Abstract
Background: Increased consumption of nuts has been advocated because of their health benefits, but the role of nuts in the treatment of obesity is unclear given their high energy density.
Objective: This study was designed to evaluate the effects of a hypocaloric, almond-enriched diet (AED) compared with a hypocaloric nut-free diet (NFD) on body weight and cardiovascular disease risk factors in the context of an 18-mo behavioral weight-management program.
Design: Overweight and obese individuals [n = 123; age = 46.8 y, BMI (in kg/m2) = 34.0] were randomly assigned to consume an AED or NFD and instructed in traditional behavioral methods of weight control. Anthropometric and metabolic measurements were made at baseline, 6 mo, and 18 mo.
Results: Those in the AED group lost slightly but significantly less weight than did those in the NFD group at 6 mo (−5.5 compared with −7.4 kg; P = 0.04), but there were no differences at 18 mo. No significant differences in body composition were found between the groups at 6 or 18 mo. The AED, compared with the NFD, was associated with greater reductions in total cholesterol (P = 0.03), total:HDL cholesterol (P = 0.02), and triglycerides (P = 0.048) at 6 mo, and no differences were observed between the groups at 18 mo.
Conclusions: The AED and NFD groups experienced clinically significant and comparable weight loss at 18 mo. Despite smaller weight loss in the AED group at 6 mo, the AED group experienced greater improvements in lipid profiles. This trial was registered at clinicaltrials.gov as NCT00194428.
INTRODUCTION
The health benefits of nuts (1) have led policymakers to recommend their regular consumption as part of a healthy diet (2). Nut consumption has positive effects on various cardiovascular disease risk factors, including improvements in triglycerides, total cholesterol (TC)5, and LDL cholesterol (3–5). Moreover, nut consumption in observational studies is associated with a lower risk of developing coronary artery disease, type 2 diabetes, and hypertension (6–11). Despite these benefits, many individuals attempting to lose weight may consciously avoid consuming nuts because of their high energy density.
Epidemiologic studies have shown a negative or inverse relation between nut consumption and body weight (12–15). Mechanisms underlying the relation between nut consumption and weight are unclear but may be related to altered resting energy expenditure, inefficient absorption of energy from nuts, or increased satiety (16–18). In addition to epidemiologic evidence, controlled feeding studies suggest that nuts do not promote significant weight gain (4, 18–21).
Only 3 randomized studies have evaluated the effect of nut consumption in the context of a weight-loss program. Wien et al (20) randomly assigned 65 participants to consume a liquid formula–based low-calorie diet (LCD) enriched with almonds or a liquid-based LCD supplemented with complex carbohydrates and found greater reductions in weight in the almond-enriched group. Li et al (4) randomly assigned 59 participants following an LCD to enrich their diet with either pistachios or pretzels and found no differences in weight change between the groups. Finally, Pelkman et al (21) compared weight-reduction outcomes for 53 participants prescribed a hypocaloric, low-fat (20% of energy) diet or a hypocaloric, moderate-fat (35% of energy) diet enriched with peanuts and found no differences in weight loss between groups. Interpretation of previous findings, however, is limited by their short duration (10–24 wk) and small sample sizes (n = 53–65) (4, 20, 21).
In the context of an obesity pandemic (22) and a public health call for increased nut consumption (2), data from larger samples over longer durations are needed to assess the effects of nut consumption in the context of obesity treatment. The purpose of the current study was to compare the effects of an almond-enriched diet (AED) with those of a nut-free diet (NFD) on body weight, body composition, and cardiovascular disease risk factors in the context of an 18-mo behavioral weight-management program in overweight and obese participants. We hypothesized that the AED would be associated with greater weight loss and improvements in cardiovascular disease risk factors than would the NFD at 6 and 18 mo.
SUBJECTS AND METHODS
Participants
Participants were 123 adults (112 women, 11 men) with a mean (±SD) age of 46.8 ± 12.4 y and a BMI (in kg/m2) of 34.0 ± 3.6. Inclusion criteria were an age of 18 to 75 y and a BMI of 27–40. Participants were excluded if they had uncontrolled hypertension (defined as a blood pressure >180/100 mm Hg), established cardiovascular disease or an inflammatory condition (eg, lupus), diabetes or use of antihyperglycemic medications, dyslipidemia requiring prescription drug therapy as defined by the National Cholesterol Education Program Adult Treatment Panel III guidelines (23), or any known allergy or sensitivity to nuts. Additional exclusion criteria were the use of medications known to affect body weight or a weight loss of ≥5 kg in the preceding 6 mo. Baseline characteristics of the sample are described in Table 1.
TABLE 1.
Baseline characteristics of the subjects1
| Variable | Almond-enriched diet (n = 61) | Nut-free diet (n = 62) |
| Sex [n (%)] | ||
| Male | 7 (11.5) | 4 (6.5) |
| Female | 54 (88.5) | 58 (93.5) |
| Race-ethnicity [n (%)] | ||
| White | 34 (55.8) | 32 (51.6) |
| Black | 21 (34.4) | 27 (43.6) |
| Asian | 0 (0) | 1 (1.6) |
| Hispanic | 1 (1.6) | 2 (3.2) |
| Other | 5 (8.2) | 0 (0) |
| Age (y) | 47.0 ± 12.02 | 46.7 ± 13.0 |
| BMI (kg/m2) | 33.9 ± 3.5 | 34.0 ± 3.7 |
| Weight (kg) | 94.0 ± 13.1 | 91.5 ± 11.9 |
| Triglycerides (mg/dL)3 | 104.9 ± 53.4 | 98.9 ± 54.7 |
| Cholesterol4 | ||
| Total (mg/dL) | 195.1 ± 30.7 | 195.0 ± 36.8 |
| VLDL (mg/dL) | 23.1 ± 15.6 | 22.4 ± 16.0 |
| LDL (mg/dL) | 115.1 ± 26.2 | 110.3 ± 28.2 |
| HDL (mg/dL) | 56.7 ± 13.3 | 61.2 ± 17.0 |
| Total:HDL cholesterol | 3.6 ± 0.8 | 3.4 ± 0.9 |
| Systolic blood pressure (mm Hg) | 123.8 ± 15.0 | 122.4 ± 17.6 |
| Diastolic blood pressure (mm Hg) | 72.2 ± 9.9 | 69.6 ± 9.6 |
| Lean mass (kg) | 56.2 ± 9.2 | 53.9 ± 6.9 |
| Fat mass (kg) | 37.8 ± 7.4 | 37.6 ± 7.4 |
There were no statistically significant differences between the 2 groups.
Mean ± SD (all such values).
To convert values for triglycerides to mmol/L, multiply by 0.01129.
To convert values for cholesterol to mmol/L, multiply by 0.02586.
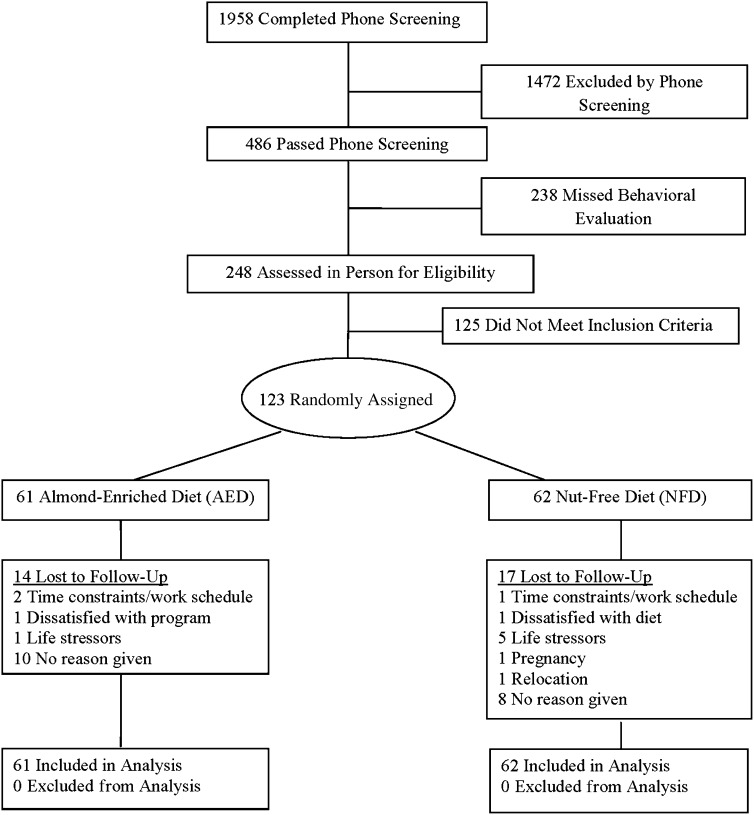
Participant flow throughout the study is shown in Figure 1. Participants who appeared, by a scripted phone screen, to meet eligibility requirements were scheduled to meet with research staff, who described the study's nature and requirements, assessed suitability for participation, and obtained written informed consent. Study visits and treatment occurred at an outpatient division of The Hospital of the University of Pennsylvania. The study protocol was in accordance with the ethical standards of the University and was approved by The University of Pennsylvania and Temple University's Institutional Review Boards.
FIGURE 1.
Participant flow throughout a randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity.
Treatment groups
Common protocol
Participants were randomly assigned, with the use of a random-number generator, to follow either the AED or the NFD as described below. During the first week of treatment, all participants were instructed to maintain their usual eating and activity habits. Thereafter, all participants were prescribed an LCD providing 1200–1500 kcal/d for women and 1500–1800 kcal/d for men. Beginning in week 4, participants in both groups were encouraged to walk for 20 min 4 times/wk, progressing to 50 min 4 times/wk by week 19. Additionally, both groups were instructed in traditional behavioral methods of weight control, such as self-monitoring and stimulus control (24, 25). Groups met weekly for 20 wk, biweekly for the next 20 wk, and every 6 wk for the remainder of 18 mo. The NFD and AED participants attended separate treatment groups to promote adherence to the intervention.
Almond-enriched, low-calorie diet
Sixty-one subjects were assigned to receive the AED. Participants were provided two 28-g packages of almonds (∼24 almonds per package) to consume daily throughout the study, which were distributed at their group meetings. Over the first 5 wk of treatment, participants received whole, raw almonds only. At week 6, roasted almonds were introduced and, over time, a variety of isocaloric, flavored almonds were used. This group was instructed to abstain from alternative nut consumption. The primary behavioral targets were adherence to the total energy intake goal and consumption of 56 g almonds/d.
Nut-free, low-calorie diet
Sixty-two subjects were assigned to receive the NFD. These participants were instructed to abstain from the consumption of nuts (eg, peanuts, peanut butter, cashews, macadamia nuts, walnuts, and pistachios). The primary behavioral target was adherence to the total energy intake goal.
Outcomes
To assess the short-term and long-term effects of an AED relative to an NFD, outcomes were collected at baseline, 6 mo, and 18 mo.
Weight
Body weight was measured on calibrated scales while the participants were wearing light-weight clothing and no shoes. Height was measured with a stadiometer at baseline only.
Plasma lipids and lipoproteins
Blood samples were obtained after subjects fasted overnight (12 h). Plasma lipids were analyzed in a lipid laboratory that participates continuously in the CDC Lipid Standardization Program. Plasma HDL cholesterol and triglycerides were measured enzymatically on a Hitachi autoanalyzer with the use of reagents from Sigma Chemical Co. VLDL-cholesterol and LDL-cholesterol concentrations were directly measured by “beta-quantification” after ultracentrifugation at a density of 1.006 g/mL to separate VLDL.
Blood pressure
Blood pressure was assessed by using automated instruments (Dinamap; GE Health Care) with cuff sizes based on measured arm circumference. After the participants sat quietly for 5 min, 2 blood pressure readings were made separated by a 1-min rest period. The average of the 2 readings was used to determine blood pressure.
Body composition
Body composition was assessed by using dual-energy X-ray absorptiometry (Hologic Discovery A, software 12.4) at baseline, 6 mo, and 18 mo.
Symptoms
We assessed symptoms with a checklist used in previous weight-loss studies (26). The checklist contains 26 symptoms rated as none, mild, moderate, or severe. Symptoms were categorized dichotomously as either absent (none) or present (mild, moderate, or severe) because most symptoms were rated as none; therefore, the data were not normally distributed.
Statistical analysis
Power and estimated sample size
To detect a 3% (SD = 5%) difference in body weight between groups with 80% power and a 2-tailed α of 0.05, 45 participants per group were required at the end of treatment.
Analyses
Between-group differences were assessed at baseline by using independent-samples t tests or Wilcoxon's rank-sum tests, as appropriate, for continuous outcomes. Categorical outcomes were assessed by using chi-square tests.
The primary analysis was an intent-to-treat linear mixed-effects model, which assessed change in each outcome at 6 and 18 mo. These models, which included time, treatment, a time-by-treatment interaction, and the respective baseline value as principal explanatory variables, posited an unrestricted structure on the variance-covariance matrix of the residuals for all 123 participants. These analyses included all observed data for each variable on all participants, regardless of attrition. Several sensitivity analyses were conducted. The first was an analysis of covariance (initial values as covariates) performed on all randomly assigned participants who reached a particular visit (ie, 6 or 18 mo), regardless of whether they subsequently dropped out of the study (ie, a completers’ analysis). The second sensitivity analysis was an intent-to-treat linear mixed-effects model performed on absolute values of the outcome, as opposed to changes in the outcome. The results from these sensitivity analyses were similar to those of the primary analysis in direction and significance. The results of the primary analysis and the completers’ analysis are reported here. Analyses were conducted by using SAS 9.2 (SAS Institute) or SPSS 19.0 (SPSS).
RESULTS
Attrition
No statistically significant differences in attrition were found between the 2 groups at 6 (P = 0.23) or 18 (P = 0.57) mo. The attrition rates were 11.5% for the AED and 19.4% for the NFD at 6 mo and 23.0% and 27.4% at 18 mo, respectively.
Attendance
No statistically significant differences in attendance were found between the 2 groups at 6 (P = 0.67) or 18 (P = 0.41) mo. Participants attended a mean of 15.7 ± 5.9 of 22 sessions (71.4%) at 6 mo and 22.9 ± 10.3 of 35 sessions (65.4%) at 18 mo.
Body weight
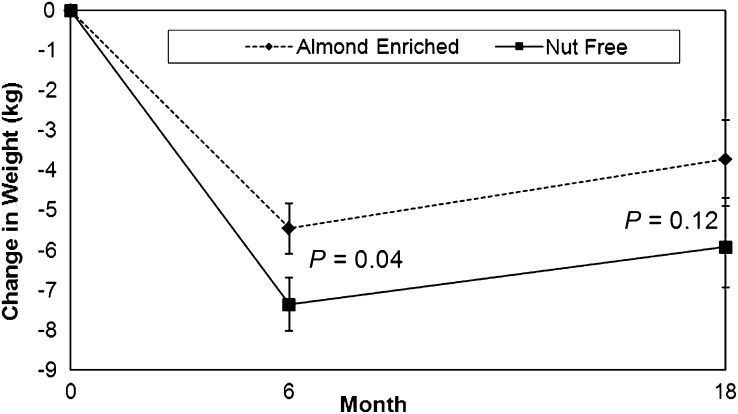
The NFD group lost slightly but significantly more weight than did the AED group at 6 mo (−7.4 compared with −5.5 kg; P = 0.04) (Table 2). Both groups experienced small and similar (1%) increases in weight between 6 and 18 mo. No significant difference in weight loss was found between the NFD (−5.9 kg) and AED (−3.7 kg) groups at 18 mo (Figure 2). Similarly, the completers’ analysis (n = 54 AED; n = 50 NFD) indicated a significantly smaller weight loss in the AED (5.5% ± 4.9%) than in the NFD (7.5% ± 4.9%) group at 6 mo (P = 0.047). At 18 mo, the completers’ analysis (n = 47 AED; n = 45 NFD) showed no significant difference in weight loss between the AED (4.7% ± 7.1%) and NFD (6.5% ± 7.1%) groups (P = 0.2226).
TABLE 2.
Adjusted mean changes by treatment condition from baseline to 6 and 18 mo1
| Variable | Almond-enriched diet | Nut-free diet | P value2 |
| Weight (kg) | |||
| 6 mo | −5.5 ± 0.6 | −7.4 ± 0.7 | 0.04 |
| 18 mo | −3.7 ± 1.0 | −5.9 ± 1.0 | 0.12 |
| Triglycerides (mg/dL) | |||
| 6 mo | −12.1 ± 4.6 | 1.0 ± 4.6 | 0.048 |
| 18 mo | −4.1 ± 6.4 | −10.3 ± 5.6 | 0.47 |
| Total cholesterol (mg/dL) | |||
| 6 mo | −8.7 ± 2.8 | −0.1 ± 2.8 | 0.03 |
| 18 mo | 3.7 ± 3.5 | 5.8 ± 3.1 | 0.64 |
| VLDL cholesterol (mg/dL) | |||
| 6 mo | −2.4 ± 1.5 | 1.4 ± 1.5 | 0.07 |
| 18 mo | 2.3 ± 1.6 | 3.5 ± 1.4 | 0.58 |
| LDL cholesterol (mg/dL) | |||
| 6 mo | −5.4 ± 2.9 | −0.2 ± 2.9 | 0.21 |
| 18 mo | −3.1 ± 2.7 | −0.1 ± 2.5 | 0.41 |
| HDL cholesterol (mg/dL) | |||
| 6 mo | 0.4 ± 1.1 | −0.6 ± 1.1 | 0.52 |
| 18 mo | 4.6 ± 1.7 | 2.3 ± 1.6 | 0.32 |
| Total:HDL cholesterol | |||
| 6 mo | −0.2 ± 0.1 | 0.04 ± 0.1 | 0.02 |
| 18 mo | −0.2 ± 0.1 | −0.1 ± 0.1 | 0.52 |
| Systolic blood pressure (mm Hg) | |||
| 6 mo | −3.9 ± 1.6 | −5.7 ± 1.7 | 0.44 |
| 18 mo | −3.2 ± 2.1 | −3.6 ± 2.0 | 0.89 |
| Diastolic blood pressure (mm Hg) | |||
| 6 mo | −0.8 ± 0.9 | −1.6 ± 1.0 | 0.56 |
| 18 mo | 0.7 ± 1.1 | −1.3 ± 1.0 | 0.19 |
| Lean mass (kg) | |||
| 6 mo | −1.8 ± 0.3 | −2.5 ± 0.3 | 0.22 |
| 18 mo | −1.4 ± 0.4 | −2.4 ± 0.4 | 0.09 |
| Fat mass (kg) | |||
| 6 mo | −3.7 ± 0.5 | −5.0 ± 0.5 | 0.06 |
| 18 mo | −3.0 ± 0.8 | −4.0 ± 0.8 | 0.39 |
All values are adjusted means ± SEs. None of the variables had a significant time-by-treatment interaction. A priori analyses were conducted at 6 and 18 mo.
P values are for between-group differences based on linear mixed-effects models; baseline values of the outcome, time, treatment, and a time-by-treatment interaction were the principal explanatory variables.
FIGURE 2.
Mean (±SE) weight change at 6 and 18 mo in a weight-management population after a hypocaloric almond-enriched diet (n = 62) or a nut-free diet (n = 61). Weight change data were analyzed by using intent-to-treat linear mixed-effects models. These analyses included all observed data on all participants, regardless of attrition.
Body composition
No differences in changes in lean mass were found between the AED and NFD groups at 6 or 18 mo (Table 2). The greater reduction in fat mass observed in the NFD group was nearly statistically significant at 6 mo (P = 0.06), but no significant differences in changes in fat mass were found between the groups at 6 or 18 mo.
Blood pressure
Systolic blood pressure decreased with weight loss in both groups, but no between-group differences were found at 6 or 18 mo (Table 2). Similarly, no significant differences in diastolic blood pressure were found between the 2 groups at 6 or 18 mo.
Plasma lipids and lipoproteins
Significantly greater reductions in triglycerides and TC were found in the AED group than in the NFD group at 6 mo but not at 18 mo (Table 2). No differences in VLDL cholesterol, LDL cholesterol, or HDL cholesterol were observed between the groups at 6 or 18 mo. The ratio of TC to HDL cholesterol (TC:HDL cholesterol) was significantly more improved in the AED group than in the NFD group at 6 mo but not at 18 mo.
Symptoms
The most common symptoms reported at 18 mo were sluggishness/tiredness (37.4%), difficulty sleeping (21.9%), and feeling tense (21.1%), but no significant differences in the presence of 26 symptoms were found between the groups at 6 or 18 mo.
DISCUSSION
There were several principal findings. First, both the AED and NFD groups experienced significant weight loss at 6 (7%) and 18 (5%) mo. These amounts of weight loss are similar to those observed in other studies (4, 21) but less than that in one study (20) that incorporated nuts into a weight-management program. The greater weight loss seen in that study (20) could have been the result of the inclusion of a portion-controlled (liquid formula) diet in both groups or a low-carbohydrate diet in the nut group only, both of which have been shown to have significant effects on short-term weight loss without the incorporation of nuts (27–29).
The NFD group experienced slightly (1.9%) but significantly greater reductions in weight than did the AED group at 6 mo. These findings were in the opposite direction of our hypothesis and may be secondary to the NFD group choosing foods lower in calories for snacks than nuts, which resulted in slightly greater energy deficits. No statistically significant differences in body weight were found between the groups at 18 mo. Whereas the difference between the groups was approximately the same at 6 and 18 mo (1.5 kg compared with 1.8 kg), the variability was greater at 18 mo. The clinical significance of the small difference in weight between groups at 6 or 18 mo (∼2%) is unclear, particularly given the lack of differences in body composition between the groups at either 6 or 18 mo. Both groups experienced minimal weight regain (1%) between 6 and 18 mo. Given that the frequency of the group sessions decreased over time, adherence to both diets may have declined.
The second principal finding was that, despite the slightly smaller weight losses at 6 mo in the AED group, triglycerides, TC, and TC:HDL cholesterol improved more in the AED group than in the NFD group. Specifically, the AED group had a 4% greater reduction in TC and a 12% greater reduction in triglycerides than did the NFD group at 6 mo. The changes are notable given that baseline lipid profiles were close to optimal ranges, which left a restricted range for improvement and/or differences between groups.
A trend toward a greater reduction in VLDL cholesterol in the AED group was observed at 6 mo, consistent with an effect (at 6 mo) of either reduced hepatic VLDL production or increased VLDL lipolysis. Finally, at 6 mo, TC:HDL cholesterol decreased significantly more in the AED group (−0.2 ± 0.1) than in the NFD group (0.04 ± 0.1), consistent with a cardioprotective effect. As in our study, Wien et al (20) found reductions in LDL cholesterol across groups but no differences between intervention groups. In our sample, the effects of almonds on LDL may have been attenuated by the effects of weight loss on LDL. Furthermore, the elevated BMI in our sample may have limited the potential cholesterol-lowering effects of nut consumption, which pooled analyses suggest to be more effective in individuals with a lower BMI (5).
Mechanistically, the compositional properties of almonds that contribute to improvements in triglycerides and TC remain unclear; however, as above, they appear to be related to effects on VLDL metabolism. Almonds are rich in unsaturated fatty acids, which can influence VLDL metabolism (30, 31). The reduction in triglycerides and cholesterol might be expected to reduce cardiovascular disease risk if maintained over a long time (32).
To our knowledge, this was the longest and largest study to date on almond consumption in the context of a weight-management program. Both groups achieved significant short-term weight reduction, which was generally maintained at 18 mo. Our study was conducted primarily with female participants, so generalization to males should be conducted with caution. It was also conducted outside of a metabolic ward, precluding objective assessments of dietary adherence, except weight loss. Whereas adherence to intake and activity were discussed in groups, data were not collected in any standardized manner. Self-reported data have been shown to be invalid when compared with objective measures such as doubly labeled water (33). The differences in lipid profiles at 6 mo, in the context of comparable weight loss, suggested that patients adhered to the energy-deficit diet and to the instruction to consume almonds (AED) or avoid nuts (NFD). The lack of lipid differences at 18 mo suggests decreased adherence over time.
In conclusion, incorporating limited portions of almonds—an energy-dense food—into a behavioral weight-loss program still resulted in significant weight reduction. Moreover, despite a smaller weight loss at 6 mo in comparison with the NFD group, the AED group experienced greater improvements in cardiovascular disease risk factors. There were no differences in weight loss or cardiovascular disease risk factor outcomes between groups at 18 mo.
Acknowledgments
The authors’ responsibilities were as follows—GDF, POS, DJR, and AG-T: designed the research; SSVV, KLS, TLO, and BSZ: conducted the research; MRL, GDF, SSVV, KLS, AV, TLO, BSZ, POS, and DJR: provided essential materials; GDF and MRL: wrote the manuscript; and GDF: had primary responsibility for the final content. GDF serves as a consultant to the Almond Board of California. None of the other authors reported a conflict of interest. The sponsor had input into the study design but had no input or involvement in the study implementation, data analysis, or interpretation of data.
Footnotes
Abbreviations used: AED, almond-enriched diet; LCD, low-calorie diet; NFD, nut-free diet; TC, total cholesterol; TC:HDL cholesterol, ratio of total to HDL cholesterol.
REFERENCES
- 1.King JC, Blumberg J, Ingwersen L, Jenab M, Tucker KL. Tree nuts and peanuts as components of a healthy diet. J Nutr 2008;138:1736S–40S [DOI] [PubMed] [Google Scholar]
- 2.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans 2010. January 2011. Available from: http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm (cited 15 August 2011)
- 3.Griel AE, Kris-Etherton PM. Tree nuts and the lipid profile: a review of clinical studies. Br J Nutr 2006;96:S68–78 [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, Thames G, Gao K, Li L, Tseng C, et al. Pistachio nuts reduce trigylcerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr 2010;29:198–203 [DOI] [PubMed] [Google Scholar]
- 5.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7 [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ 1998;317:1341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992;152:1416–24 [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev 2001;59:103–11 [DOI] [PubMed] [Google Scholar]
- 9.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–60 [DOI] [PubMed] [Google Scholar]
- 10.Djoussé L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr 2009;28:10–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu FB, Stampfer MJ. Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Curr Atheroscler Rep 1999;1:204–9 [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Gonzalez MA, Bes-Rastrollo M. Nut consumption, weight gain and obesity: Epidemiological evidence. Nutr Metab Cardiovasc Dis 2011;21:S40–5 [DOI] [PubMed] [Google Scholar]
- 13.Mattes RD, Kris-Etherton P, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr 2008;138:1741S–5S [DOI] [PubMed] [Google Scholar]
- 14.Sabaté J. Nut consumption and body weight. Am J Clin Nutr 2003;78:647S–50S [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alper CM, Mattes RD. Effects of chronic peanut consumption on energy balance and hedonics. Int J Obes Relat Metab Diord 2002;26:1129–37 [DOI] [PubMed] [Google Scholar]
- 17.Mattes RD. The energetics of nut consumption. Asia Pac J Clin Nutr 2008;17(suppl):337–9 [PubMed] [Google Scholar]
- 18.Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr 2007;98:651–6 [DOI] [PubMed] [Google Scholar]
- 19.Zaveri S, Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J Hum Nutr Diet 2009;22:461–8 [DOI] [PubMed] [Google Scholar]
- 20.Wien MA, Sabate´ JM, Ikle DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord 2003;27:1365–72 [DOI] [PubMed] [Google Scholar]
- 21.Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, Kris-Etherton PM. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr 2004;79:204–12 [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 24.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr 2005;82:230S–5S [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007;132:2226–38 [DOI] [PubMed] [Google Scholar]
- 26.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Ann Intern Med 2010;153:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia. Ann Intern Med 2004;140:769–77 [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003;27:537–49 [DOI] [PubMed] [Google Scholar]
- 29.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–90 [DOI] [PubMed] [Google Scholar]
- 30.Spiller GA, Miller A, Olivera K, Reynolds J, Miller B, Morse SJ, Dewell A, Farquhar JW. Effects of plant-based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. J Am Coll Nutr 2003;22:195–200 [DOI] [PubMed] [Google Scholar]
- 31.Yada S, Lapsley K, Huang G. A review of composition studies of cultivated almonds: macronutrients and micronutrients. J Food Compost Anal 2011;24:469–80 [Google Scholar]
- 32.Pearson TA, Blair SE, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, et al. AHA Guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Circulation 2002;106:388–91 [DOI] [PubMed] [Google Scholar]
- 33.Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfeld SB. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992;327:1893–8 [DOI] [PubMed] [Google Scholar]