Abstract
In order to follow the spatial and temporal evolution of neuronal damage, cellular activation and stress responses subsequent to lithium–pilocarpine seizures of various durations in the adult rat, we analyzed the expression of Fos protein and local cerebral glucose utilization as markers of cellular activation, HSP72 immunoreactivity and acid fuchsin staining as indicators of cellular stress and injury, and Cresyl violet staining for the assessment of neuronal damage. The expression of Fos appeared very early, 2–30 min after the onset of polyspikes and intensified during the following 4 h. Fos immunoreactivity was especially high in the hippocampus, cerebral cortex, amygdala and anterior olfactory nuclei. Local cerebral glucose utilization measured during the second hour of seizures was largely increased (350–580%) over control levels in cortical areas, amygdala, dentate gyrus, caudate nucleus and mediodorsal thalamus. HSP72 immunoreactivity never appeared earlier than 40–50 min after the onset of polyspikes, and was most prominent in hippocampal CA3 area, cerebral cortex (except the piriform cortex) and anterior olfactory nuclei. Acid fuchsin staining was maximal in the piriform cortex and the polymorphic layer of the dentate gyrus. Staining was moderate in the sensorimotor cortex and the amygdala. Neuronal damage was extensive in the piriform and entorhinal cortices, the hippocampal CA3 area and the polymorphic layer of the dentate gyrus, basal amygdala, mediodorsal thalamus and anterior olfactory nuclei. In conclusion, the present study shows that brain regions with the highest expression of Fos and the largest metabolic activation were also highly stained with acid fuchsin and most heavily damaged. Conversely, there is no clear relationship between HSP72 expression, cellular activation and neuronal damage.
Keywords: Limbic seizure, Lithium–pilocarpine, Fos protein, HSP72, Stress response, Neuronal damage, 2-Deoxyglucose
1. Introduction
The lithium–pilocarpine model is a well-studied model of partial limbic seizures that progress to secondary generalized status epilepticus (SE) [3,12,24,29,38]. SE starts usually within 50–60 min after pilocarpine injection and lasts for up to 8–12 h, is followed by recurrent seizure episodes for up to 24–48 h. This acute seizure period is followed by a ‘silent’ seizure-free phase lasting for 4–44 days, after which all animals exhibit spontaneous recurrent seizures [3]. In this model of epilepsy, neuronal damage is preferentially located within the hippocampal formation, the piriform and entorhinal cortices. Cell loss is also recorded in the septum, olfactory tubercle, thalamus, amygdaloid complex, neocortex and substantia nigra [3,5,12,38]. The extent of neuronal damage in the hippocampal formation correlates directly with the duration of the initial SE. No damage is observed in rats with seizures lasting less than 1 h [15]. However, there are no data on the spatiotemporal evolution of neuronal stress, injury and damage in this model of SE.
The measurement of local cerebral metabolic rates for glucose (LCMRglcs) provides a precise mapping of the areas of cellular activation [34,35]. Likewise, the c-fos proto-oncogene is a reliable marker of cellular hyperactivation and undergoes a rapid, transient increase following a variety of external stimuli. The expression of its encoded protein product, Fos, appears in specific brain regions after the induction of various types of seizures [6,14,21,22,31]. Brain cells are able to express HSP72 (heat shock protein) in response to various types of insults, such as hyperthermia [16], hypoxia–ischemia [7,9,37] and seizures [7,18,30,39]. The expression of HSP72 represents a response to excitation-induced stress and potential brain cell injury. Finally, acid fuchsin staining is a reliable marker of dying neurons after hypoglycemia [1], seizures [18,32,36] or ischemia [37].
Since there is a correlation between the duration of the seizures and the extent of neuronal damage in the pilocarpine model of SE [15], we wondered how cellular activation, stress and injury could be affected by the interruption of the seizures at different times after their onset. Therefore, in the present study, the expression of all the markers cited above was mapped after different durations of SE induced by lithium–pilocarpine in adult rats.
2. Materials and methods
2.1. Animals and treatment
For the study, a total number of 79 adult male Sprague–Dawley rats weighing 200–290 g were used. In the experiments related to the study of the expression of Fos and HSP72 proteins, and the staining with acid fuchsin, 43 rats were implanted over the right and left frontoparietal cortices with 4 electrodes under light halothane anesthesia and were allowed a 4-day period of recovery. For the measurement of local cerebral metabolic rates for glucose (LCMRglc), catheters were inserted into the femoral artery and vein of 4 saline- and 6 lithium–pilocarpine-exposed rats on the day before the experiment. The 26 rats used for Cresyl violet staining did not undergo any surgery. Lithium chloride (3 meq/kg) was administered i.p. to all rats 18–24 h before the subcutaneous injection of pilocarpine (30 mg/kg) to the experimental group or saline to the control group. The electroencephalographic (EEG) activity was recorded continuously from freely moving animals, starting 30 min before the injection of pilocarpine, and lasting throughout the entire duration of SE and recovery periods, in order to assess the exact onset and duration of SE. Because of the incomplete efficacy of conventional anticonvulsants such as phenobarbital, diazepam or clonazepam, which stop the behavioral signs of lithium–pilocarpine-induced SE while abnormal paroxysmal discharges persist on the EEG, paraldehyde was used (800 mg/kg) to stop SE at different time lengths ranging from 2 to 240 min [23]. SE duration was defined as the duration of continuous polyspikes and periodic paroxysmal activity lasting for 2 min or more on the EEG. All paroxysmal EEG activity was totally suppressed within a few min after paraldehyde injection. All experimentation was conducted in conformity with the ‘Guiding Principles for Research Involving Animals and Human Beings’.
2.2. Fos and HSP72 immunocytochemistry
Since we were interested in the spatiotemporal evolution of Fos and HSP72 expression according to the duration of the initial seizure event, both markers were studied at a single time point corresponding to their maximal expression, i.e., 4 h or 24 h after the cessation of the seizures consecutive to the injection of paraldehyde, for Fos or HSP72, respectively.
The immunocytochemical detection of Fos protein was performed in one group of control rats (n = 2) and two groups of lithium–pilocarpine-treated animals, subjected to a 2–30 min (n = 6) or a 30–240 min long SE (n = 6). Within the latter group, two rats were used for immunocytochemical control.
The study of HSP72 expression was performed in one group of control animals (n = 2) and in four groups of rats with continuous seizures. In the first group (n = 7), SE lasted for 8–38 min, and in the second (n = 2), continuous polyspikes were recorded for 40–50 min before being stopped with paraldehyde. In the third group (n = 6), the duration of SE ranged from 90 to 180 min, while the fourth group of rats, which did not receive any antiepileptic treatment, had continuous polyspikes for 8–10 h (n = 12). Brains were removed, immediately frozen in isopentane and stored at −80°C until being cut into 25-µm serial coronal sections in a cryostat.
Brain sections were fixed for 7 min in 4% paraformaldehyde dissolved in phosphate buffer saline (PBS) at pH 7.4. Sections were then sequentially incubated twice in PBS, once in 0.6% hydrogen peroxide in PBS, and twice in PBS containing 0.4% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for Fos or horse serum (Vector) for HSP, 0.25% Triton-X100 and 1.5% serum albumin (BSA). Sections were then incubated overnight at 20°C with the primary antibody, a rabbit affinity-purified polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:500 in PBS containing goat serum) for Fos or a monoclonal anti-72-kDa HSP antibody (Amersham, Les Ulis, France; dilution 1:200 in PBS containing horse serum) for the detection of HSP72. The sections were rinsed twice in PBS containing the appropriate serum and incubated for 1 h at 20°C with the secondary antibody, biotinylated goat anti-rabbit antibody, dilution 1:400 for Fos (Vector) and biotinylated anti-mouse antibody, dilution 1:50 for HSP72 (Vector) in the corresponding serum/Triton-X100/BSA/PBS mixture. Sections were rinsed twice in the latter medium and covered with the ABC reagent (Vectastain Kit, Vector) for 1 h at 20°C. Sections were rinsed twice in PBS and incubated for 5–8 min in a mixture of 0.025% diaminobenzidine, 0.01% nickel chloride and 0.05% hydrogen peroxide in PBS. Thereafter, sections were dehydrated in ethanol and coverslipped. In addition to control animals exposed to lithium and saline, immunocytochemical control sections from 2 animals subjected to a 8–10 h SE (2 for Fos and 2 for HSP72) underwent the procedures described above except for the exposure to the primary antibody.
The distribution of positive neurons was recorded from the forebrain to the cerebellum. Direct visual counting of the density of Fos or HSP72-expressing neurons was performed without knowledge of seizure duration by using a grading scale of 0–3, with 0 corresponding to the absence of reactive cells and 1, 2 and 3 to low-, moderate- and high-density labeled cells, respectively, according to the examples shown in Fig. 1 in the anterior cingulate cortex. The nomenclature used was that described by Paxinos [28].
Fig. 1.
Temporal evolution and grading scale of the density of HSP72 positive neurons in the anterior cingulate cortex. (A) grade 1 recorded after 90 min of SE, (B) grade 2 recorded after 2 h of SE and (C) grade 3 recorded after 4 h of SE. Scale bar: 100 µm.
2.3. Measurement of local cerebral glucose utilization
LCMRglcs were measured by the [14C]2-deoxyglucose (2DG) [35]. The 2DG (4.625 MBq/kg; spec. act., 1.65–2.04 GBq/mol; Isotopchim, Ganagobie, France) was injected as an i.v. pulse (125 µl/100 g) only at one time, i.e., at 1 h after the onset of SE, or an equivalent time after saline injection in controls. Timed arterial blood samples were drawn during the following 45 min for determination of the plasma concentration of 2DG and glucose. At approximately 45 min after the pulse of 2DG, the animals were killed by decapitation. Brains were rapidly removed and frozen in isopentane chilled to −30°C and stored at −80°C. Coronal brain sections, 20-µm thick, were autoradiographed along with calibrated [14C]methylmethacrylate standards [35]. Adjacent sections were fixed and stained with thionin for histological identification of specific nuclei. The autoradiographs were analyzed by quantitative densitometry. Tissue 14C concentrations were determined in 18 brain structures from the optical densities of the autoradiographic representations of the tissues and a calibration curve obtained from the standards. LCMRglcs were then calculated from local tissue concentration of 14C, time courses of the plasma 2DG and glucose concentrations, and appropriate constants [35].
2.4. Acid fuchsin and Cresyl violet staining
Acid fuchsin staining was performed on dry mounted frozen sections from animals sacrificed 24 h after the onset of SE in four groups of rats subjected to various durations of SE, as well as in a control group sacrificed together with the experimental ones. For acid fuchsin staining, brain sections used were adjacent to those that were used for the immunocytochemical detection of HSP72. Sections were placed sequentially in 4% paraformaldehyde for 20 min, PBS for 10 min and water for 2 min. Slides were then dipped into a solution of 10 mg/ml acid fuchsin and 0.1% acetic acid in water for 20 s, washed in water twice for 20 s, dehydrated in 100% ethanol for 2 min and coverslipped [4]. The distribution of stained neurons was recorded from the forebrain to the cerebellum. Direct visual counting of bright pink neurons was performed using a grading scale of 0–3, corresponding to the density of acid fuchsin stained neurons.
Cresyl violet staining was performed on dry mounted sections from frozen brains obtained from control rats (n = 4) and animals that underwent SE for either 1 h (n = 9) or 8–10 h (n = 13). Half of the animals were sacrificed at 24 h and the other half at 6 days after pilocarpine administration.
3. Results
3.1. Behavior and EEG
Lithium–pilocarpine affected both behavior and EEG as previously described [3,29,38]. Within 5 min after pilocarpine injection, rats developed diarrhea, piloerection and other signs of cholinergic stimulation. During the following 15–20 min, rats exhibited stereotyped and exploratory behavior, such as head bobbing, scratching and chewing, but they remained reactive to external stimuli. During that period, the cortical EEG was unaltered.
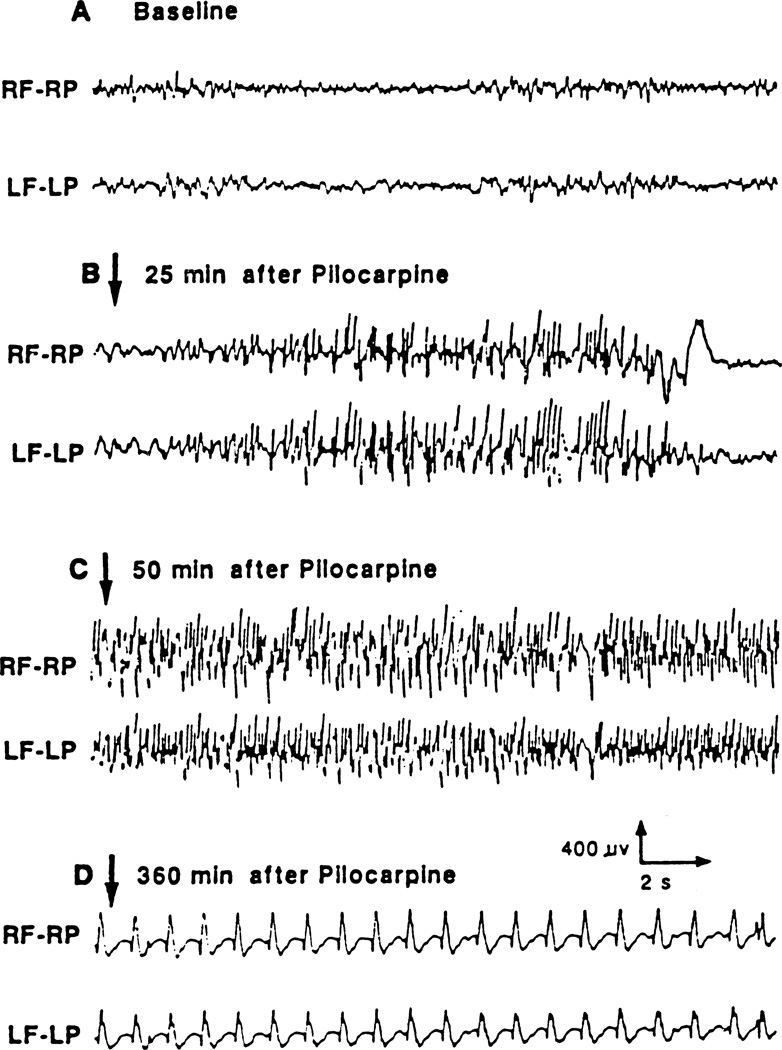
Recurrent limbic and motor seizures started around 20–25 min after pilocarpine administration, and lasted for about 10 min. The motor seizures included alternating episodes of bilateral forelimb clonus with rearing and falling lasting less than 1 min and accompanied by high-voltage polyspikes on the EEG (Fig. 2). These recurrent seizures progressed to SE at about 50 min after pilocarpine. At that time, the animals developed head and forelimb myoclonus concurrent with a continuous train of high-voltage spikes lasting for about 2 1/2 h followed by 1 1/2 h of polyspikes and waves (1–1.5 Hz). After that period, the EEG was characterized by sharp/slow waves of 0.5 Hz amplitude that lasted until the end of SE. Paraldehyde was effective in stopping continuous polyspikes within a few min after injection.
Fig. 2.
Typical example of cortical EEG recording of seizure activity induced by the administration of lithium (3 meq/kg) and pilocarpine (60 mg/kg) in the adult rat. The EEG was recorded through frontoparietal epidural electrodes. Abbreviations: RF–RP: right frontal–right parietal, LF–LP: left frontal–left parietal.
3.2. Expression of c-Fos
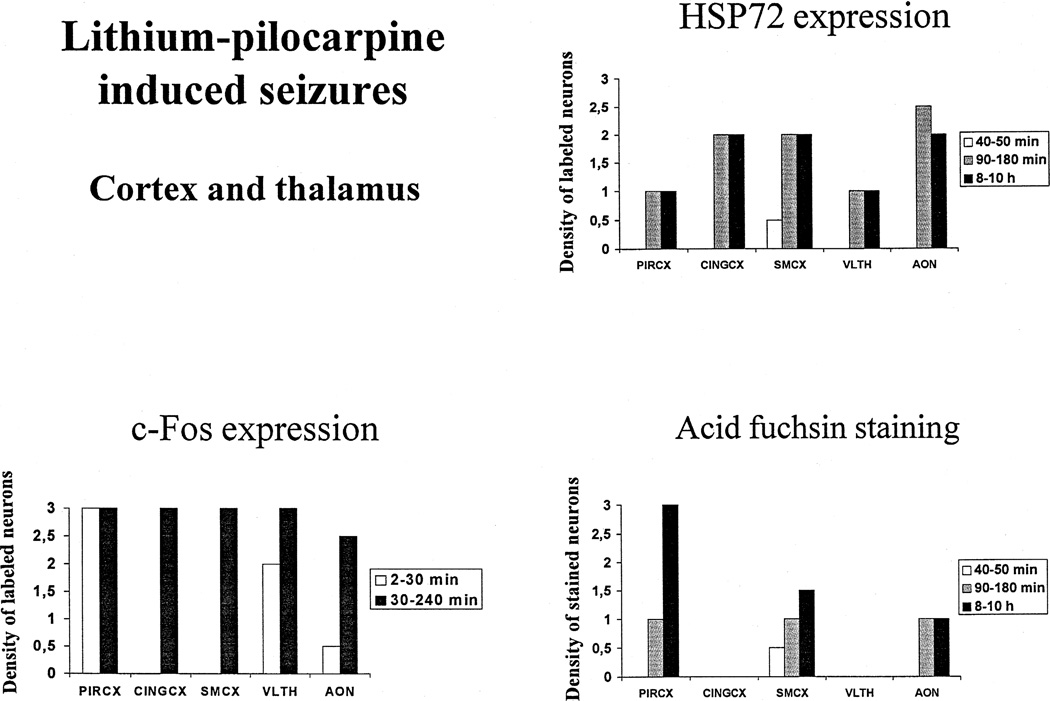
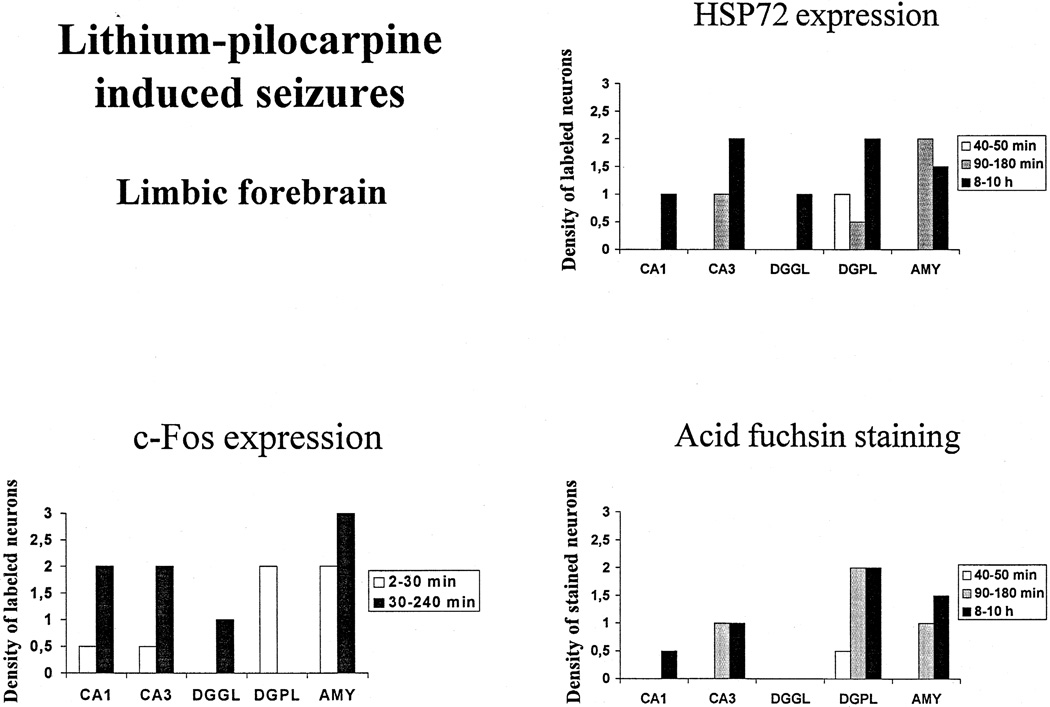
No Fos immunoreactivity was detected in control animals. In rats injected with pilocarpine, the induction of Fos was very rapid, occurring as early as within 2 min of polyspikes. Strong Fos immunoreactivity was present in the piriform cortex (Figs. 3 and 5a) and a moderate density of Fos occurred in the polymorphic layer of the dentate gyrus and the amygdala (Figs. 4 and 6a) and the dorsomedial thalamus. At this early time point, all other regions exhibited a weak and inconsistent expression of Fos that was not present in all animals. Between 30 and 240 min of SE, the expression of Fos spread to all the regions studied, and was especially prominent in the piriform, cingulate and sensorimotor cortices, the amygdala and the anterior olfactory nuclei (Figs. 3–5a). In the hippocampal subfields, moderate Fos labelling could be seen in CA1, CA3, and weak c-Fos expression was recorded in the granular layer of the dentate gyrus (Fig. 4). Within thalamus, Fos expression was high in the ventrolateral (Fig. 3) and very weak in the dorsomedial and dorsolateral nuclei.
Fig. 3.
Effect of increased durations of lithium–pilocarpine-induced seizures on c-Fos and HSP72 immunoreactivity, and acid fuchsin staining in the cortex and thalamus of adult rats. Values are expressed as arbitrarily labeled cell density units as defined in Section 2 and shown in Fig. 1. Abbreviations: PIRCX: piriform cortex, CINGCX: anterior cingulate cortex, SMCX: sensorimotor cortex, DMTHAL: dorsomedial thalamus, AON: anterior olfactory nuclei.
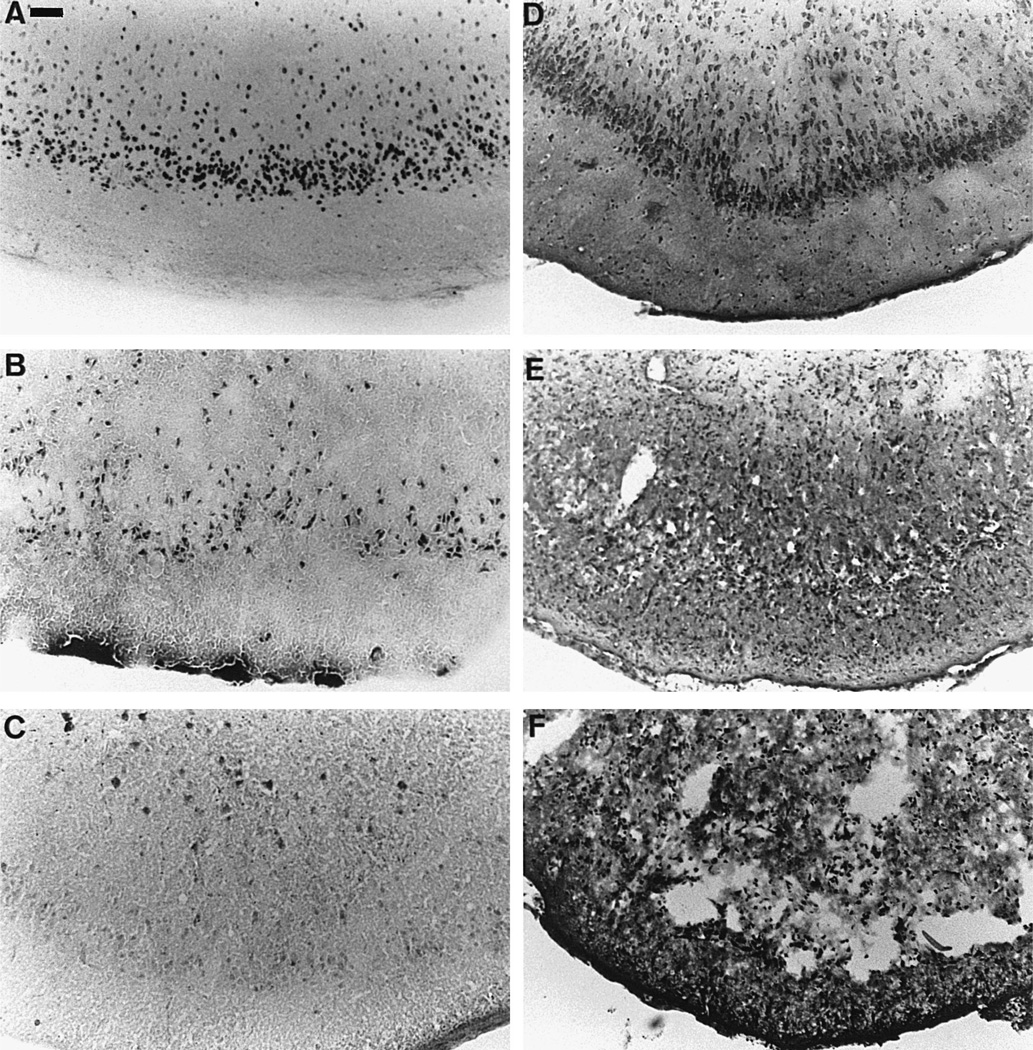
Fig. 5.
Expression of activation, stress and injury markers in sections taken at the level of the piriform cortex. (A) expression of Fos protein in animals sacrificed after 4 h of SE, (B) acid fuchsin staining in animals subjected to 8 h of SE and sacrificed 24 h after SE onset, (C) HSP72 immunoreactivity in animals exposed to 8 h of SE and sacrificed 24 h after SE onset, (D) Cresyl violet staining in control rats, (E) Cresyl violet staining in animals subjected to 8 h of SE and sacrificed 24 h after SE onset and (F) Cresyl violet staining in rats undergoing 8 h SE and sacrificed 144 h after SE onset. Note the high number of nuclei expressing Fos in layer IV of the piriform cortex (A), together with a quite elevated number of neurons stained with acid fuchsin in that area (B) where neuronal damage is also highest (E and F). Conversely, HSP72 is not expressed in layer IV of the piriform cortex, and there are only a few scattered HSP72 positive nuclei in the deeper layers of the cortex (C). Note also the extensive neuronal damage with a complete disorganization and destruction of the piriform cortex in the animals subjected to complete SE and sacrificed 144 h later (F) compared to more limited damage in those sacrificed at 24 h (E). Scale bar: 60 µm.
Fig. 4.
Effect of increased durations of lithium–pilocarpine-induced seizures on c-Fos and HSP72 immunoreactivity, and acid fuchsin staining in the limbic forebrain of adult rats expression of HSP72. Values are expressed as arbitrarily labeled cell density units as defined in Section 2 and shown in Fig. 1. Abbreviations: CA1, CA3: CA1, CA3 hippocampal subfields, DGGL: granular layer of the dentate gyrus, DGPL: polymorphic layer of the dentate gyrus, AMY: amygdala.
Fig. 6.
Expression of activation, stress and injury markers in sections taken at the level of the dentate gyrus. (A) expression of Fos protein in animals sacrificed after 4 h of SE, (B) acid fuchsin staining in animals subjected to 8 h of SE and sacrificed 24 h after SE onset, (C) HSP72 immunoreactivity in animals exposed to 8 h of SE and sacrificed 24 h after SE onset, (D) Cresyl violet staining in control rats, (E) Cresyl violet staining in animals subjected to 8 h of SE and sacrificed 24 h after SE onset and (F) Cresyl violet staining in rats undergoing 8 h of SE and sacrificed 144 h after SE onset. Note the high number of nuclei expressing Fos in the polymorphic layer of the dentate gyrus (A), together with a quite elevated number of neurons stained with acid fuchsin in that area (B) where neuronal damage is quite marked, as shown by the almost complete disappearance of neurons (E and F). Conversely, HSP72 is not expressed in the polymorphic layer of the dentate gyrus, but rather in the hippocampal CA3 area (C). Note also the extensive neuronal damage in the animals subjected to complete SE and sacrificed at 144 h after the seizures in which all hippocampal subfields show an increased number of very dark, swollen and pycnotic neurons (F). Scale bar: 60 µm.
3.3. Local cerebral metabolic rates for glucose
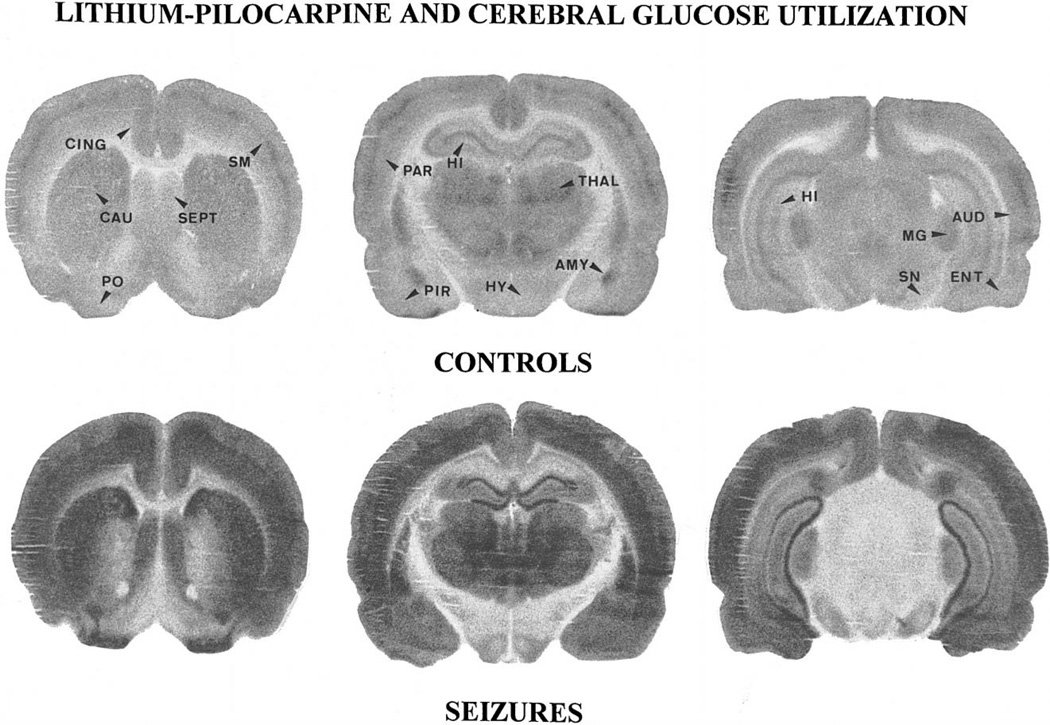
LCMRglcs measured during SE were largely increased over control values in 17 of the 18 brain regions studied (Table 1 and Fig. 7). Metabolic increases were highest (> 300%) in all cortical areas, especially in the piriform cortex, in the nucleus accumbens, amygdala and dentate gyrus, caudate nucleus, substantia nigra and mediodorsal thalamic nucleus. Metabolic increases were lowest (74–98%) in the hippocampal CA1 area and cerebellar cortex. LCMRglcs were similar in control and lithium–pilocarpine-treated animals in a single brain region, the inferior colliculus.
Table 1.
Effect of lithium–pilocarpine induced seizures on local cerebral glucose utilization in rats
| Brain structure | Control (n = 4) | Seizures (n = 6) | Variation (%) |
|---|---|---|---|
| Cerebral cortex | |||
| Frontal cortex | 71.0 ± 7.8 | 375.9 ± 32.4*** | +429 |
| Cingulate cortex | 75.7 ± 5.3 | 330.9 ± 23.0**** | +337 |
| Olfactory cortex | 69.1 ± 6.0 | 288.0 ± 24.8**** | +317 |
| Piriform cortex | 47.5 ± 3.1 | 288.3 ± 26.6**** | +507 |
| Limbic structures | |||
| Nucleus accumbens | 47.7 ± 5.0 | 226.6 ± 49.8*** | +376 |
| Amygdala | 33.2 ± 3.3 | 226.9 ± 34.6*** | +582 |
| Hippocampus, CA1 | 51.9 ± 6.6 | 103.3 ± 8.7** | +98 |
| Hippocampus, CA3 | 68.3 ± 4.4 | 243.6 ± 18.4**** | +257 |
| Dentate gyrus | 40.2 ± 3.3 | 223.2 ± 23.7*** | +455 |
| Striatum and related areas | |||
| Caudate nucleus | 66.6 ± 5.9 | 319.1 ± 43.3*** | +379 |
| Substantia nigra pars reticulata | 40.0 ± 2.7 | 167.2 ± 30.6*** | +318 |
| Hypothalamus | |||
| Anterior | 40.1 ± 5.7 | 118.7 ± 15.5*** | +196 |
| Ventromedian | 41.9 ± 3.6 | 126.7 ± 23.4*** | +203 |
| Thalamus | |||
| Ventrolateral | 78.7 ± 6.6 | 233.7 ± 38.4**** | +197 |
| Mediodorsal | 80.7 ± 5.5 | 374.5 ± 30.1**** | +364 |
| Medial geniculate body | 86.3 ± 9.8 | 231.0 ± 41.3*** | +168 |
| Brainstem | |||
| Inferior colliculus | 138.9 ± 24.3 | 113.8 ± 18.8 | −19 |
| Cerebellar cortex | 43.6 ± 5.1 | 76.0 ± 15.3* | +74 |
Values are means ± S.D. of the number of animals in parentheses.
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001, statistically significant differences from controls (Student’s t-test).
Fig. 7.
Autoradiographic sections taken at the level of the caudate nucleus (CAU), the hippocampus (HI) and the substantia nigra (SN) of control and lithium–pilocarpine exposed rats. Compared to control animals, the grain density was largely increased especially in the whole neocortex, the piriform (PIR) and the primary olfactory cortex (PO), as well as in caudate nucleus, amygdala (AMY), thalamus (THAL), hippocampus and septum (SEPT). In the dorsal hippocampus, the increase was prominent in dentate gyrus (DG), while the pyramidal cell layer of the CA3 area was no longer visible. A large increase in tracer levels also occurred in thalamus (THAL) and substantia nigra, while grain density increased moderately in ventromedian hypothalamus (HY) and medial geniculate body (MG). Abbreviations: CING: anterior cingulate cortex, SM: sensorimotor cortex, PAR: parietal cortex, AUD: auditory cortex, ENT: entorhinal cortex.
3.4. Expression of HSP72
No expression of HSP72 could be detected in control rats, as well as in animals with continuous polyspikes lasting less than 40 min. The first low-density expression of HSP72 occurred after 40–50 min of polyspikes, and was located only in the polymorphic layer of the dentate gyrus (Figs. 4 and 6c) and in the sensorimotor cortex (Fig. 3). After 90–180 min of polyspikes, the expression of HSP72 was strong in amygdala, cingulate and sensorimotor cortices, and anterior olfactory nuclei, and moderate in hippocampal CA3 area, piriform cortex and ventral thalamus (Figs. 3 and 4). The hippocampal CA1 area and the granular layer of the dentate gyrus were devoid of any HSP72 immunoreactive cells. After 8–10 h of SE, HSP72 expression was quite prominent in hippocampal CA3 area, the polymorphic layer of the dentate gyrus, amygdala, cingulate and sensorimotor cortices, as well as anterior olfactory nuclei (Figs. 3 and 4). Within thalamus, the ventrolateral and paraventricular thalamic nuclei expressed HSP72 moderately already at 90–180 min after pilocarpine (Fig. 3), while the dorsal nuclei exhibited HSP72 staining very weakly and only after 8–10 h of SE.
The globus pallidus, ventral pallidum and nucleus accumbens never expressed HSP72. After several hours of SE, occasional stained neurons were found in the caudate-putamen and the deep layers of the piriform cortex (Fig. 5c). The substantia nigra was devoid of HSP72 immunoreactivity in all animals but 2, in which the expression was weak and located in astrocytes.
3.5. Acid fuchsin staining
No acid fuchsin staining was observed in control rats and in animals with SE lasting less than 40 min. The first brain regions stained by this marker were the polymorphic cells of the dentate gyrus (Figs. 4 and 6b), and the sensorimotor cortex (Fig. 3) at 40–50 min after the onset of continuous polyspikes. After 90–180 min of seizures, staining was strong in the polymorphic layer of the dentate gyrus (Figs. 4 and 6b) and moderate in hippocampal CA3 area, amygdala, piriform and sensorimotor cortices, as well as the anterior olfactory nuclei (Figs. 3–5b). After 8–10 h SE, acid fuchsin staining was observed in all regions except for the granular layer of the dentate gyrus, anterior cingulate cortex and ventrolateral thalamus. The staining was robust in the polymorphic layer of the dentate gyrus (Figs. 4 and 6b) and the piriform cortex, and moderate in amygdala, sensorimotor cortex, hippocampal CA3 area, dorsomedial thalamus and anterior olfactory nuclei (Figs. 3 and 4).
3.6. Neuropathology
Neuronal damage observed on Cresyl violet stained sections was more extensive in rats subjected to protracted SE than in those in which SE was stopped after 1 h. Neuronal damage was already observed at 24 h after SE and fully developed by 6 days (Fig. 5d,e, Fig. 6d,e), and was similar to previous reports [12,38]. Briefly, in some areas such as piriform (Fig. 5d,e) and entorhinal cortices, hippocampal CA3 area and the polymorphic layer of the dentate gyrus (Fig. 6d,e), basal and cortical amygdala, mediodorsal thalamus and anterior olfactory nucleus, there was almost a complete neuronal loss with edema. The few remaining neurons and astrocytes were swollen or dark. Less affected areas showed a partial neuronal loss with shrunken and pycnotic neurons. These regions were all other neocortical areas, the ventrolateral thalamus, nucleus accumbens, lateral septum and substantia nigra pars reticulata. In the remaining cerebral areas, only isolated degenerating neurons could be seen.
4. Discussion
The results of the present study show the very early onset of c-Fos expression occurring as soon as 2 min after the first seizures and spreading to most brain regions within 30 min. Conversely, the first and weak expression of HSP72 immunoreactivity and acid fuchsin positivity could only be recorded after 50 min of seizure activity. Several hours of SE were necessary to allow the spread of these markers to the whole brain.
4.1. Localization of neuronal activation induced by lithium-pilocarpine SE
Lithium–pilocarpine SE is a model of limbic seizures induced by the injection of a cholinergic muscarinic agonist [26] whose potency is enhanced by lithium. This model is characterized by early behavioral and EEG changes confirming the primary involvement of the limbic system. In the present study, strong Fos protein expression was evident in the piriform cortex, as well as in the amygdala within 2 min after the onset of polyspikes. At that time, Fos immunoreactivity could also be detected in the polymorphic layer of the dentate gyrus. The early expression of Fos protein in the hippocampus, the amygdala and the piriform cortex correlates with the limbic onset of lithium–pilocarpine seizures in the brain regions containing a high density of muscarinic receptors [13,25]. These data are also in accordance with previous studies showing that isolated focal seizures induced by moderate doses of pilocarpine induce Fos expression in the piriform cortex, the amygdala and the olfactory tubercle [2]. Likewise, large increases in c-fos mRNA expression have been reported in the cerebral cortex and the hippocampus after lithium–pilocarpine seizures [41]. Thus, all of these data including ours, support the initiation of lithium–pilocarpine induced seizures in the piriform cortex, the amygdala and the dentate gyrus, among which the piriform cortex could be the primary structure potentially involved in the generation of the seizures [2]. Conversely, using the 2DG method, Clifford et al. [5] focused on the role of the hippocampus and the nucleus accumbens as pacemakers for seizure activity in the lithium–pilocarpine model. However, a 45-min experimental time window is necessary for the full quantification of LCMRglcs by the 2DG technique. Therefore, this method provides less accurate information about the putative structures involved in the initiation and propagation of the seizures. More recently, a semi-quantitative study using 2DG accumulation over 10 min showed the primary involvement of rostral and piriform cortices, basolateral amygdaloid nuclei, but also of some striatal areas in the initiation of lithium–pilocarpine seizures [10]. These results are similar to the data of the early Fos expression reported in the present study, except that the rate of 2DG uptake in the hippocampus did not differ between control and lithium–pilocarpine-treated rats over the first 10 min after the pilocarpine injection [10] as opposed to the early, but modest expression of Fos protein observed in that structure in the present study.
In the subsequent, generalized seizure phase of the lithium–pilocarpine model, i.e., between 30 and 240 min after the onset of SE, Fos expression spread all over the brain reflecting prolonged generalized cellular activation. During this phase, Fos immunoreactivity reached high levels in all cortical areas, including the piriform cortex, as well as in anterior olfactory nuclei, hippocampus, and dorsal thalamic nuclei. These data are in accordance with previous studies on the regional distribution of Fos immunoreactivity [2] and the expression of the c-fos gene during SE induced by pilocarpine or lithium–pilocarpine [40,41].
In most brain regions, there was good concordance between the expression of Fos and the increase in LCMRglcs. Indeed, metabolic activation measured quantitatively over 45 min during the second hour of SE was particularly marked in the structures where the highest expression of Fos was detected. LCMRglcs were mostly increased over control levels (> 450%) in the piriform cortex, the amygdala and the dentate gyrus, and also largely enhanced (260–380%) in all cortical regions, nucleus accumbens, hippocampal CA3 area, striatum and mediodorsal thalamus. These data are in agreement with the regional increases in cerebral metabolic activity reported previously during pilocarpine or lithium–pilocarpine-induced SE [5,10,11,27]. However, metabolic activity was also significantly increased compared to control animals in regions where Fos expression was very weak or absent, such as substantia nigra and ventrolateral thalamic nuclei. The anatomical discrepancy between activated LCMRglcs and Fos expression likely reflects the fact that Fos immunocytochemistry reveals gene activation within specific cell nuclei at the site of activation [21], while changes in metabolic levels reflect changes in the activity of the whole cell body, and can reflect either the activity at the site of action of the drug, along the whole neuronal pathway affected, or only at the target area [34]. Moreover, as shown recently, glucose is primarily metabolized by astrocytes before being released as lactate to neurons, and the 2DG accumulation may rather reflect increased astrocyte metabolic activity [20].
4.2. Neuronal stress and injury induced by lithium–pilocarpine SE
The initial brain structures expressing HSP72 and stained with acid fuchsin were the polymorphic layer of the dentate gyrus and the sensorimotor cortex. The expression of the two markers appeared simultaneously in those two regions after 40–50 min SE. Thus, a longer duration of SE is required for HSP72 as compared to the 2 min of SE required for Fos activation. The primary expression of HSP72 and acid fuchsin neuronal staining in the dentate gyrus is likely related to the primary limbic expression of the seizures and to the high density of muscarinic receptors in that area [13,25]. The expression of HSP72 and acid fuchsin staining in sensorimotor cortex probably reflects the rapid appearance of recurrent motor seizures occurring immediately after partial limbic seizures. In the subsequent phase of generalized seizure activity, i.e., at 1 1/2–10 h after the onset of SE, HSP72 immunoreactivity spread throughout the brain and was particularly intense in the hippocampal CA3 area, the granular layer of the dentate gyrus, the amygdala, the anterior olfactory nuclei and the neocortex. Conversely, HSP72 expression was weak in hippocampal CA1 area, the polymorphic layer of the dentate gyrus, piriform cortex and dorsomedial thalamus.
In the lithium–pilocarpine model of SE, neuronal damage was mainly described in the hippocampus, piriform and entorhinal cortices, central and basal amygdaloid nuclei and dorsal thalamus [Refs. [5,12,26,38] and the present study]. Acid fuchsin staining was especially strong in the piriform and sensorimotor cortices, the polymorphic layer of the dentate gyrus, and the amygdala. This dye positivity has been shown to correlate with neuronal death after various insults to the adult brain such as hypoglycemia [1], traumatic brain injury [19], seizures [18,32,36] or ischemia [37]. In the present study, acid fuchsin staining was present mainly in the structures that undergo damage after lithium–pilocarpine induced SE, i.e., piriform cortex, amygdala, the polymorphic layer of the dentate gyrus and mediodorsal thalamus. These were also the regions in which the metabolic increases recorded during lithium–pilocarpine induced SE were the largest. A similar relationship was previously shown in seizure models induced by systemic bicuculline [8,33] or kainic acid [17]. Conversely, acid fuchsin staining was quite moderate in the hippocampal CA1 and CA3 areas that are also largely damaged in this model, but undergo a more moderate metabolic increase.
The comparison of the regional distribution of HSP72 expression and acid fuchsin staining shows a quite heterogeneous distribution of both markers. However, within the cerebral cortex, HSP72 immunoreactivity was strong in anterior cingulate and sensorimotor regions, while acid fuchsin staining and neuronal damage was mainly observed in piriform cortex, as reported previously [5,12,26]. Likewise, in the thalamus and amygdaloid complex, HSP72 expression and neuronal damage were not distributed in the same subregions. Indeed, in amygdala, HSP72 immunoreactivity was essentially present in the laterodorsal nucleus, while acid fuchsin staining was recorded in the basal and medial parts. In the thalamus, the ventrolateral and paraventricular nuclei expressed HSP72, whereas acid fuchsin staining was located in the dorsomedial part. Conversely, in the hippocampal CA1 and CA3 and in the polymorphic layer of the dentate gyrus that undergo neuronal damage in this model, both HSP72 expression and acid fuchsin staining are recorded, while there is only an expression of HSP72 in the granular layer of the dentate gyrus. Thus, as shown in Figs. 4 and 5, there was some overlap between HSP72 expression and acid fuchsin staining in most brain areas after lithium–pilocarpine induced SE in the adult rat. Therefore in this model of SE, there is no clear relationship between HSP72 expression and neuronal injury.
In conclusion, the present study demonstrates that Fos induction is very rapid after lithium–pilocarpine-induced seizures, while HSP72 immunoreactivity appears only with prolonged 40–50 min of polyspikes. During SE, brain areas with highest expression of Fos, acid fuchsin staining and largest metabolic activation are those in which most damage occurs. Conversely, there is no clear relationship between HSP72 expression, cellular activation and neuronal damage.
Acknowledgements
This work was supported by a grant from the American Committee for the American Memorial Hospital of Reims, grant NS17117 from the NINDS and by the Institut National de la Santé et de la Recherche Médicale (U 398). The authors wish to thank S. Boyet for excellent technical assistance.
References
- 1.Auer RN, Wieloch T, Olsson Y, Siesjö BK. The distribution of hypoglycemic brain damage. Acta Neuropathol. 1984;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- 2.Barone P, Morelli M, Cicarelli G, Cozzolino A, DeJoanna G, Campanella G, DiChiara G. Expression of c-Fos protein in the experimental epilepsy induced by pilocarpine. Synapse. 1993;14:1–9. doi: 10.1002/syn.890140102. [DOI] [PubMed] [Google Scholar]
- 3.Cavalheiro EA. The pilocarpine model of epilepsy. Ital. J. Neurol. Sci. 1995;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- 4.Chang D, Baram TZ. Status epilepticus results in reversible neuronal injury in infant rat hippocampus: novel use of a marker. Dev. Brain Res. 1994;77:133–136. doi: 10.1016/0165-3806(94)90220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium–pilocarpine and high-dose pilocarpine seizures. Neuroscience. 1987;23:953–958. doi: 10.1016/0306-4522(87)90171-0. [DOI] [PubMed] [Google Scholar]
- 6.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferriero DM, Soberano HQ, Simon RP, Sharp FR. Hypoxia–ischemia induces heat shock protein-like (HSP72) immunoreactivity in neonatal rat brain. Dev. Brain Res. 1990;53:145–150. doi: 10.1016/0165-3806(90)90136-m. [DOI] [PubMed] [Google Scholar]
- 8.Folbergrova J, Ingvar M, Siesjö BK. Metabolic changes in cerebral cortex hippocampus and cerebellum during sustained bicuculline-induced seizures. J. Neurochem. 1981;37:1228–1238. doi: 10.1111/j.1471-4159.1981.tb04673.x. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez MF, Shiraishi K, Hisanaga K, Sagar SM, Mandabach M, Sharp FR. Heat shock proteins as markers of neuronal injury. Mol. Brain Res. 1989;93:93–100. doi: 10.1016/0169-328x(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 10.Handforth A, Treiman DM. Functional mapping of the early stages of status epilepticus: a 14C-2-deoxyglucose study in the lithium–pilocarpine model in rat. Neuroscience. 1995;64:1057–1073. doi: 10.1016/0306-4522(94)00376-g. [DOI] [PubMed] [Google Scholar]
- 11.Handforth A, Treiman DM. Functional mapping of the late stages of status epilepticus in the lithium–pilocarpine model in rat: a 14C-2-deoxyglucose study. Neuroscience. 1995;64:1075–1089. doi: 10.1016/0306-4522(94)00377-h. [DOI] [PubMed] [Google Scholar]
- 12.Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi RM, Palkovits M, Hruska RE, Rothschild R, Yamamura HI. Regional distribution of muscarinic receptors in rat brain. Brain Res. 1978;154:13–23. doi: 10.1016/0006-8993(78)91047-8. [DOI] [PubMed] [Google Scholar]
- 14.Le Gal La Salle G. Long-lasting and sequential increase of c-Fos oncoprotein expression in kainic acid-induced status epilepticus. Neurosci. Lett. 1988;88:127–130. doi: 10.1016/0304-3940(88)90112-7. [DOI] [PubMed] [Google Scholar]
- 15.Lemos T, Cavalheiro EA. Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp. Brain Res. 1995;102:423–428. doi: 10.1007/BF00230647. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Chopp M, Yoshida Y, Levine SR. Distribution of 72-kDa heat-shock protein in rat brain after hyperthermia. Acta Neuropathol. 1992;84:94–99. doi: 10.1007/BF00427221. [DOI] [PubMed] [Google Scholar]
- 17.Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic behavioral electroencephalographic and neuropathological correlates. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 18.Lowenstein DH, Simon RP, Sharp FR. The pattern of 72-kDa heat shock protein-like immunoreactivity in the rat brain following flurothyl-induced status epilepticus. Brain Res. 1990;531:173–182. doi: 10.1016/0006-8993(90)90771-3. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magistretti PJ, Pellerin L, Martin JL. Brain energy metabolism. An integrated cellular perspective. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 657–670. [Google Scholar]
- 21.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 22.Morgan JI, Curran T. Proto-oncogene transcription factors and epilepsy. Trends Neurosci. 1991;12:459–462. doi: 10.1016/0165-6147(91)90594-i. [DOI] [PubMed] [Google Scholar]
- 23.Morrisett RA, Jope RS, Snead OC., III Effects of drugs on the initiation and maintenance of status epilepticus induced by administration of pilocarpine to lithium-pretreated rats. Exp. Neurol. 1987;97:193–200. doi: 10.1016/0014-4886(87)90293-7. [DOI] [PubMed] [Google Scholar]
- 24.Morrisett RA, Jope RS, Snead OC., III Status epilepticus is produced by administration of cholinergic agonists to lithium-treated rats: comparison with kainic acid. Exp. Neurol. 1987;98:594–605. doi: 10.1016/0014-4886(87)90268-8. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka R, Moroji T. Quantitative autoradiography of muscarinic cholinergic receptors in the rat brain. Brain Res. 1984;296:295–303. doi: 10.1016/0006-8993(84)90065-9. [DOI] [PubMed] [Google Scholar]
- 26.Olney JW, de Gubareff T, Labruyere J. Seizure-related brain damage induced by cholinergic agents. Nature. 1983;301:520–522. doi: 10.1038/301520a0. [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Evans MC, Meldrum BS. Development of pilocarpine-induced seizures in the rat as mapped with [3H]2-deoxy-D-glucose. J. Cereb. Blood Flow Metab. 1989;9(Suppl. 1):S90. [Google Scholar]
- 28.Paxinos G. The Rat Nervous System. 2nd edn. New York: Academic Press; 1995. [Google Scholar]
- 29.Persinger MA, Makarec K, Bradley JC. Characteristics of limbic seizures evoked by peripheral injections of lithium and pilocarpine. Physiol. Behav. 1988;44:27–37. doi: 10.1016/0031-9384(88)90342-3. [DOI] [PubMed] [Google Scholar]
- 30.Planas AM, Soriano MA, Ferre I, Farré ER. Regional expression of inducible heat shock protein-70 mRNA in the rat brain following administration of convulsant drugs. Mol. Brain Res. 1994;27:127–137. doi: 10.1016/0169-328x(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 31.Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimosaka S, Yuen TS, Simon RP. Distribution of HSP72 induction and neuronal death following limbic seizures. Neurosci. Lett. 1992;138:202–206. doi: 10.1016/0304-3940(92)90915-t. [DOI] [PubMed] [Google Scholar]
- 33.Siesjö BK, Abdul-Rahman A. A metabolic basis for the selective vulnerability of neurons in status epilepticus. Acta Physiol. Scand. 1979;106:377–378. doi: 10.1111/j.1748-1716.1979.tb06414.x. [DOI] [PubMed] [Google Scholar]
- 34.Sokoloff L. The radioactive deoxyglucose method. Theory procedure and applications for the measurement of local glucose utilization in the central nervous system. In: Agranoff BW, Aprison MH, editors. Advances in Neurochemistry. New York: Plenum; 1982. pp. 1–82. [Google Scholar]
- 35.Sokoloff L, Reivich M, Kennedy C, Desrosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory procedure and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;27:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, Simon RP. The pattern of neuronal injury following seizures induced by intranigral kainic acid. Neurosci. Lett. 1994;176:205–208. doi: 10.1016/0304-3940(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 37.Tomioka C, Nishioka K, Kogure K. A comparison of induced heat shock protein in neurons destined to survive and those destined to die after transient ischemia in rats. Brain Res. 1993;612:216–220. doi: 10.1016/0006-8993(93)91663-d. [DOI] [PubMed] [Google Scholar]
- 38.Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioral electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- 39.Vass K, Berger ML, Nowack TS, Welch WJ, Lassman H. Induction of stress protein HSP70 in nerve cells after status epilepticus in the rat. Neurosci. Lett. 1988;100:259–264. doi: 10.1016/0304-3940(89)90695-2. [DOI] [PubMed] [Google Scholar]
- 40.Weiner ED, Kalasapudi VD, Papolos DF, Lachman HM. Lithium augments pilocarpine-induced fos gene expression in rat brain. Brain Res. 1991;553:117–122. doi: 10.1016/0006-8993(91)90238-q. [DOI] [PubMed] [Google Scholar]
- 41.Williams MB, Jope RS. Distinctive rat brain immediate early gene responses to seizures induced by lithium plus pilocarpine. Mol. Brain Res. 1994;25:80–89. doi: 10.1016/0169-328x(94)90281-x. [DOI] [PubMed] [Google Scholar]