Abstract
Systemic lupus erythematosus (SLE) is considered a prototype of systemic autoimmune diseases; however, despite considerable advances in recent years in the understanding of basic mechanisms in immunology, little progress has been made in elucidating the etiology and pathogenesis of this disease. This even holds for inbred mice, such as the lupus-prone New Zealand Black/New Zealand White (NZB/NZW) F1 mice, which are all genetically programmed to develop lupus at a predetermined age. This frustrating state of affairs calls for a fundamental change in our scientific thinking, and the opening of new directions in lupus research. Here, we suggest that intrinsic B cell tolerance mechanisms are not grossly impaired in lupus-prone mice, but that an unusually strong positive selection event recruits a small number of autoreactive B cells to the germinal centers. This event could be facilitated by nucleic acid–protein complexes that are created by somatic changes in the susceptible animal.
Keywords: B cell tolerance, Lupus, Anti-DNA, NZB/NZW mice, Retroelements
A substantial amount of experimental work has been done in recent years, in both human subjects and mouse models, centering on B cell tolerance mechanisms and their apparent failure in autoimmune disease. One major conclusion from these research efforts has been the notion that B cell tolerance is not confined to immature B cells (central tolerance) as originally postulated by Burnet (1), but that a large number of autoreactive B cells reach the blood and the secondary immunological organs. These cells are said to be subject to a series of tolerogenic “checkpoints” at various stages of their maturation and activation (peripheral tolerance). The tolerance checkpoints, combined with T cell tolerance, protect the organism against autoimmune diseases, such as systemic lupus erythematosus (SLE). For example, Goodnow et al. (2) have counted as many as 10 checkpoints for B cell tolerance, from the immature B cell to the plasma cell stage, and a similar number of checkpoints for T cell tolerance. The mechanisms responsible for the elimination of autoreactive B cells at each of these checkpoints remain largely unknown.
Among the large number of mouse strains that serve as models for SLE, some develop lupus spontaneously at a well-defined age (e.g., NZB/NZW F1, MRL/lpr/lpr, BXSB.Yaa, (3)); others can be induced to develop the disease by knocking out specific genes (e.g., CD22, Lyn, FcγRIIb) or by introducing genes (e.g., BAFF, Bcl2) that are involved in B cell regulation and/or activation. Among the spontaneous mouse models, the oldest NZB/NZW F1 (abbreviated B/W) mouse, discovered over 50 years ago, has disease properties that are most similar to those of human SLE (4). This mouse is characterized by a strong female-to-male bias, fatal immune-mediated glomerulonephritis and high titers of anti-nuclear autoantibodies, including high-affinity IgG antibodies to dsDNA. The disease in B/W mice is apparently not dominated by a single gene product, such as Fas (as in MRL/lpr/lpr mice) or TLR7 (as in BXSB.Yaa mice), but is controlled by multiple genetic elements (4), as is the case in human lupus.
Each SLE mouse model has different characteristics; however, in this review we discuss mostly, but not exclusively, the spontaneous B/W model. We describe an experimental system in which a pre-rearranged H chain derived from a high-affinity IgG anti-DNA hybridoma (D42) of B/W origin (5, 6) was site-directed to the mouse germ line of the C57BL/6 mouse and subsequently backcrossed onto the genetic background of the original autoimmune strain (7, 8). This work has implications for several controversial issues in autoimmunity and has culminated in the recent analysis of single B cells from distinct bone marrow and peripheral populations, illuminating the fate of high-affinity autoreactive B cells in health and disease.
The D42 H chain is encoded by the VH11 gene of the small S107 VH family of the mouse (5, 6). The gene products of VH11 (S107) represent at least 5% of the H chains expressed in anti-DNA antibodies of B/W mice (9, 10). The CDR3 of the D42 H chain is rich in arginine residues that are encoded by the D segment Sp2, read in an unusual reading frame, as well as by flanking N sequences (6). These arginine residues in CDR3 are important for DNA binding (11–14). The D42 H chain has two somatic mutations—one in CDR1 and one in CDR2—that increase the affinity for DNA by about 10-fold (11). The acquisition of mutations that increase the affinity for DNA is consistent with T cell dependence and selection by the autoantigen. However, the D42 antibody presumably binds dsDNA even in its H and L chain–unmutated (germ line) forms (8)—although it has been argued that due to the presence of non-templated (N-region) junctional sequences in CDR3, an unmutated configuration of an H chain cannot be determined with absolute certainty (15). However, other mouse anti-DNA antibodies, such as those encoded by the highly DNA-specific BW16 VH gene, also bind DNA with apparently unmutated H and L chain configurations (13, 14). These include antibodies that do not contain arginine or other presumed DNA-binding amino acid residues (e.g., lysine, asparagine) in their H chain CDR3, so that any putative somatic mutation in their N-regions is not likely to affect their DNA binding affinity. The specificity of the D42 antibody is strictly limited to ss- and dsDNA. We have not observed even a low level of cross-reaction with closely related antigens, such as RNA and cardiolipin (5). This high specificity for DNA may result from the strong preference for polynucleotides containing deoxythymidine sequences (5).
The high affinity of the D42 antibody for dsDNA, the increase in affinity for the cognate antigen by each of the two H chain somatic mutations, and the strict DNA specificity with its poly (dT) fine specificity support the view that the autoimmune response to DNA mimics a secondary immunization with a foreign antigen (9, 12, 13). It was recently proposed that anti-DNA autoantibodies arise by somatic mutations, generated in the course of a B cell response to a foreign antigen (15, 16). While this mechanism has been demonstrated in several cases, it may not apply to high-affinity anti-DNA antibodies. It is also not supported by the elegant in vivo experiment of Scharff and coworkers in B/W mice (10). These investigators followed up on their widely cited initial observation (17) that the S107 myeloma protein encoded by the V1 (T15) VH gene of the S107 family had changed its specificity in vitro from phosphorylcholine (PC) to DNA by a mutation leading to a single amino acid change. They further immunized prediseased B/W mice with phosphorylcholine–keyhole limpet hemocyanin (PC-KLH) to encourage them to produce anti-DNA autoantibodies by introducing somatic mutations in the widely expressed V1 gene. Surprisingly, however, the diseased lupus-prone mice utilized, exclusively, the closely related V11(S107) VH gene and not the readily available V1 gene for generating their anti-DNA autoantibodies (10). This experiment shows that at least in this case, anti-DNA autoantibodies are not generated by simply mutating binding sites of antibodies to foreign antigens, even when these antigens are structurally related to the autoantigen and the elicited antibodies are encoded by highly related germ line genes.
In our initial experiments, the site-directed D42 H chain was expressed in its unmutated or mutated form in C57BL/6 mice. The majority of B cells in blood and spleen of these mice expressed the targeted H chain, but we measured no significant anti-DNA activity in the serum, suggesting very efficient B cell tolerance mechanisms in the normal animals (7). When backcrossed onto the B/W genetic background, transgenic IgM anti-DNA antibodies appeared in the serum of 3-month-old female mice, while high titers of both IgM and IgG anti-DNA were measured in 7-month-old mice, concomitant with the development of clinical disease. The age-dependent switch from IgM to IgG anti-DNA at the onset of clinical disease in B/W mice was observed over 30 years ago by Talal and coworkers (18) and is consistent with the view that IgG autoantibodies, mostly of the IgG2a and IgG2b subclasses, are essential components in the pathogenesis of clinical nephritis in murine lupus.
The difference in DNA binding activity between the C57BL/6 and B/W D42H transgenic mice was reflected by the L chains that could associate with the targeted D42H (8). While many different L chains associated with the D42H in hybridomas of spontaneous, presumably activated C57BL/6 spleen cells and gave rise to low affinity for DNA, the great majority (85%) of D42H hybridomas from diseased, female B/W mice were associated with a single L chain, VκRF-Jκ5. This Vκ gene segment represents a one-gene Vκ family and encodes the same L chain as the original D42 hybridoma. This exceptionally strong selection for one particular HxL pairing that confers DNA binding with high affinity and specificity was present in every single diseased D42H B/W mouse analyzed. In contrast, not a single VκRF-expressing hybridoma was ever identified in D42H transgenic C57BL/6 mice.
Since we were interested in B cell tolerance mechanisms, namely, clonal deletion (1), clonal anergy (19) and receptor editing (20), and their apparent failure in autoimmune mice, we further generated several HxL double-knock-in mice on normal and B/W genetic backgrounds (21, 22). These included the D42H transgene, combined with Vκ1-Jκ1, Vκ4-Jκ4 or Vκ8-Jκ5. The L chain-targeted BALB/c mice were constructed by Weigert and coworkers and had a variable number of Jκ segments available for secondary rearrangements. The DNA affinities of the three double transgenic constructs were very similar and were about 1.5 orders of magnitude lower than that of the canonical D42H/VκRF-Jκ5 HxL pair. On a normal mouse background, (C57BL/6xBALB/c)F1, we found that the double transgenic mice edited their L chains extensively, and that the extent of receptor editing depended greatly on the availability of the unrearranged Jκ segments on the targeted L chain allele. Thus, D42H/Vκ1-Jκ1 B cells, having three remaining unrearranged Jκ segments, edited their L chain in almost every recovered hybridoma of LPS-activated spleen cells; D42H/Vκ4-Jκ4 B cells with only one remaining Jκ were only partially edited, and D42H/Vκ8-Jκ5 B cells remained essentially unedited (21). Surprisingly, when backcrossed to the B/W autoimmune background, the double transgenic mice edited their L chains to the same extent as the normal mice (22). This suggested that the mechanism of B cell receptor editing is intact in diseased female B/W mice. Here, the anti-DNA transgenic B/W mice differed somewhat from anti-MHC class I transgenic MRL/lpr/lpr mice. In these mice, Feeney and coworkers noted a decrease in the extent of receptor editing (23), although the mice were as efficient as normal mice in deletion of the transgenic, autoreactive B cells (24).
Strikingly, about 25% of D42H/Vκ1-Jκ1 hybridomas of spontaneously (non-LPS-) activated cells from the transgenic B/W mice did not utilize non-DNA-binding “editor” L chains, but rather the very high affinity anti-DNA canonical VκRF-Jκ5 L chain (22). In contrast, no hybridoma from the non-autoimmune transgenic C57BL/6xBALB/c mice had the VκRF-Jκ5 L chain. The fraction of VκRF-edited hybridomas was again dependent on the available Jκ segments. Despite a similar initial affinity of Vκ1, Vκ4 and Vκ8 L chains combined with the D42 H chain, the DNA binding capacity in the serum of D42H/Vκ1-Jκ1 B/W female mice was several orders of magnitude higher than that of D42H/Vκ8-Jκ5 mice (22). Apparently, receptor editing in diseased B/W mice is not only fully functional, but it also operates to make the autoimmune disease worse by combining random editing with a powerful positive selection of the highest-affinity autoreactive B cells. Weigert and coworkers reported very similar results in MRL/lpr/lpr knock-in mice, targeted with the high-affinity anti-DNA H chain 3H9 H76R (25).
The low-affinity anti-DNA D42H/Vκ8-Jκ5 B cells that could not edit their L chains were found to be anergic and populated the spleens of normal and B/W mice in large numbers. We looked at several parameters of anergy, such as B cell receptor (BCR) density, tyrosine phosphorylation of BCR-dependent signaling components, activation by LPS or CpG, calcium immobilization and Ig secretion in B cells taken ex vivo from the spleens of D42H/Vκ8-Jκ5 HxL mice. We found that the B/W transgenic B cells were as anergic as the corresponding D42H/Vκ8 B cells that were derived from normal mice (26). This suggests that clonal anergy, like receptor editing, is virtually intact in the lupus-prone mice.
With the hybridoma work having clarified the final outcome of B cell tolerance and the disease process in the autoimmune mice, we still did not know the fate of the high-affinity anti-DNA autoreactive B cells through the various stages of B cell maturation in the bone marrow and the periphery. This was done by Davidson and coworkers (27), who performed single-cell analysis and quantitative (q)PCR expression studies of the relevant anti-DNA H and L chains in young and old B/W mice, targeted with the unmutated D42 H chain. Young B/W mice serve as a particularly good control for diseased, old mice, because they have the same genetic background but do not show any features of clinical disease. B cells from young B/W mice, however, are hyperactive, possibly as a result of unexplained polyclonal activation (28). This could give rise to the relatively early anti-DNA binding activity in the serum, which could be mediated by polyreactive IgM antibodies. This early anti-DNA binding activity is found prior to the IgG switch in activity, which occurs at about 5 months of age (18).
Huang et al. (27) analyzed the L chain repertoire of unmutated D42H B cells from T1, T2, marginal zone, follicular, and germinal center (GC) populations in 8-week-old and 32-week-old female B/W mice. Several L chains were associated with the D42 H chain in the various peripheral B cell compartments. One dominant L chain, 55.01, constituted a large fraction of the repertoire in all the compartments except the GC. This HxL combination did not differ in abundance between young (healthy) and diseased mice. The same HxL combination had been previously identified in a large number of edited D42H B/W hybridomas (22). The antibody product of these hybridomas is a polyreactive IgM that binds DNA with very low affinity, as well as phosphatidylcholine, phosphatidylserine and actin. Such polyreactive IgM autoantibodies were recently shown to protect MRL/lpr/lpr mice from lupus nephritis and to improve their survival (29). Strikingly, the VκRF-Jκ5 L chain, which is the only mouse L chain that confers anti-DNA binding upon the D42 H chain with high affinity and specificity, was detected only in cells derived from one B cell compartment, the GC, and exclusively in old mice; no D42H/VκRF HxL combination could be found in 8-week-old B/W mice in any of the peripheral B cell compartments, including the GC. This suggests that the high-affinity anti-DNA B cells are perfectly regulated in young B/W mice (as well as in normal mice) and throughout B cell maturation in diseased B/W mice up to the GC stage. This analysis may also suggest that the anti-DNA B cells that secrete polyreactive IgM anti-DNA antibodies and are common in young and old B/W mice may constitute only a minor component of the precursor B cells that are recruited to the GC.
Perhaps even more revealing is the qPCR analysis of the same study (27), in which different B cell populations from bone marrow and spleen were examined specifically for the expression ratio of VκRF to D42H. A high ratio of about 1 was observed in immature B cells in the bone marrow. This is somewhat surprising, as there is no good reason for the preferential selection of high-affinity anti-DNA B cells at this developmental stage. One explanation could be a preferential association of these H and L chains over other HxL combinations. Another possibility is the requirement for binding to negative charges on bone marrow stromal cells that induces preferential B cell proliferation. Such binding to stromal cells has been shown for the positively charged non-Ig portion of λ5 in the pre-B cell receptor (30). In any event, the expression ratio of VκRF to D42H fell by 20- to 50-fold in peripheral B cell populations of both young and old mice, suggesting that the high-affinity autoreactive B cells in the bone marrow are mostly (but not completely) eliminated by central tolerance, mediated by receptor editing and/or by clonal deletion.
In young B/W mice, residual autoreactive B cells apparently never make it to the GC and plasma cell compartments, possibly due to a lack of T cell help. Alternatively, these cells may be eliminated in the GC by a Fas-dependent tolerance mechanism that operates for a membrane-bound self-antigen and is defective in Fas-deficient MRL/lpr mice (31). Strikingly, however, in the diseased B/W mice, a much higher expression ratio of VκRF to D42H reappears in the GC. This inevitably leads to the emergence of the high-affinity, class-switched and somatically mutated D42H/VκRF IgG anti-DNA plasma cells in the spleen and bone marrow. It follows that only two tolerance checkpoints are apparent in this experimental system: central tolerance and GC tolerance. The difference between young and old B/W mice lies in the remarkable activation and positive selection of residual autoreactive B cells from the follicular compartment into the GC. Similar results were obtained with human tonsil autoreactive B cells that were recruited to the GC of SLE patients, but not to the GC of rheumatoid arthritis patients or healthy control subjects (32). Although it has been suggested that L chain secondary rearrangements could also take place in the periphery (25), it is unlikely that the VκRF in the GC is reintroduced by L chain receptor editing, since no RAG mRNA was detected by qPCR in the follicular and GC compartments (27); rather, receptor editing in the bone marrow probably adds to the small number of high-affinity autoreactive B cells that escape central tolerance in the diseased B/W mice.
Lupus in B/W mice does not correspond to human SLE in every respect, but it is interesting to note the results of Nussenzweig and coworkers, who analyzed and expressed single B cell antibodies from human SLE patients and healthy control subjects (33). They found that antibodies from new emigrant B cells in the blood of SLE patients were not significantly different in anti-nuclear antibody (ANA) reactivity from the antibodies of healthy donors, whereas the antibodies from mature naïve SLE B cells were different from those in controls (40 vs. 20% ANA); however, these populations had high frequencies of polyreactive antibodies with binding to DNA, LPS and insulin. As we have suggested, these presumably (rarely measured) low-affinity and polyreactive IgM-producing B cells may not be precursors of the high-affinity, class-switched memory and plasma cells in these patients. In fact, Nussenzweig and coworkers found that the increased number of self-reactive naïve B cells in SLE does not have a strong impact on the general level of autoreactivity in the IgG+ memory B cell pool. They concluded that defects leading to abnormalities in IgG+ memory B cell tolerance in human SLE may be independent of the earlier defects in B cell tolerance (34).
It could be argued that tolerance is abrogated due to a B cell defect within the GC of lupus-prone mice, as is the case in Fas-deficient animals (31); however, unlike earlier B cell maturation stages, recruitment to the GC depends on the availability of antigen and T cell help. We prefer to assume that the residual anti-DNA follicular B cells that escape central tolerance are either “ignorant” (35) or subject to clonal anergy, which is readily reversible upon provision of T cell help. This was clearly demonstrated by Erikson and coworkers in anti-DNA transgenic BALB/c and MRL/lpr/lpr mice (36). The autoreactive B cells are totally dependent on signals from helper and follicular helper CD4+ T cells for their recruitment into GC and their survival in the GC, respectively (37). Thus, follicular exclusion of autoreactive B cells in mice (38) and humans (32) could also be explained by the lack of T cell help (2). The mechanisms of T cell tolerance and activation in murine and human lupus have been investigated to a much lesser extent than B cell mechanisms. Nevertheless, T cells play a decisive role in the disease process (39). For example, one of the NZW contributing loci on chromosome 7, Sle3, disregulates CD4+ T cells (40). High-affinity anti-DNA antibodies are class-switched and somatically mutated and thus also suggest a T cell-dependent secondary immune response (9, 13). Lupus in B/W mice was reversed by treatment with anti-T cell monoclonal antibody, indicating a T cell help requirement for disease development (41). In human SLE, T cells provide excessive help to B cells. Engagement of the T cell receptor CD3 leads to an enhanced signaling response, manifested by increased calcium flux and tyrosine phosphorylation (42). Patients with SLE contain abnormally high levels of the proinflammatory cytokine IL-17 in their serum. IL-17 increases the survival of B cells and contributes to the formation of germinal centers and the production of antibody-secreting cells (43).
In the absence of any major B cell defect, there could be abnormal T cell help. But this possibility does not answer the question of why the positive antigen selection discussed here would occur only in old but not young mice. We consider this question a crucial one in the etiology of spontaneous autoimmune disease. Another crucial question is: What is the initiating antigen? Most lupus researchers would agree that it is DNA, alone or in complex with nuclear proteins. In line with this, mice defective in the clearance of apoptotic cells develop a lupus-like disease (44), and a subgroup of SLE patients do not clear apoptotic cells properly (45) (reviewed in (46), together with defects in complement-dependent clearance of necrotic cells). But where does the antigenic DNA suddenly come from in B/W mice, and if it has been there all along, why does it suddenly lead to a GC reaction? We suggest that in B/W mice, endogenous recombinant murine leukemia virus (MLV) plays a decisive role in the initiation of autoimmunity. More generally, endogenous retroelements would mediate both hereditary and spontaneous forms of SLE.
How B cells would be stimulated specifically by DNA has not been solved, but nucleic acids—whether they are introduced from the outside or are cell-intrinsic—are key structures sensed by the innate immune system (47). In lupus, both RNA and DNA seem to contribute to the disease, and as single agents, retroelements provide both, in structures different enough from their cellular counterparts, and located in different cellular compartments. In the B/W mouse, the observed hyperproliferation could be explained by stimulation through TLR4 by the envelope of MLV (48). The nucleic acids of MLV may contribute as well. When introduced from the outside, the TLR9 and TLR7 pathways sense CpG-containing DNA and ssRNA, respectively, and these pathways can promote autoantibody formation (49–51). Unfortunately, results in lupus-prone mice have been paradoxical. Despite the absence of anti-DNA antibodies in TLR9-deficient MRL/lpr/lpr animals, disease is worsened. In contrast, TLR7 deficiency has no effect on anti-DNA and anti-nucleosome antibodies, but clinical disease is partially ameliorated (50). In lupus-prone mice, we note that TLR7 also controls the GC response to exogenous (52) and endogenous (53) MLV (as demonstrated in non-autoimmune mice). But mouse strains with spontaneous autoimmune disease, including NZB, NZW, and MRL/lpr/lpr, produce large amounts of retrovirus even in the presence of TLR7 and TLR9. In NZB mice, for example, there are at least 84 endogenous non-ecotropic proviruses, 28 of which are unique to that strain (54).
When the (xenotropic) NZB virus was discovered, investigators hypothesized that it constitutes an “autoimmune virus” (55) that initiates antibodies to red blood cells and dsDNA and eventually causes nephritis—similar to that of the B/W mouse, where these symptoms develop faster. However, in experiments with NZBxSWR crosses, virus titers did not correlate with anti-DNA antibody titers or nephritis (56). The results left the immunology community less enthusiastic about the retroelement hypothesis. However, even barring a direct role of MLV in the autoimmune disease, we are still left with the question, Why is retroviral antigen a preferred autoantigen in diseased B/W mice? There, levels of gp70–antibody complexes are strongly associated with the development of glomerulonephritis (57). Mouse serum gp70 (produced by the liver) is very similar to MLV gp70 and is present in virtually every mouse strain, but only lupus-prone strains produce autoantibodies to (retroviral) gp70 (57). In fact, B/W mice produce antibodies to the gp70 of the inducible NZB-X2 MLV (58) that cross-react with endogenous murine gp70. This is not to say that the gp70–antibody complexes necessarily cause the disease, but it shows the importance of MLV as an autoantigen.
Recently, a seminal paper provided a fascinating glimpse of potentially expansive views on autoimmunity (59); it showed how retroelements can stimulate the interferon stimulatory DNA (ISD) response (60), an intracellular DNA sensing mechanism, which leads to the activation of innate and adaptive immune responses in autoimmune disease. Mice deficient in the DNA exonuclease Trex1 generate ANA and develop glomerulonephritis (61), and most of them die of circulatory failure caused by severe inflammatory myocarditis (62); this is dependent on the type I ISD pathway and IFN-dependent mobilization of lymphocytes (59). In humans, both Aicardi-Goutières syndrome (63) and a cutaneous subtype of SLE called familial chilblain lupus (64) can result from mutations in TREX1. Furthermore, mutations in TREX1 are present in 2.6% of SLE patients, but only 0.1% of control subjects (65). In the cells of the hearts of Trex1-deficient mice, cDNA of endogenous retroviruses and retrotransposons accumulates, and the sera contain autoantibodies to heart tissue. Reverse transcriptase inhibitors prevent disease in these mice, suggesting that the retroelements contribute to this form of autoimmunity (66). If not properly metabolized, retroelements are potential sources of endogenous nucleic acids that may trigger an antiviral response (67). Similarly, B/W mice treated with a drug that increases the cDNA concentration of MLV succumb to disease earlier than their non-treated littermates (68).
Almost half of the genome of mice and humans consists of retroelements, which include retroviruses and retrotransposons. The biochemical nature of retroelement DNA in the genome is no different from that of the part of the genome that includes classical genes, regulatory RNAs and intervening sequences, and thus antibodies to DNA and RNA will react to retroelement nucleic acids as well. However, apart from being replicated as part of the genome, retroelements also replicate with their own life cycle, which includes infection (for retrovirus), reverse transcription, recombination and hypermutation. This is important on several counts: placing viral RNA and DNA into the cytoplasm, where it is sensed by the innate immune system; creating new viruses with different tropism and effector functions; potentially interfering with the expression of activating and tolerizing genes; and creating new (cross-reacting) epitopes.
The special nature of retroelement replication, with its high frequency of mutation and recombination, may also provide an explanation for the strict time line of the onset of autoimmune disease and death in B/W mice. Unlike an undefined “error catastrophe” as a result of relentless accumulation of unknown defects in tolerization, the accumulation of mutations (including recombination and hypermutation of the retroviral genome and insertional mutagenesis of the cellular genome) follows a linear time line. Although stochastic in nature, the large number of events ensures predictability. For example, de novo insertions of MLV in the genome are silenced in cell culture with a strict time line that depends on the genomic site but is independent of culture conditions (69). In AKR mice, T lymphoma formation is mediated by de novo recombinant (endogenous) MLV and its mutagenic insertions into cellular DNA, yet the time by which 50% of mice are dead can be predicted. The emergence of a mutated antigen would thus satisfy the requisite properties needed to explain both the positive selection and the time line in the autoimmune response of lupus-prone mice. It may also represent the environmental (somatic) factor needed for the development of disease in human patients, as the concordance rate for lupus among monozygotic twins is only about 25% (70).
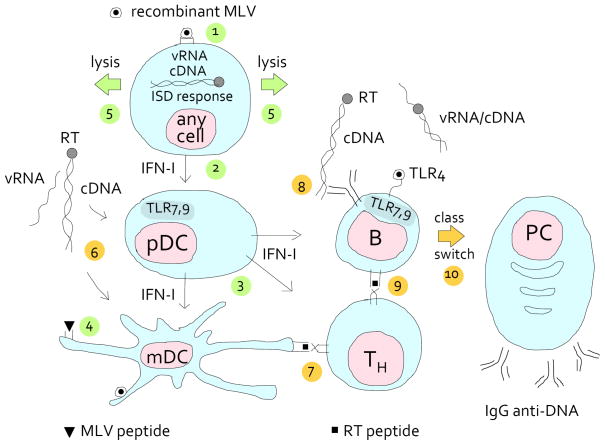
Retroelements are induced by a variety of stimuli, including UV radiation, chemicals and infection. But what exactly would this retroviral autoantigen be? Because it is not presented by MHC molecules, DNA as an antigen for high-affinity antibodies poses a problem that is not relevant to other autoantigens. Proteins associated with the nucleic acids may provide T cell help. But we also cannot exclude the possibility that the reverse transcriptase, with its mutation rate equivalent to that of activation-induced deaminase, generates altered retroviral peptides or peptide mimics of nucleic acids (71). In Fig. 1, we present one of many possibilities of how MLV initiates autoimmunity in B/W mice.
FIGURE 1.
Activation of an anti-DNA specific B cell by mutated endogenous retrovirus. NZB and NZW mice produce endogenous, infectious, replication competent, but xenotropic MLV (76), which cannot infect mouse cells unless pseudotyped with ecotropic or polytropic envelope. These mice also encode many MLVs that are defective in various other ways. In addition, NZW mice also produce ecotropic MLV (77, 78), although it is inefficiently activated from their cells. In the B/W mice, this diversity can lead to phenotypic mixing and generate recombinant viruses. We suggest that some recombinant viruses provide peptides that bind to MHC class I or class II and that are recognized as non-self. As a consequence, B cells will be killed, helped, or both (depending on whether or not they are infected with recombinant virus), resulting in lymphopenia, hyperproliferation and/or autoimmunity. MLV may also infect most other cells, including myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC).
Because the T cells are tolerant to the endogenous virus early in life, sufficiently different recombinant virus has to be generated before cells are killed. In the scheme, the steps leading to lysis are numbered 1 to 5 and colored green. In step 1, recombinant MLV (circle with filled core) enters a hematopoietic or non-hematopoietic cell. If the cDNA fails to integrate, it is stabilized by circularization and triggers the type I interferon stimulatory DNA (ISD) response. (Containing double-stranded RNA, the tRNA-viral RNA (vRNA) priming complex may also be sensed by TLR3; and In B lymphocytes, the vRNA is sensed by TLR7). As a result, type I interferon (IFN-I) is secreted (step 2), which stimulates the plasmacytoid dendritic cell (pDC) to secrete copious amounts of IFN-I, which, in turn, stimulates mDCs (step 3). The mDC presents MLV peptides (step 4) to T helper cells (not shown) that help cytotoxic T cells (not shown) to lyse the MLV-infected cell (step 5).
With the lysis of the infected cell, retroviral cDNA complexed with (mutated) reverse transcriptase (RT) is released (step 6; this and the following steps depict the events that lead to anti-DNA antibody formation and are colored yellow). The cDNA complexes are taken up by pDCs, which secrete IFN to help B and T cells, and by mDCs, which license T helper (TH) cells (step 7). T cell help for B cells with DNA specificity is available due to a recombination event that generates a class II-binding epitope of the RT (RT peptide) that is sufficiently different from self. In step 8, a B cell receptor specific to DNA endocytoses cDNA complexed with RT and presents an RT peptide on MHC class II (step 9). (In a variation on the theme, instead of cDNA, a retro-viral protein mimetope of DNA is bound to the B cell receptor.) This activates the B cell, and after class switching (step 10) the plasma cell produces antibodies to dsDNA. The cognate immune response is helped by the innate immune response to (mutated or non-mutated) retroviral envelope via TLR4 (48, 79); to ss retroviral RNA (vRNA) via TLR7 (53), sensed as part of an endocytosed virus; and to cDNA, via the ISD response (60) or TLR9.
Retroelements are ancient companions of the human genome. Although in healthy persons the vast majority of retroelements are truncated and/or highly mutated and no longer encode functional genes, the most recently active retroviruses that have integrated into the human germ line contain intact open reading frames in some or all genes that encode functional proteins expressed in various tissues (72). There are 80–100 retrotransposition-competent LINE-1 elements in an average human being. Remarkably, most of the retrotransposition capability is present in six highly active elements (73). It does not take a great leap of faith to assume that in some instances, retrotranspositions of such elements in somatic cells will have consequences, causing cancer or autoimmune disease. Furthermore, 38 of 86 LINE-1s are polymorphic in human populations—ample ground for variations in outcome. Lupus patients would have allelic forms of one or several retroelements that are pathogenic. Some may lack a methylase that silences functional retroelements (74). Genome-wide association studies filter out and thus disregard any contribution of repetitive elements, which are, to a great extent, retroelements. But even if the repetitive sequences were not filtered out, it would be difficult to pinpoint a putative retroelement in such studies—the same retroelement need not be responsible for disease in every patient. Studies in lupus-prone families would reduce this complexity.
Finally, there is a well-documented link between lupus and cancer. Human SLE patients have an increased risk of non-Hodgkin’s lymphoma (75), and in the B/W mouse there is a concomitant incidence of lupus and lymphoma. In our view, mutated B cells would be responsible for this link. In the B/W mouse, the mutations would be generated by de novo proviral insertions. The requirement for more than one mutation within a given cell may explain why cancer is preceded by autoimmunity, which may be initiated by a single event.
Conclusion
A fundamental unresolved question in autoimmune disease is: What is the driving force? Specifically, what causes the emergence of plasma cells that secrete high-affinity IgG anti-DNA antibodies in SLE? In the B/W mice, which develop spontaneous lupus, we did not find any major intrinsic defect in currently known B cell tolerance mechanisms (deletion, anergy, and receptor editing). Instead, in old but not young mice, a GC reaction produces anti-DNA antibodies. In the model presented here, rather than antibodies fortuitously mutating toward DNA specificity, we suggest that a mutated endogenous retrovirus would activate T and B cells. We have sketched an example of how recombinant retroviral epitopes can lead to high-affinity antibodies to DNA, helped by the innate immune system. The innate immune receptors are stimulated by retroviral envelope; by unmethylated cDNA released from dying cells; by single-stranded viral RNA and double-stranded RNA of the tRNA priming complex introduced via infection; and by viral DNA in the cytoplasm, where it is sensed as ectopic.
Acknowledgments
We thank Louis Du Pasquier for discussions, David Nemazee and Tony Marion for critical reading of the manuscript and Mary McKenney for editing it.
Abbreviations used in this paper
- ANA
anti-nuclear antibody
- GC
germinal center
- ISD
interferon stimulatory DNA response
- MLV
murine leukemia virus
- qPCR
quantitative PCR
- SLE
systemic lupus erythematosus
Footnotes
This work was supported by grants from the Israel Science Foundation, Founded by the Academy of Sciences and Humanities to DE; from NIH (R01AI041570) and the Lupus Research Institute to MW; and from the United States–Israel Binational Science Foundation (BSF) to MW and DE.
References
- 1.Burnet FM. The Clonal Slection Theory. Nashville: Vanderbilt University Press; 1959. [Google Scholar]
- 2.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 3.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 4.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 5.Eilat D, Hochberg M, Pumphrey J, Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984;133:489–494. [PubMed] [Google Scholar]
- 6.Eilat D, Webster DM, Rees AR. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988;141:1745–1753. [PubMed] [Google Scholar]
- 7.Pewzner-Jung Y, Friedmann D, Sonoda E, Jung S, Rajewsky K, Eilat D. B cell deletion, anergy, and receptor editing in “knock in” mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol. 1998;161:4634–4645. [PubMed] [Google Scholar]
- 8.Friedmann D, Yachimovich N, Mostoslavsky G, Pewzner-Jung Y, Ben-Yehuda A, Rajewsky K, Eilat D. Production of high affinity autoantibodies in autoimmune New Zealand Black/New Zealand white F1 mice targeted with an anti-DNA heavy chain. J Immunol. 1999;162:4406–4416. [PubMed] [Google Scholar]
- 9.Tillman DM, Jou NT, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB × NZW)F1 mice. J Exp Med. 1992;176:761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behar SM, Lustgarten DL, Corbet S, Scharff MD. Characterization of somatically mutated S107 VH11-encoded anti-DNA autoantibodies derived from autoimmune (NZB × NZW)F1 mice. J Exp Med. 1991;173:731–741. doi: 10.1084/jem.173.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pewzner-Jung Y, Simon T, Eilat D. Structural elements controlling anti-DNA antibody affinity and their relationship to anti-phosphorylcholine activity. J Immunol. 1996;156:3065–3073. [PubMed] [Google Scholar]
- 12.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 14.Eilat D, Anderson WF. Structure-function correlates of autoantibodies to nucleic acids. Lessons from immunochemical, genetic and structural studies. Mol Immunol. 1994;31:1377–1390. doi: 10.1016/0161-5890(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond B, Scharff MD. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984;81:5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papoian R, Pillarisetty R, Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977;32:75–79. [PMC free article] [PubMed] [Google Scholar]
- 19.Nossal GJ. Clonal anergy of B cells: a flexible, reversible, and quantitative concept. J Exp Med. 1996;183:1953–1956. doi: 10.1084/jem.183.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yachimovich N, Mostoslavsky G, Yarkoni Y, Verbovetski I, Eilat D. The efficiency of B cell receptor (BCR) editing is dependent on BCR light chain rearrangement status. Eur J Immunol. 2002;32:1164–1174. doi: 10.1002/1521-4141(200204)32:4<1164::AID-IMMU1164>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Yachimovich-Cohen N, Fischel R, Bachar N, Yarkoni Y, Eilat D. Autoimmune NZB/NZW F1 mice utilize B cell receptor editing for generating high-affinity anti-dsDNA autoantibodies from low-affinity precursors. Eur J Immunol. 2003;33:2469–2478. doi: 10.1002/eji.200324025. [DOI] [PubMed] [Google Scholar]
- 23.Lamoureux JL, Watson LC, Cherrier M, Skog P, Nemazee D, Feeney AJ. Reduced receptor editing in lupus-prone MRL/lpr mice. J Exp Med. 2007;204:2853–2864. doi: 10.1084/jem.20071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kench JA, Russell DM, Nemazee D. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J Exp Med. 1998;188:909–917. doi: 10.1084/jem.188.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Li H, Tian Q, Beardall M, Xu Y, Casanova N, Weigert M. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol. 2006;176:5183–5190. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 26.Kat I, Makdasi E, Fischel R, Eilat D. B-cell anergy is maintained in anti-DNA transgenic NZB/NZW mice. Int Immunol. 2010;22:101–111. doi: 10.1093/intimm/dxp120. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Moisini I, Bethunaickan R, Sahu R, Akerman M, Eilat D, Lesser M, Davidson A. BAFF/APRIL inhibition decreases selection of naive but not antigen-induced autoreactive B cells in murine systemic lupus erythematosus. J Immunol. 2011;187:6571–6580. doi: 10.4049/jimmunol.1101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165:1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannoor K, Matejuk A, Xu Y, Beardall M, Chen C. Expression of Natural Autoantibodies in MRL-lpr Mice Protects from Lupus Nephritis and Improves Survival. J Immunol. 2012;188:3628–3638. doi: 10.4049/jimmunol.1102859. [DOI] [PubMed] [Google Scholar]
- 30.Vettermann C, Herrmann K, Albert C, Roth E, Bosl MR, Jack HM. A unique role for the lambda5 nonimmunoglobulin tail in early B lymphocyte development. J Immunol. 2008;181:3232–3242. doi: 10.4049/jimmunol.181.5.3232. [DOI] [PubMed] [Google Scholar]
- 31.Ait-Azzouzene D, Kono DH, Gonzalez-Quintial R, McHeyzer-Williams LJ, Lim M, Wickramarachchi D, Gerdes T, Gavin AL, Skog P, McHeyzer-Williams MG, Nemazee D, Theofilopoulos AN. Deletion of IgG-switched autoreactive B cells and defects in Fas(lpr) lupus mice. J Immunol. 2010;185:1015–1027. doi: 10.4049/jimmunol.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 37.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 38.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 39.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- 41.Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- 42.Crispin JC, Liossis SN, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang YT, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 44.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 45.Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, Voll RE, Kalden JR, Herrmann M. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 46.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Marshak-Rothstein A, I, Rifkin R. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 52.Browne EP. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog. 2011;7:e1002293. doi: 10.1371/journal.ppat.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–145. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frankel WN, Lee BK, Stoye JP, Coffin JM, Eicher EM. Characterization of the endogenous nonecotropic murine leukemia viruses of NZB/B1NJ and SM/J inbred strains. Mamm Genome. 1992;2:110–122. doi: 10.1007/BF00353859. [DOI] [PubMed] [Google Scholar]
- 55.Levy JA. Autoimmunity and neoplasia. The possible role of C-type viruses. Am J Clin Pathol. 1974;62:258–280. doi: 10.1093/ajcp/62.2.258. [DOI] [PubMed] [Google Scholar]
- 56.Datta SK, Manny N, Andrzejewski C, Andre-Schwartz J, Schwartz RS. Genetic studies of autoimmunity and retrovirus expression in crosses of New Zealand black mice I. Xenotropic virus. J Exp Med. 1978;147:854–871. doi: 10.1084/jem.147.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izui S, McConahey PJ, Clark JP, Hang LM, Hara I, Dixon FJ. Retroviral gp70 immune complexes in NZB × NZW F2 mice with murine lupus nephritis. J Exp Med. 1981;154:517–528. doi: 10.1084/jem.154.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker RM, Roark CL, Santiago-Raber ML, Izui S, Kotzin BL. Association between nuclear antigens and endogenous retrovirus in the generation of autoantibody responses in murine lupus. Arthritis Rheum. 2004;50:3626–3636. doi: 10.1002/art.20623. [DOI] [PubMed] [Google Scholar]
- 59.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr, Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G, Lindahl T, Barnes DE. Gene-targeted mice lacking the Trex1 (DNase III) 3′-->5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 64.Lee-Kirsch MA, Chowdhury D, Harvey S, Gong M, Senenko L, Engel K, Pfeiffer C, Hollis T, Gahr M, Perrino FW, Lieberman J, Hubner N. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J Mol Med. 2007;85:531–537. doi: 10.1007/s00109-007-0199-9. [DOI] [PubMed] [Google Scholar]
- 65.Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, de Silva U, Bailey SL, Witte T, Vyse TJ, Kere J, Pfeiffer C, Harvey S, Wong A, Koskenmies S, Hummel O, Rohde K, Schmidt RE, Dominiczak AF, Gahr M, Hollis T, Perrino FW, Lieberman J, Hubner N. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet. 2007;39:1065–1067. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- 66.Beck-Engeser GB, Eilat D, Wabl M. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology. 2011;8:91. doi: 10.1186/1742-4690-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stetson DB. Connections between antiviral defense and autoimmunity. Curr Opin Immunol. 2009;21:244–250. doi: 10.1016/j.coi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beck-Engeser GB, Eilat D, Harrer T, Jack HM, Wabl M. Early onset of autoimmune disease by the retroviral integrase inhibitor raltegravir. Proc Natl Acad Sci U S A. 2009;106:20865–20870. doi: 10.1073/pnas.0908074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang CL, Wabl M. Hypermutation rate normalized by chronological time. J Immunol. 2005;174:5650–5654. doi: 10.4049/jimmunol.174.9.5650. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan KE. Genetics of systemic lupus erythematosus. Clinical implications. Rheum Dis Clin North Am. 2000;26:229–256. v–vi. doi: 10.1016/s0889-857x(05)70137-x. [DOI] [PubMed] [Google Scholar]
- 71.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garaud S, Youinou P, Renaudineau Y. DNA methylation and B-cell autoreactivity. Adv Exp Med Biol. 2011;711:50–60. doi: 10.1007/978-1-4419-8216-2_5. [DOI] [PubMed] [Google Scholar]
- 75.Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, Ginzler E, Urowitz M, Gladman D, Fortin PR, Petri M, Edworthy S, Barr S, Gordon C, Bae SC, Sibley J, Isenberg D, Rahman A, Aranow C, Dooley MA, Steinsson K, Nived O, Sturfelt G, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, El-Gabalawy H, McCarthy T, St Pierre Y, Ramsey-Goldman R, Clarke A. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1481–1490. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 76.Levy JA. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973;182:1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- 77.Stephenson JR, Reynolds RK, Tronick SR, Aaronson SA. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975;67:404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- 78.Chattopadhyay SK, Lander MR, Rands E, Lowy DR. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980;77:5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, Arnoult C, Tron F, Gilbert D, Musette P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]