILC | H | CCL27 | Ccl27a | CCR10 | ||
| – | – | H | – | Ccl27b | |||
| CCL28 | MEC | H | CCL28 | Ccl28 | CCR10, CCR3 | ||
| XC subfamily | |||||||
| XCL1 | Lymphotactin ATAC, SCM-1α |
D | XCL1 | Xcl1 | lymphotactin | XCR1 | |
| XCL2 | SCM-1β | D | XCL2 | – | XCR1 | ||
| CX3C subfamily | |||||||
| CX3CL1 | Fractalkine | D | CX3CL1 | Cx3cl1 | fractalkine neurotactin | CX3CR1 | |
I, inflammatory chemokines; H, homeostatic chemokines; D, dual chemokines; P, plasma or platelet chemokines that are activated by cleavage; U, unknown. Mouse genes whose human counterparts are reassigned by synteny analysis are highlighted by underline. H4, histamine receptor type 4; PITPNM3, phosphatidylinositole transfer protein membrane associated 3; FPRL-1, formyl peptide receptor like-1.
Besides the structural criteria, chemokines may be categorized into several functional groups (Moser et al., 2004). Inflammatory chemokines (termed “I” in Table 1) are those upregulated under inflammatory conditions and are mainly involved in the recruitment of leukocytes to inflamed tissues. Among them, CXC chemokines with an ELR (glu-leu-arg) motif just prior to the 1st cysteine residue are angiogenic, a property likely mediated via the chemokine receptors CXCR1 and CXCR2, while the ligands CXCL4, CXCL9, CXCL10 and CXCL11 are non-ELR motif angiostatic chemokines (Kiefer and Siekmann, 2011). Homeostatic chemokines (termed “H”) are those expressed constitutively in lymphoid or other organs and normally mediate homeostatic migration and homing of various cells (including lymphocytes). Some chemokines overlap both fields, and are called dual-function chemokines (termed “D” in Table 1). These classifications are at best operational, and not mutually exclusive. Thus, some inflammatory chemokines may have homeostatic functions, while some homeostatic chemokines may be upregulated under certain ‘emergency’ conditions.
Some chemokines, such as CCL14 and CCL15, are present at high concentrations in serum, have an extended N-terminus, and are converted to high affinity ligands by N-terminal cleavage. CCL23 also has a long N-terminus, is closely related to CCL15, and, along with them, is likely to form a subgroup of inflammatory chemokines termed plasma chemokines (“P” for plasma or precursor) (Nomiyama et al., 2010). Yet another group of chemokines represented by CXCL4, CXCL4L1, CXCL7 and CCL5 are stored at high concentrations in α-granules of platelets and quickly released upon platelet activation (Flad and Brandt, 2010). They likely have important roles as first-line inflammatory mediators upon vascular injury. One of these, CXCL4 (PF4), is one of the oldest members of the chemokine superfamily. However, its biological significance is still obscure. Although CXCR3-B (a CXCR3 slice variant) is believed to mediate the angiostatic activity of CXCL4 and other non-ELR CXC chemokines (CXCL9, CXCL10, CXCL11)(Lasagni et al., 2003), the mechanism remains unknown. CXCL4L1 is the non-allelic variant of CXCL4, differs from CXCL4 by only 3 amino acids within the C terminus, and is more angiostatic than CXCL4 (Struyf et al., 2004). CCL5 is one of the most versatile chemokines, acting on as many as three chemokine receptors CCR1, CCR3 and CCR5, and highly inducible in many cells. Furthermore, it is abundantly stored in α-granules of platelets and in cytoplasmic granules of memory or effector CD8+ T cells as well (Catalfamo et al., 2004). Functionally, CXCL4 and CCL5 appear to be closely associated, possibly through the formation of heteroligomers. CXCL7 is stored in inactive form and upon release is progressively processed at the N-terminus by neutrophil cathepsin G from Platelet Basic Protein PBP (aa 35–128) to Connective Tissue Activating Peptide CTAP-III (aa 44–128), to Beta Thromboglobulin β-TG (aa 48–128), and finally to neutrophil activating peptide NAP-2 (aa 59–128), a high-affinity CXCR2 ligand. Thus, neutrophil attraction and proteolytic activation of CXCL7 form an amplification loop. Meanwhile, PBP and its processed products, along with CXCL4, have potent antimicrobial activities, and are also called thrombocidins. Collectively, these chemokines are often termed platelet chemokines (termed “Pt” for platelet in Table 1) (Flad and Brandt, 2010).
The differences between inflammatory and homeostatic chemokines have an evolutionary origin, and it centers on the rapid evolution of the former (Nomiyama et al., 2010). Inflammatory chemokines are clustered in chromosome 4 and chromosome 17, respectively, in the human (Fig 1). The reason for such rapid evolution in inflammatory chemokines is not clear but likely is a result of strong selective pressure to increase their members when early humans (or mice) faced a new pathogen that represented a serious threat to their survival. Furthermore, individual inflammatory chemokines can also differ markedly in their function between species (Islam et al., 2011). Finally, many inflammatory chemokines sometimes exist only in mouse or human (for example, CXCL8, Cxcl15, etc (see Table 1)); this is likely the result of different pathogen-driven evolutionary experiences of the mouse and human lineages. We should note that the original nomenclature was aimed at the human chemokines, designated in capital letters; the mouse chemokines are designated in lower case letters (Table 1).
The rapid evolution of inflammatory chemokines often makes the orthologous relationships obscure by phylogenic analysis, even between human and mouse (Nomiyama et al., 2010). For example, the three human paralogues CXCL1, CXCL2 and CXCL3 are structurally similar, and the same applies to their mouse counterparts (Nomiyama et al., 2010). It is therefore impossible to determine their orthologous relationships just from phylogenic analyses. In such cases, analysis of the syntenic regions between the two genomes can be used to correctly assign species counterparts. The latter approach reveals that the mouse orthologues of human CXCL6, CXCL4L1, CXCL1 and CXCL3 correspond to Cxcl5, Cxcl4, Cxcl3 and Cxcl1, respectively (Nomiyama et al., 2010). Indeed, mouse Cxcl5 is closer to human CXCL6 than CXCL5, and recently the rat gene symbol Cxcl5 has been renamed Cxcl6 (Nomiyama et al., 2010). Similar analyses in the CC chemokines indicate that the mouse homologues of human CCL15 and CCL23 are Ccl9 and Ccl6, respectively (Nomiyama et al., 2010).
Islam et al. (Islam et al., 2011) have shown that, while mouse Ccl8 is considered a member of the MCP (Monocyte Chemotactic Protein) subfamily, it does not signal via Ccr2 but is instead a potent agonist for Ccr8, until now considered the sole receptor for Ccl1. However, human CCL8 is not a CCR8 agonist. These divergent results may be explained by the fact that mouse Ccl8 is actually not the counterpart of human CCL8, but is instead a mouse-specific chemokine like mouse Ccl12 (Table 1). Conversely, human CCL13 has no mouse counterpart. The chromosomal gene order of the human MCP-1 locus is CCL2-CCL7-CCL11-CCL8-CCL13-CCL1, while in the mouse it is Ccl2-Ccl7-Ccl11-Ccl12-Ccl8-Ccl1 (Nomiyama et al., 2010). Thus, the correct relationships, if any, of these genetically related chemokines still need to be determined. The importance of these observations is obvious when studying the effects of these chemokines in mouse models and their relevance to human disease.
In contrast, homeostatic chemokines are located singly or in mini-clusters in different chromosomes (Fig. 1). They are relatively ancient in evolutionary terms, well conserved between species and function in a more predictable manner. For these reasons, the conclusions of studies in genetically modified mice deficient in homeostatic chemokines are more likely to apply to human physiology.
Chemokine receptors
Chemokine receptors are class A GPCRs coupled with the Gαi class of the heterotrimeric G proteins. They are also grouped into 4 subfamilies according to the subfamily of their major chemokine ligands (Zlotnik et al., 2006). Like the ligand counterpart genes, the chemokine receptor genes also form clusters. A large cluster is located in human chromosome 3 (Fig. 1), suggesting a rapid evolution through repeated gene duplications. However, compared to the ligands, chemokine receptors are relatively well conserved between species (including human and mouse)(Nomiyama et al., 2011). Table 2 summarizes all known chemokine receptors. So far, 18 chemokine receptors with the standard Gαi-dependent chemotactic activity have been identified in human and mouse. Furthermore, 5 atypical (non-chemotactic, recycling or scavenging) chemokine receptors have been described. It is apparent that the chemokine receptors of inflammatory chemokines tend to have large numbers of chemokine ligands, and some ligands are also shared by multiple receptors (Nomiyama et al., 2011).
Table 2.
Human and mouse chemokine receptors
| Human gene symbol | Other names | Category | Mouse gene symbol | Ligands | |
|---|---|---|---|---|---|
| Agonistic | Antagonistic | ||||
| CXCR subfamily | |||||
| CXCR1 | IL8RA | I | Cxcr1 | CXCL6, CXCL7, CXCL8, acPGP | |
| CXCR2 | IL8RB | I | Cxcr2 | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8, acPGP, MIF | |
| CXCR3 | GPR9 | I | Cxcr3 | CXCL4 (CXCR3-B), CXCL9, CXCL10, CXCL11, CXCL13 CCL21 (mouse) |
CCL11 |
| CXCR4 | LESTER, Fusin | H | Cxcr4 | CXCL12, MIF, ubiquitin | |
| CXCR5 | BLR1 | H | Cxcr5 | CXCL13 | |
| CXCR6 | STRL33, BONZO | H | Cxcr6 | CXCL16 | |
| CCR subfamily | |||||
| CCR1 | CC-CKR-1 | I | Ccr1 | CCL3, CCL3L1, CCL5 CCL7, CCL8, CCL13, CCL14 CCL15, CCL16, CCL23 |
CCL26 |
| CCR2 | CC-CKR-2 | I | Ccr2 | CCL2, CCL7, CCL8, CCL13 CCL16, β-defensin 2,3 |
CCL11, CCL26 |
| CCR3 | CC-CKR-3 | I | Ccr3 | CCL3L1, CCL5, CCL7, CCL11 CCL13, CCL14, CCL15, CCL24 CCL26, CCL28 |
CXCL9, CXCL10, CXCL11, CCL18 |
| CCR4 | CC-CKR-4 | D | Ccr4 | CCL17, CCL22 | |
| CCR5 | CC-CKR-5 | I | Ccr5 | CCL3, CCL3L1, CCL4, CCL4L1 CCL5, CCL8, CCL11, CCL16 |
CCL7, CXCL11 CCL26 |
| CCR6 | STRL22, GPR29 | I | Ccr6 | CCL20, β-defensin-2 | |
| CCR7 | EBI1, BLR2 | H | Ccr7 | CCL19, CCL21 | |
| CCR8 | TER1, GPR-CY6 | H | Ccr8 | CCL1, CCL16, CCL8 (mouse) | |
| CCR9 | GPR-9-6 | H | Ccr9 | CCL25 | |
| CCR10 | GPR2 | H | Ccr10 | CCL27, CCL28 | |
| XCR subfamily | |||||
| XCR1 | GPR-5 | D | Xcr1 | XCL1, XCL2 | |
| CX3CR subfamily | |||||
| CX3CR1 | V28, GPR13 | D | Cx3cr1 | CX3CL1, CCL26 | |
| Atypical (non-chemotactic, recycling or scavenger receptors) | |||||
| CXCR7 | RDC1, GPR159 | Cxcr7 | CXCL11, CXCL12 | ||
| CCBP2 | D6 | Ccbp2 | CCL2, CCL3, CCL3L1, CCL4, CCL4L1, CCL5, CCL7, CCL8, CCL11, CCL12, CCL13, CCL14 CCL17, CCL22, CCL23, CCL24 |
||
| CCRL1 | CCX-CKR | Ccrl1 | CCL19, CCL21, CCL25, CXCL13 | ||
| CCRL2 | HCR, CRAM | Ccrl2 | CCL19, chemerin | ||
| DARC | Duffy | Darc | CXCL1, CXCL2, CXCL3, CXCL7, and CXCL8, CCL2, CCL5, CCL11, CCL13, CCL14, CCL17 | ||
acPGP, N-acetyl Pro-Gly-Pro; MIF, macrophage migration inhibitory factor.
In another twist, some chemokines can also function as natural chemokine antagonists of other ligand-receptor pairs (Table 2). For example, ligands of CXCR3 and CCR3 are reciprocally natural antagonists (Loetscher et al., 2001; Weng et al., 1998). Thus, CXCL9, 10 and 11 are natural antagonists for CCR3, while CCL11 is a natural antagonist for CXCR3. Given that CXCR3 is a T helper-1 (Th1) cell-associated chemokine receptor, while CCR3 is expressed by Th2 cells and eosinophils, this suggests that these chemokines can form mutually-exclusive microenvironments that favor either Th1 or Th2 cell differentiation (Sallusto and Lanzavecchia, 2000).
Non-chemokine ligands can also bind certain chemokine receptors. Though still controversial, N-acetyl Pro-Gly-Pro (acPGP), a neutrophil protease-mediated degradation product of extracellular matrix, can be a Cxcr1 and Cxcr2 agonist (Weathington et al., 2006). Thus, production of this tripeptide by infiltrating neutrophils may recruit yet more neutrophils via Cxcr1 and Cxcr2. Macrophage migration inhibitory factor (MIF) is an evolutionary conserved cytokine with proinflammatory functions, and has also been claimed to be a non-canonical CXCR2 and CXCR4 ligand (Bernhagen et al., 2007). Extracellular ubiquitin, which has anti-inflammatory properties, is another non-chemokine CXCR4 ligand (Saini et al., 2010). Finally, the nuclear protein HMGB1 can also interact with CXCL12 and mediates mononuclear cell recruitment in vivo via CXCR4 (Schiraldi et al., 2012).
Antimicrobial peptides are natural antibiotics of multicellular organisms and often exibit chemotactic activity. β-defensins are antimicrobial peptides expressed by epithelial cells of the body surface (Ouellette, 2011). Like CCL20, β-defensin-2 attracts immature dendritic and memory T cells via CCR6 (Yang et al., 1999). β-defensins 2 and 3 also function as noncanonical ligands for CCR2 (Rohrl et al., 2010). Conversely, a growing number of chemokines mediate direct antimicrobial activities (Eliasson and Egesten, 2008). It is therefore possible that chemokines and antimicrobial peptides share a common evolutionary origin. Alternatively, the selective pressures exerted by the pathogens on their hosts may have led the chemokines and antimicrobial peptides to perform overlapping functions.
In contrast to inflammatory chemokine receptors, homeostatic or dual function chemokine receptors show more restricted ligand usages: just one or two ligands (Table 2). Furthermore, even in the case of two ligands, most are not just redundant but appear instead to have specific roles. For example, CCR4, which is expressed by Th2 cells, skin-homing T cells and regulatory T cells, has two ligands: CCL17 and CCL22. In inflamed skin tissues, CCL17 is expressed by skin microvascular endothelial cells, while CCL22 is expressed by dermal dendritic cells. Thus, CCR4-expressing T cells are first exposed to CCL17 on skin endothelial cells and then guided into skin tissues by CCL22 (Mariani et al., 2004). This sequential interaction of ligands seems to be possible because CCL17 does not induce desensitization of CCR4, while CCL22, being a stronger ligand for CCR4, can override the prior effect of CCL17 to guide T cells away from CCL17 (Mariani et al., 2004). Another example is CCR7 and its two ligands, CCL19 and CCL21, which play an essential role in the homing of lymphocytes and dendritic cells to secondary lymphoid tissues. Although both CCL19 and CCL21 are co-expressed in the T cell zone of secondary lymphoid tissues, only CCL21 is expressed or presented by high endothelial venules (HEV). Similarly, only CCL21 is expressed on afferent lymphatic vessels. CCL21 but not CCL19 can also be immobilized to the cell surface through its highly charged 40 amino acid extension at the C-terminus. On the other hand, only CCL19 but not CCL21 can desensitize and internalize CCR7 (Nandagopal et al., 2011; Schumann et al., 2010). Thus, the likely scenario is that CCL21 guides lymphocytes and dendritic cells expressing CCR7 into lymph nodes and T cell zones via HEVs and afferent lymphatics. Then, CCL19 overrides CCL21-mediated migration and desensitizes CCR7. Downregulation of CCR7 is also necessary for B and follicular helper T cells expressing CXCR5 to redirect their migration to the B cell zone via CXCL13 expressed by follicular dendritic cells (Haynes et al., 2007). These examples illustrate how a set of a single receptor with a pair of chemokines can play a combinatorial or functionally differential role in target cell migration.
Atypical Chemokine Receptors
There are also 5 atypical chemokine receptors (Table 2). They can bind a large number of chemokine ligands. Recently, atypical or decoy chemokine receptors have attracted attention because of their influence on the availability and function of both inflammatory and homeostatic chemokines. They are not just chemokine binding proteins, but standard heptahelical membranespanning receptors similar to classical chemokine receptors. Still, they do not transduce the full spectrum of chemokine receptor signals that lead to chemotactic and other cellular responses. This is partly because of either modification or lack of the typical DRY motif in the second intracellular loop (DRYLAIV) known to be important for efficient coupling with the Gαi class G-proteins. Their functions include either chemokine scavenger or decoy (interceptor) receptors or transporters. There are excellent reviews on this subject (Mantovani et al., 2006; Ulvmar et al., 2011). Briefly, DARC (Duffy Antigen Chemokine Receptor) is the classic example of an atypical chemokine receptor. DARC binds a large number of CXC and CC inflammatory but not homeostatic chemokines, is abundantly expressed by erythrocytes, and functions as a chemokine sink. DARC is also expressed by venular endothelial cells and mediates chemokine transcytosis from the basolateral to the apical side. This may promote leukocyte emigration at inflammation sites (Pruenster and Rot, 2006). CCBP2(D6) is another atypical chemokine receptor binding a large number of inflammatory CC chemokines. Given that D6 is expressed by the endothelial cells of afferent lymphatic vessels, its main role may be scavenging chemokines in the lymphatic flow to prevent leukocyte activation within the lymphatics. Indeed, Ccpb2−/− mice exhibited increased inflammatory responses in D6-expressing tissues such as skin, placenta, lung, etc., when challenged with various stimuli (Graham, 2009). CXCR7 is an interesting atypical chemokine receptor (Maksym et al., 2009). Together with CXCR4 and its ligand CXCL12, CXCR7 is conserved well across the vertebrate species including sea lamprey (Nomiyama et al., 2011). It binds CXCL11 and CXCL12. Importantly, CXCR7 can heterodimerize with CXCR4 and modulate the effect of CXCL12 on CXCR4. Furthermore, CXCR7 can also signal in some cell types although not by the classical G protein-coupled receptor pathways (Maksym et al., 2009). During embryogenesis, CXCR7 is necessary for proper migration of primordial germ cells to CXCL12 via CXCR4 by acting as a sink for CXCL12 (Boldajipour et al., 2008). CXCR7 is also involved in cardiac tissue development along with CXCR4 and CXCL12 (Sierro et al., 2007). Although poorly expressed in normal adult tissues, CXCR7 is widely expressed in a variety of tumors and tumor-associated vessels and appears to promote tumor growth and neoangiogenesis partly by signaling via mitogen activated protein kinase (MAPK) cascades (Maksym et al., 2009). CCRL1 and CCRL2 differ from other atypical receptors in their ability to bind and scavenge homeostatic chemokines, particularly the CCR7 axis (Comerford et al., 2006; Leick et al., 2010). Thus, they are considered to play a regulatory role in the homeostatic migration of lymphocytes and dendritic cells via CCR7. Interestingly, chemerin, a chemoattractant for myeloid-lineage cells including myeloid and plasmacytoid dendritic cells and potential adipokine signaling via ChemR23, also binds CCRL2 without signaling (Bondue et al., 2011). CCRL2 has been shown to play an important role in lung DC trafficking (Otero et al., 2010).
Chemokines and the Immune System
T cell Plasticity
Chemokine receptors have been associated with specific polarized subsets of CD4+ T cells that participate in various immune responses. Th1) cells express CCR5 and CXCR3, while Th2 cells express CCR4 (Sallusto and Lanzavecchia, 2000) and CCR8 (Zingoni et al., 1998). Th17 cells express CCR6 (Singh et al., 2008) and this is likely to be the signal that recruits these cells to the small intestine when it becomes colonized by the microbiome (Esplugues et al., 2011), while T regulatory (Treg) cells express CCR4 (Hirahara et al., 2006) and also CCR6 (Kitamura et al., 2010). These observations indicate that different chemokine ligands, likely produced by antigen presenting or immunomodulatory cells, have the capacity to direct the migration of these different subsets within the body (Sallusto and Baggiolini, 2008). Chemokines can also influence macrophage plasticity and their interaction with lymphocytes (Biswas and Mantovani, 2010).
Skin Chemokines
Several chemokines have evolved to be present in these tissues. The most skin-associated chemokine is CCL27. Other chemokines expressed in the skin include CXCL14, and several inflammatory chemokines that are expressed in the skin under inflammatory conditions. The roles of CCL17 and CCL22 as well as their receptor CCR4 in the skin were discussed above.
CCL27 was originally called CTACK for cutaneous T cell attracting chemokine (Morales et al., 1999). It is specifically and strongly expressed in the skin by keratinocytes. CCL27 binds CCR10 (GPR2) a class A G-protein couple receptor (Wang et al., 2000). CCR10 is not widely expressed as other chemokine receptors; instead, in the skin it is expressed strongly by melanocytes (and also by melanoma). This skin expression pattern suggests that the CCL27/− CCR10 axis participates in skin development, although a Ccr10−/− mouse does not exhibit skin abnormalities (Morteau et al., 2008). A well documented function of the CCL27-CCR10 axis in the skin is the recruitment of a subset of T cells to the skin that express cutaneous lymphocyte antigen (CLA)(Morales et al., 1999). The function of these skin-specific T cells remains obscure, but they are believed to represent memory cells that participate in local responses (Clark et al., 2006)
CXCL14 is a very interesting chemokine. It was originally reported as BRAK (breast and kidney chemokine) because of its high expression in these tissues (Hromas et al., 1999). It is, along with CXCL12. one of the most conserved chemokines (Zlotnik et al., 2006), suggesting that it has important evolutionary functions. It exhibits microbicidal activity (Maerki et al., 2009), suggesting that this may be its main role in the skin.
Mucosal Chemokines
Two chemokines are well known as mucosal chemokines (CCL25 and CCL28) while two other chemokines (CXCL14 and CXCL17) are also expressed in the mucosa but are not widely known as mucosal chemokines. CCL25 was originally reported as TECK (thymus expressed chemokine) because it was originally isolated from the thymus where it exhibits very strong expression (Vicari et al., 1997) and participates in T cell development (see below). The only other site where it is strongly expressed is the small intestine (Vicari et al., 1997). Its receptor is CCR9, another class A GPCR that does not exhibit a wide expression pattern (Norment et al., 2000). Like the CCL27-CCR10 axis, the CCL25-CCR9 axis is responsible for recruiting a certain subset of T cells to the lamina propria of the intestine (Kunkel et al., 2000) that express both CCR9 and α4β7 integrin (Papadakis et al., 2000). These cells have become important because they are involved in the pathogenesis of Crohn’s disease and ulcerative colitis. In fact, several companies have been developing CCL25-CCCR9 axis inhibitors and some of these are in clinical trials in Crohn’s disease (Eksteen and Adams, 2010).
Another interesting aspect of the CCL25-CCR9 axis is the expression of CCR9 in a subset of melanomas (Amersi et al., 2008). The expression of certain homeostatic chemokine receptors in tumor cells influences their metastatic potential (Zlotnik et al., 2011). If tumor cells of a certain cancer express a given homeostatic chemokine receptor, they may preferentially metastasize to organs where the ligand of that receptor is expressed. A dramatic example is the expression of CCR9 in a subset of melanomas. Melanoma does not usually metastasize to the small intestine; however, if melanoma tumor cells express CCR9, then intestinal metastases are observed (Amersi et al., 2008).
CCL28 was originally identified as a product of epithelial cells and it also binds CCR10 (Wang et al., 2000). It is one of the most mucosal-associated chemokines. It is highly expressed in the salivary gland, the female reproductive tissues and the mammary gland (Bourges et al., 2008). It is detectable in saliva and exhibits microbicidal activity (Hieshima et al., 2003). Interestingly, its expression in the mammary gland is induced upon the onset of lactation (Wilson and Butcher, 2004). CCL28 attracts plasmablasts that produce IgA through CCR10 (Kunkel et al., 2003) and is responsible for the production of IgA in certain mucosal sites (Hieshima et al., 2004; Morteau et al., 2008). A Ccr10−/− mouse, exhibits defective IgA responses in the mammary gland (Morteau et al., 2008).
Less is known about the other two mucosal chemokines, CXCL14 and CXCL17. CXCL14 is expressed in several mucosal sites (Meuter and Moser, 2008; Shellenberger et al., 2004). In some sites CXCL14 can exhibit surprising expression specificity. For example, in the tongue, CXCL14 is strongly expressed in the taste buds but not in lingual epithelium (Hevezi et al., 2009) although its function there is currently unknown. CXCL14 is also expressed in the intestines, and a Cxcl14−/− mouse shows metabolic abnormalities (Nara et al., 2007) and also grows at a slower rate (Tang et al., 2010). Since CXCL14 is a strongly conserved chemokine it is possible that these defects reflect developmental abnormalities. Importantly, its receptor has not been identified yet.
CXCL17 was the last chemokine to be reported and characterized (Pisabarro et al., 2006) and there is little information about it in the literature. Originally reported as DMC (dendritic cell and macrophage chemokine) it is the last human chemokine ligand of the CXC family. It is expressed in stomach and trachea (Pisabarro et al., 2006). It has recently been confirmed to be a mucosal chemokine since its expression is restricted to bronchus and trachea, tongue, oral cavity, stomach, small intestine and colon and it exhibits microbicidal activity (Burkhardt et al., 2012). In contrast to CXCL14 that in the tongue is expressed in the taste buds, CXCL17 is expressed in the lingual epithelium but not in the taste buds (Burkhardt et al 2012). This in an example of highly specific expression patterns that chemokines can have in the mucosa, although its functional significance is currently unknown. It attracts dendritic cells and monocytes (Pisabarro et al., 2006) and is induced by VEGF (vascular endothelial growth factor) (Weinstein et al., 2006). Like CXCL14, its receptor is currently unknown.
Chemokines in T cell development
Several chemokines are highly expressed in sites of lymphoid development like the thymus, bone marrow, or fetal liver. There are specific examples of chemokines important in lymphoid development. One of these is the CCL25-CCR9 axis, which plays an important role in T cell development in the thymus (Vicari et al., 1997).
T cell development in the thymus commences when hematopoietic precursors from the bone marrow colonize the thymus. Some chemokines participate in the seeding of the thymus rudiment by hematopoietic bone marrow precursors. Ccr7−/− and Ccr9−/− mice were shown to exhibit defective thymus colonization (Krueger et al., 2009; Zlotoff et al., 2009). A more recent study used a triple deficient mouse (CXCR4, CCR7 and CCR9) and observed a more profound defect (Calderon and Boehm, 2011), suggesting that several chemokines participate in this seeding process.
Once early T cell progenitors enter the thymus they are CD4−CD8− (double negative, DN), and are not yet committed to the T cell lineage (Godfrey and Zlotnik, 1993). These are called DN1. As they commit to the T cell lineage, they become DN2 and start to rearrange the β chain of the T cell receptor (TCR) (Godfrey et al., 1994) and subsequently become DN3. DN3 thymocytes that successfully rearrange the TCRβ chain in frame express this chain with the pre- T alpha (Kometani et al., 2008) and undergo ‘beta selection’, a step that allows the developing DN3 thymocytes to proceed become DN4. As a result of beta selection, DN3 thymocytes start to express CCR9 (Norment et al., 2000) and this allows them to become CD4+CD8+ thymocytes. CCR9 is also required for progression from the CD4+CD8+ stage to mature CD4+ thymocytes (Svensson et al., 2008). Other chemokine receptors like CCR7 are also involved in T cell development in the thymus (Misslitz et al., 2006).
Chemokines and Stem Cells
Hematopoietic stem cells (HSC) are known to reside in the bone marrow. Broxmeyer et al. (Broxmeyer et al., 2005) reported that the CXCR4 inhibitor AMD3100 induced release of HSC from the bone marrow, indicating that the CXCR4-CXCL12 axis is a key signal maintaining HSC in the bone marrow. This observation had potential clinical utility, as certain cancer patients are candidates for whole body irradiation followed by reconstitution of the hematopoietic compartment using HSC. Thus, the ability of the CXCR4 antagonist AMD3100 to release HSC from the bone marrow meant that these ‘mobilized’ HSC could be isolated from the blood of the patient in sufficient numbers to permit repopulation with autologous HSC (Uy et al., 2008). Interestingly, AMD3100 synergizes with granulocyte colony stimulating factor to mobilize HSC, improving the effectiveness of the approach and allowing the isolation of enough cells for autologous cell transplantation. This combined approach has been used in patients with non- Hodgkin’s lymphoma and with multiple myeloma (Pusic and DiPersio, 2010). Other CXCR4- based therapeutics have been reviewed recently (Peled et al., 2012).
These developments underscore the important roles that CXCR4 plays in development. Other studies have documented that the CXCL12-CXCR4 axis plays pivotal roles in homing of various stem cells to particular places of the embryo during development. One of the most useful experimental models to understand these mechanisms has been the zebrafish, where CXCR4 is the signal that guides stem cells that give rise to the gonads of the developing fish embryo (Doitsidou et al., 2002; Knaut et al., 2003). More recent studies have documented the coordinated roles of CXCR4, CXCL12 and CXCR7 in the development of the central nervous system in the zebrafish (Diotel et al., 2010; Tiveron and Cremer, 2008). Thus, certain homeostatic chemokine receptors play key roles in the homing of various stem cells. The ability of the CXCR4-CXCL12 axis in directing stem cell homing is probably related to its prominent role in directing the metastatic destination of tumor cells as well (Zlotnik et al., 2011).
Chemokine receptor antagonists as therapeutics
As discussed above, inflammatory chemokines are involved in control of inflammatory responses while several homeostatic chemokines are important in the control of immune responses, and their roles in development and cell migration also make them compelling targets for drug development. However, only recently have 2 drugs that target chemokine receptors been approved. One of these is a CCR5 antagonist that is approved for inhibition of entry of human immunodeficiency virus (HIV) into CCR5 positive cells (Gilliam et al., 2011). The other is a CXCR4 antagonist (AMD3100) that has been approved for mobilization of human hematopoietic stem cells from the bone marrow (Keating, 2011). The latter therapeutic use of a CXCR4 antagonist was predicted following the observation that blocking CXCR4 resulted in release of hematopoietic stem cells from the bone marrow (Broxmeyer et al., 2005). This approach is now used to obtain enough hematopoietic stem cells for use in autologous bone marrow transplants in patients with non-Hodgkin lymphoma or in multiple myeloma (Pusic and DiPersio, 2010). Despite the predominant role that chemokines play in inflammatory responses, no chemokine-based drugs have been approved for autoimmune or inflammatory diseases. One possibility, as discussed above, is that animal models used for preclinical studies aimed to evaluate the role of inflammatory chemokines may not translate well for human diseases. Another possibility is that more focused targets will succeed in specific indications. For example, CCR9 antagonists are currently in clinical trials for inflammatory bowel diseases (Proudfoot et al., 2010), while CXCR3 antagonists (presumably because they target Th1 cell responses) also show promise in other indications (Liu et al., 2011). CCR4 may also be a target for adult T cell leukemia-lymphomas (Tobinai et al., 2012).
Can Chemokines be Biomarkers?
Molecular characterization of various human diseases using genearrays indicates that despite the complexity of this superfamily, chemokines usually exhibit remarkable specificity in their association with certain human diseases. This suggests another possible use of chemokines, namely, as potential biomarkers.
One example is Hepatitis C, which is caused by the Hepatitis C virus that infects the human liver. Until recently the treatment for chronic carriers was a combination therapy of interferon alpha (IFN-α, and ribavirin, but 50% of the patients failed to clear the virus following this treatment (Tanaka et al., 2009). Infection with the Hepatitis C virus modulates the expression of many genes in the infected liver, including two chemokines, CXCL9 and CXCL10, which are strongly induced in the HCV-infected human liver (Hevezi et al., 2011). IL-28B is a cytokine that has been associated with the response to pegylated IFN-α and ribavirin in chronic Hepatitis C patients (Ge et al., 2009; Tanaka et al., 2009). Darling et al (Darling et al., 2011) found that lower CXCL10 serum concentrations correlate with sustained virological response, whereas high pretreatment CXCL10 amounts in serum correlate with nonresponders. Thus, the combination of IL-28B single nucleotide polymorphism (SNP) analyses along with CXCL10 serum concentrations may be effective predictors of response to treatment in Hepatitis C patients (Albert et al., 2011).
Alterations in plasma CXCL10 concentrations have also been associated with onset of transplant rejection (Romagnani and Crescioli, 2012). Given that CXCL10 is a ligand of CXCR3 and the latter is associated with Th1 cell responses (Sallusto and Lanzavecchia, 2000), it is possible that serum CXCL10 concentrations may reflect Th1 cell response activity in vivo. High CXCL10 concentrations may also correlate with poor prognosis and metastasis in colorectal cancer (Toiyama et al., 2012). More information on this subject can be found here (Amanatidou et al., 2011; Balkwill, 2012; Melve et al., 2011).
Conclusion
In order to understand the chemokine superfamily and its functions in the organism it is better to study it from an evolutionary perspective. While the function of inflammatory chemokines is more likely linked to resistance to infectious agents, homeostatic chemokines exhibit specific functions related to their expression site(s). Chemokines participate in the development of the immune system, inflammatory responses, and in innate and acquired immune responses. The discoveries and accumulated knowledge in chemokine biology in the last 25 years have led to important advances in our understanding of immune responses and organization of the immune system. It is our hope that this review will help readers better understand the chemokine superfamily.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Albert ML, Casrouge A, Chevaliez S, Hezode C, Rosa I, Renard P, Mallet V, Fontanet A, Pawlotsky JM, Pol S. Interferon induced protein 10 remains a useful biomarker of treatment failure in patients stratified for the interleukin-28B rs12979860 haplotype. Hepatology. 2011;53:1410–1411. doi: 10.1002/hep.24055. [DOI] [PubMed] [Google Scholar]
- Amanatidou V, Zaravinos A, Apostolakis S, Spandidos DA. Chemokines in respiratory viral infections: focus on their diagnostic and therapeutic potential. Crit Rev Immunol. 2011;31:341–356. doi: 10.1615/critrevimmunol.v31.i4.40. [DOI] [PubMed] [Google Scholar]
- Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res. 2008;14:638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Bourges D, Meurens F, Berri M, Chevaleyre C, Zanello G, Levast B, Melo S, Gerdts V, Salmon H. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol. 2008;45:3354–3362. doi: 10.1016/j.molimm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt AM, Tai KI, Flores-Gutierrez JP, Vilches-Cisneros M, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Ouellette AJ, Zlotnik A. CXCL17 is a mucosal chemokine with antimicrobial activity upregulated in idiopathic pulmonary fibrosis. J Immunol. 2012 doi: 10.4049/jimmunol.1102903. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon L, Boehm T. Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc Natl Acad Sci U S A. 2011;108:7517–7522. doi: 10.1073/pnas.1016428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo M, Karpova T, McNally J, Costes SV, Lockett SJ, Bos E, Peters PJ, Henkart PA. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity. 2004;20:219–230. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol. 2006;36:1904–1916. doi: 10.1002/eji.200535716. [DOI] [PubMed] [Google Scholar]
- Darling JM, Aerssens J, Fanning G, McHutchison JG, Goldstein DB, Thompson AJ, Shianna KV, Afdhal NH, Hudson ML, Howell CD, et al. Quantitation of pretreatment serum interferon-gamma-inducible protein-10 improves the predictive value of an IL28B gene polymorphism for hepatitis C treatment response. Hepatology. 2011;53:14–22. doi: 10.1002/hep.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N, Vaillant C, Gueguen MM, Mironov S, Anglade I, Servili A, Pellegrini E, Kah O. Cxcr4 and Cxcl12 expression in radial glial cells of the brain of adult zebrafish. J Comp Neurol. 2010;518:4855–4876. doi: 10.1002/cne.22492. [DOI] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Eksteen B, Adams DH. GSK-1605786, a selective small-molecule antagonist of the CCR9 chemokine receptor for the treatment of Crohn’s disease. IDrugs. 2010;13:472–781. [PubMed] [Google Scholar]
- Eliasson M, Egesten A. Antibacterial chemokines--actors in both innate and adaptive immunity. Contrib Microbiol. 2008;15:101–117. doi: 10.1159/000136317. [DOI] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W, Jr, Rongvaux A, Van Rooijen N, Haberman AM, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flad HD, Brandt E. Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol Life Sci. 2010;67:2363–2386. doi: 10.1007/s00018-010-0306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, et al. Genetic variation in IL28B predicts hepatitis C treatmentinduced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Gilliam BL, Riedel DJ, Redfield RR. Clinical use of CCR5 inhibitors in HIV and beyond. J Transl Med. 2011;9(Suppl 1):S9. doi: 10.1186/1479-5876-9-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Graham GJ. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol. 2009;39:342–351. doi: 10.1002/eji.200838858. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene- 1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, et al. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One. 2009;4:e6395. doi: 10.1371/journal.pone.0006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevezi PA, Tom E, Wilson K, Lambert P, Gutierrez-Reyes G, Kershenobich D, Zlotnik A. Gene expression patterns in livers of Hispanic patients infected with hepatitis C virus. Autoimmunity. 2011;44:532–542. doi: 10.3109/08916934.2011.592881. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, Yoshie O. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–3675. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skinhoming receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Christopherson K, 2nd, Azam M, Hou YH. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem Biophys Res Commun. 1999;255:703–706. doi: 10.1006/bbrc.1999.0257. [DOI] [PubMed] [Google Scholar]
- Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL- 5+ T(H)2 cells. Nat Immunol. 2011;12:167–177. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM. Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs. 2011;71:1623–1647. doi: 10.2165/11206040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Siekmann AF. The role of chemokines and their receptors in angiogenesis. Cell Mol Life Sci. 2011;68:2811–2830. doi: 10.1007/s00018-011-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Farber JM, Kelsall BL. CCR6 marks regulatory T cells as a colontropic, IL-10-producing phenotype. J Immunol. 2010;185:3295–3304. doi: 10.4049/jimmunol.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Kometani K, Moriyama M, Nakashima Y, Katayama Y, Wang SF, Yamasaki S, Saito T, Hattori M, Minato N. Essential role of Rap signal in pre-TCR-mediated beta-selection checkpoint in alphabeta T-cell development. Blood. 2008;112:4565–4573. doi: 10.1182/blood-2008-06-164517. [DOI] [PubMed] [Google Scholar]
- Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2009;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Absecreting cells. J Clin Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leick M, Catusse J, Follo M, Nibbs RJ, Hartmann TN, Veelken H, Burger M. CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAMB. Immunology. 2010;129:536–546. doi: 10.1111/j.1365-2567.2009.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, Stiles JK. CXCL10/IP- 10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- Maerki C, Meuter S, Liebi M, Muhlemann K, Frederick MJ, Yawalkar N, Moser B, Wolf M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol. 2009;182:507–514. doi: 10.4049/jimmunol.182.1.507. [DOI] [PubMed] [Google Scholar]
- Maksym RB, Tarnowski M, Grymula K, Tarnowska J, Wysoczynski M, Liu R, Czerny B, Ratajczak J, Kucia M, Ratajczak MZ. The role of stromal-derived factor-1--CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625:31–40. doi: 10.1016/j.ejphar.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur J Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- Melve GK, Ersvssr E, Kittang AO, Bruserud O. The chemokine system in allogeneic stem-cell transplantation: a possible therapeutic target? Expert Rev Hematol. 2011;4:563–576. doi: 10.1586/ehm.11.54. [DOI] [PubMed] [Google Scholar]
- Meuter S, Moser B. Constitutive expression of CXCL14 in healthy human and murine epithelial tissues. Cytokine. 2008;44:248–255. doi: 10.1016/j.cyto.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Misslitz A, Bernhardt G, Forster R. Trafficking on serpentines: molecular insight on how maturating T cells find their winding paths in the thymus. Immunol Rev. 2006;209:115–128. doi: 10.1111/j.0105-2896.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, Law Y, Distelhorst K, Nielsen EM, Hill ED, et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol. 2008;181:6309–6315. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Nandagopal S, Wu D, Lin F. Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PLoS One. 2011;6:e18183. doi: 10.1371/journal.pone.0018183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara N, Nakayama Y, Okamoto S, Tamura H, Kiyono M, Muraoka M, Tanaka K, Taya C, Shitara H, Ishii R, et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem. 2007;282:30794–30803. doi: 10.1074/jbc.M700412200. [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Hieshima K, Osada N, Kato-Unoki Y, Otsuka-Ono K, Takegawa S, Izawa T, Yoshizawa A, Kikuchi Y, Tanase S, et al. Extensive expansion and diversification of the chemokine gene family in zebrafish: identification of a novel chemokine subfamily CX. BMC Genomics. 2008;9:222. doi: 10.1186/1471-2164-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010;21:253–262. doi: 10.1016/j.cytogfr.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev Comp Immunol. 2011;35:705–715. doi: 10.1016/j.dci.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol. 2000;164:639–648. doi: 10.4049/jimmunol.164.2.639. [DOI] [PubMed] [Google Scholar]
- Otero K, Vecchi A, Hirsch E, Kearley J, Vermi W, Del Prete A, Gonzalvo-Feo S, Garlanda C, Azzolino O, Salogni L, et al. Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood. 2010;116:2942–2949. doi: 10.1182/blood-2009-12-259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cell alpha-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215–2229. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- Peled A, Wald O, Burger J. Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs. 2012;21:341–353. doi: 10.1517/13543784.2012.656197. [DOI] [PubMed] [Google Scholar]
- Pisabarro MT, Leung B, Kwong M, Corpuz R, Frantz GD, Chiang N, Vandlen R, Diehl LJ, Skelton N, Kim HS, et al. Cutting edge: novel human dendritic cell- and monocyteattracting chemokine-like protein identified by fold recognition methods. J Immunol. 2006;176:2069–2073. doi: 10.4049/jimmunol.176.4.2069. [DOI] [PubMed] [Google Scholar]
- Proudfoot AE, Power CA, Schwarz MK. Anti-chemokine small molecule drugs: a promising future? Expert Opin Investig Drugs. 2010;19:345–355. doi: 10.1517/13543780903535867. [DOI] [PubMed] [Google Scholar]
- Pruenster M, Rot A. Throwing light on DARC. Biochem Soc Trans. 2006;34:1005–1008. doi: 10.1042/BST0341005. [DOI] [PubMed] [Google Scholar]
- Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12- CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–326. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol. 2010;184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Romagnani P, Crescioli C. CXCL10: A candidate biomarker in transplantation. Clin Chim Acta. 2012 doi: 10.1016/j.cca.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Shellenberger TD, Wang M, Gujrati M, Jayakumar A, Strieter RM, Burdick MD, Ioannides CG, Efferson CL, El-Naggar AK, Roberts D, et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res. 2004;95:855–857. doi: 10.1161/01.RES.0000146674.38319.07. [DOI] [PubMed] [Google Scholar]
- Svensson M, Marsal J, Uronen-Hansson H, Cheng M, Jenkinson W, Cilio C, Jacobsen SE, Sitnicka E, Anderson G, Agace WW. Involvement of CCR9 at multiple stages of adult T lymphopoiesis. J Leukoc Biol. 2008;83:156–164. doi: 10.1189/jlb.0607423. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–755. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Cremer H. CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol. 2008;18:237–244. doi: 10.1016/j.conb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tobinai K, Takahashi T, Akinaga S. Targeting Chemokine Receptor CCR4 in Adult TCell Leukemia-Lymphoma and Other T-Cell Lymphomas. Curr Hematol Malig Rep. 2012 doi: 10.1007/s11899-012-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiyama Y, Fujikawa H, Kawamura M, Matsushita K, Saigusa S, Tanaka K, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int J Oncol. 2012;40:560–566. doi: 10.3892/ijo.2011.1247. [DOI] [PubMed] [Google Scholar]
- Ulvmar MH, Hub E, Rot A. Atypical chemokine receptors. Exp Cell Res. 2011;317:556–568. doi: 10.1016/j.yexcr.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy GL, Rettig MP, Cashen AF. Plerixafor, a CXCR4 antagonist for the mobilization of hematopoietic stem cells. Expert Opin Biol Ther. 2008;8:1797–1804. doi: 10.1517/14712598.8.11.1797. [DOI] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) J Biol Chem. 2000;275:22313–22323. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, Westlin MM, Mazzarella R. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350:74–81. doi: 10.1016/j.bbrc.2006.08.194. [DOI] [PubMed] [Google Scholar]
- Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, Gould SL, Springer MS, DeMartino JA. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288–18291. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200:805–809. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organspecific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2009;115:1897–905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]