Abstract
Background: By modulating immune function, vitamin D might increase innate immunity and inhibit the growth of initial bacterial invasion and protect against tuberculosis infection.
Objective: We examined the effect of vitamin D supplementation on tuberculin skin test (TST) conversion.
Design: A double-blind, placebo-controlled study was conducted in 120 Mongol schoolchildren. We estimated the prevalence of latent tuberculosis infection at baseline and examined the effect of vitamin D (800 IU/d) on serum concentrations of 25-hydroxyvitamin D [25(OH)D] and TST conversion.
Results: At baseline, the mean (±SD) 25(OH)D concentration was 7 ± 4 ng/mL, and all concentrations were <20 ng/mL. Vitamin D supplementation increased serum 25(OH)D by a mean of 12.7 ng/mL compared with placebo (P < 0.0001). At baseline, 16 children in the vitamin D group and 18 in the placebo group were TST positive (P = 0.7). Over 6 mo, TSTs converted to positive in 5 (11%) children receiving vitamin D compared with 11 (27%) receiving placebo (RR: 0.41; 95% CI: 0.16, 1.09; P = 0.06). Only one TST conversion occurred among those whose serum 25(OH)D concentration increased to >20 ng/mL, whereas 8 TST conversions occurred in those whose final 25(OH)D concentration remained <10 ng/mL (P = 0.05). The mean increase in stature was 2.9 ± 1.6 cm in the vitamin D group and 2.0 ± 1.7 cm in the placebo group (95% CI: 2.16, 2.81; P < 0.003).
Conclusions: Vitamin D supplementation for 6 mo had significant favorable effects on serum 25(OH)D concentrations and on growth in stature. A trend was seen toward fewer TST conversions in the vitamin D group. This trial was registered at clinicaltrials.gov as NCT01244204.
INTRODUCTION
Tuberculosis might have been the world's leading cause of death from infectious disease over the past 4 centuries (1, 2). Mongolia, a land-locked country in northern Central Asia, has one of the highest tuberculosis burdens in the Western Pacific region. Tuberculosis is among the top 10 causes of mortality in Mongolia. Its incidence increased from 79 to 141 per 100,000 between 1990 and 2001. WHO estimates the current incidence at 230 per 100,000.
Mongols are at high risk of vitamin D deficiency because they live above 45°N latitude, where there is too little ultraviolet light to induce cutaneous vitamin D synthesis in winter (3), they must keep their skin covered during the winter, and they have no access to vitamin D–fortified foods. In addition, the recent movement of many formerly nomadic people to the city has resulted in reduced exposure to sunlight and greater exposure to air pollution, which can also reduce cutaneous vitamin D synthesis (4).
Sunshine and cod liver oil were both mainstays of the treatment of tuberculosis before the availability of antibiotics (5). In more recent reports, low serum 25-hydroxyvitamin D [25(OH)D]4 concentrations are shown to be associated with increased susceptibility to tuberculosis (6, 7), and a vitamin D receptor gene polymorphism and vitamin D metabolism have been related to resistance to tuberculosis (8, 9). Gibney et al (10) reported an association in asymptomatic people between higher vitamin D status and negative tuberculin status. Recently, Arnedo-Pena et al (11) showed in cross-sectional and case-control phases of their study that sufficient serum 25(OH)D concentrations are inversely associated with tuberculin skin test (TST) conversion.
Infection with Mycobacterium tuberculosis most commonly results in a latent infection. Because only a subset of infected immunocompetent persons develops active tuberculosis, and the risk of disease is vastly higher in immunocompromised individuals, adequate immune function likely plays a role in staving off disease. The biologically active form of vitamin D—1,25-dihydroxyvitamin D (calcitriol)—is a modulator of macrophage function that helps suppress the intracellular growth of M. tuberculosis (12, 13). Liu et al (14) showed that serum low in 25(OH)D could not upregulate induction of the antimicrobial peptide cathelicidin and the killing of intracellular M. tuberculosis. By modulating immune function, vitamin D may increase innate immunity (5, 14) and inhibit the growth of the initial bacterial invasion and protect against tuberculosis infection.
We are not aware of any clinical trials studying the ability of vitamin D supplementation to increase resistance to tuberculosis infection. However, Martineau et al (5) investigated the effect of vitamin D supplementation on antimycobacterial responses in a clinical trial of tuberculosis contacts with positive results. In 2009–2010, we conducted a randomized, double-blind, placebo-controlled feasibility trial of supplemental vitamin D among healthy school-age children in Mongolia.
SUBJECTS AND METHODS
Our subjects were selected from among public schoolchildren in the district with the highest incidence of tuberculosis (National Statistics). Children between the ages of 12 and 15 y who resided in Ulaanbaatar were enrolled in the study. Children and parents who agreed to participate completed forms asking about demographic information, economic background, history of contact with tuberculosis-infected persons, and a history of Bacillus Calmette-Guérin (BCG) vaccination. We had a 92% enrollment rate, and 97% of enrolled children had been vaccinated with BCG. The BCG vaccine used in Mongolia comes from a strain maintained by a BCG laboratory in Japan. We did not measure BCG scar size, and, as evidence of BCG immunization, we looked for the presence of a BCG scar.
For each child in the study, the school doctor reviewed the child's medical history, focusing on such symptoms as fever, cough, change in appetite, and change in weight. Research assistants weighed the children with the use of standard double-beam scales. They also measured the children's heights while they were standing shoeless with their backs against a vertical surface by using a level, right-angled rod brought to the crown of the head. BMI was calculated as body weight divided by height squared (kg/m2). Midupper arm circumference was measured with a plastic tape (15). Triceps and subscapular skinfold thicknesses were measured with a Holtain skinfold caliper (Holtain Ltd) and were recorded to the nearest 0.2 mm. Each site was measured 3 times, on the left side (15).
Intradermal Mantoux tuberculin testing was done with purified protein derivative–standard (0.1 mL, 2 TE liquid purified protein derivative; Biolek Enterprise Production Immunobiology and Medications, Kharkiv, Ukraine), which was applied to all enrolled children at baseline. Testing was repeated after 6 mo, at the end of the intervention. The diameter of induration was measured to the nearest millimeter at 72 h by experienced physicians and nurses. A transverse diameter of induration of <10 mm was considered negative. For purposes of data analysis, a conversion was defined as an increase in induration to ≥10 mm at follow-up. Those with skin test conversions had blood drawn for T-SPOT.TB testing (an assay of T cell function; Oxford Immunotec) and underwent a physical examination, a chest X-ray, and routine blood tests. They were then referred to the district tuberculosis doctor for further evaluation and possible treatment.
To confirm latent tuberculosis infection (LTBI), we used antigen-stimulated interferon-γ production assays that are more specific for tuberculosis infection than is purified protein derivative testing. We used the T-SPOT.TB assay (Oxford Immunotec) because of its reportedly superior ability to detect M. tuberculosis in tuberculosis-endemic areas (16). Only children whose TSTs were positive at baseline or whose TSTs converted from negative to positive between the baseline measurement and the final measurement at 6 mo had the T-SPOT.TB assay. LTBI was defined as a positive Mantoux test result (diameter: ≥10 mm) and a positive T-SPOT.TB test, with no evidence of clinical tuberculosis or history of clinical tuberculosis.
The study was designed to be a randomized, double-blind, placebo-controlled feasibility trial to generate preliminary data to justify a larger, longer study to be conducted at a later time. The population was chosen to be representative of the group of schoolchildren from whom we would recruit subjects for the larger study. The size of the sample was limited by our budget but was ultimately determined from the magnitude of the changes (mean ± SD) in vitamin D concentrations that we found in our preliminary data. We had 80% power to detect a difference as small as 2.63 ng/mL and 90% power to detect a difference as small as 3.89 ng/mL in mean vitamin D between the pre- and postintervention arms [given a type 1 error probability (α) of 0.05]. We used PASS statistical software for our calculations. We used the SDs in 25(OH)D concentrations found in our pilot data, in which we observed mean (±SD) 25(OH)D concentrations of 17.3 ± 4.8 ng/mL at the outset of the intervention and 26.5 ± 4.5 ng/mL at the end of 1 mo of the intervention. These data are comparable to those gathered from adolescents in Boston during the winter (20.2 ± 9.9 ng/mL) (17) and from children in northern Spain in March (12.6 ± 5.5 ng/mL) (18). At baseline, participants were randomly assigned to receive a daily capsule of 800 IU vitamin D3 (cholecalciferol) or placebo, provided by the vitamin D capsule manufacturer, which matched the vitamin D capsules in color and appearance and were packed in identical but coded bottles. After informed consent was obtained at the baseline visit, research personnel assigned eligible children to the active or placebo regimen on the basis of a table we constructed from a computer-generated list of random numbers. An on-site research assistant not involved with the human subjects stored the coded randomization list in a locked file cabinet. The research assistants, the school doctors, research nurses, and participants were unaware of the assignment groups. During the school week, participants took their assigned vitamin D or placebo and reported their intake in a diary that we provided. To ensure adherence, a trained monitor recorded the intake report data on a daily basis and reported the results to study personnel weekly. During holidays and weekends, the children took the supplements at home under their parents’ supervision. At the follow-up visits, capsules remaining in the supplied bottles were counted, to assess compliance. The Tischon Corporation (Salisbury, MD) prepared the capsules but was not involved in the study design, implementation, analysis, or reporting of findings. The study protocol was approved by the institutional review board of the Mongolian Ministry of Health and the institutional review board of the Harvard School of Public Health. No significant adverse events were observed. The project funders had no role in the design, implementation, analysis, or interpretation of the data.
The intervention ran for 6 of the coldest months of the Mongolian year, from November 2009 to May 2010. Each subject was followed up once in January 2010 and again at the end of the intervention, in May 2010. Blood was collected for serum 25(OH)D assays at each visit. Approximately 8 mL blood was obtained from each child in standard red-top tubes. After the blood was centrifuged, the aliquots were frozen and shipped to the United States for serum 25(OH)D measurement (with the use of the LIAISON 25 OH D Vitamin D TOTAL Assay). This assay uses a chemoluminescence immunoassay technology to quantitatively determine the concentration of circulating 25(OH)D. Baseline and midstudy samples were assayed in April 2010, and the final samples were assayed in June 2010.
Descriptive statistics were calculated for all measured variables; t tests were used to compare normally distributed variables between the groups, and chi-square tests or Fisher exact tests were used for qualitative variables. In addition, t tests were used to test for the difference in the magnitude of the changes from baseline serum 25(OH)D concentrations in the vitamin D as compared with the placebo group. Statistical analyses were performed by using SPSS 8.0 (SPSS Inc) for Windows.
RESULTS
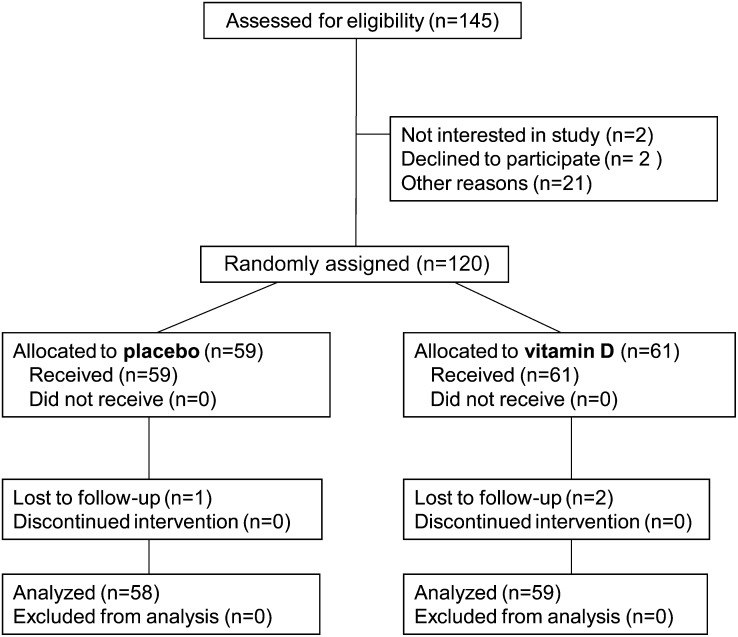
A total of 120 children were recruited for the study. All except 2 of 61 (97%) children who were randomly assigned to the vitamin D group completed the study, compared with all except 1 of 58 (98%) children in the placebo group (Figure 1). These 3 children transferred schools during the study follow-up. In addition, 4 students were excluded from the analysis because they missed the blood drawing at the first (n = 1) or third (n = 3) visit. Compliance with pill taking was excellent. Mean compliance (percentage of prescribed pills taken) was 96% in the vitamin D group and 95% in the placebo group. Only 2 participants from the vitamin D group had compliance <90% and both of them were at 70%. At baseline, the children in the vitamin D and placebo groups were similar in age, height, weight, BMI, midupper arm circumference, and skinfold thickness. Proportions of TST- and T-SPOT.TB-positive and -negative children were similar in both groups (Table 1). Slightly fewer participants of the vitamin D group (28% or 46%) than of the placebo group (31% or 53%) were male.
FIGURE 1.
Flow of participants throughout the trial.
TABLE 1.
Baseline characteristics of the participants1
| Treatment arm |
||
| Vitamin D (n = 61) | Placebo (n = 59) | |
| Age (y) | 13.0 ± 1.12 | 13.1 ± 1.5 |
| Male (%) | 45.9 | 52.5 |
| Type of residence (%) | ||
| Yurt | 50.8 | 49.2 |
| 1- or 2-bedroom apartment | 1.6 | 1.7 |
| House without central heating | 47.5 | 49.2 |
| Weight (kg) | 42.8 ± 7.8 | 44.8 ± 9.5 |
| Height (cm) | 151.6 ± 8.1 | 152.4 ± 8.8 |
| BMI (kg/m2) | 18.5 ± 2.1 | 19.1 ± 2.5 |
| Midupper arm circumference (cm) | 23.2 ± 2.5 | 23.6 ± 3.0 |
| Triceps skinfold thickness | 11.7 ± 4.9 | 12.3 ± 5.1 |
| Subscapular skinfold thickness | 10.0 ± 4.3 | 10.2 ± 4.6 |
| Serum 25(OH)D | 7.0 ± 3.6 | 7.3 ± 3.9 |
| TST positive (n) | 16 | 18 |
| TST negative (n) | 45 | 41 |
| T-SPOT.TB3 positive (n) | 9 | 5 |
| T-SPOT.TB3 negative (n) | 7 | 12 |
None of the variables were significantly different at baseline. TST, tuberculin skin test; 25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
Oxford Immunotec.
LTBI at baseline
Of the 120 children who had a TST administered at baseline, 117 (97%) had a visible BCG immunization scar, and 34 (28%) had an induration diameter of ≥10 mm. Of these 34 TST-positive children, 14 (41%) had positive T-SPOT.TB tests. (One child's T-SPOT.TB assay could not be evaluated because of an insufficient number of cells in the blood sample.) Thus, 14 (12%) of the 120 children given TSTs underwent diagnosis by the most rigorous criteria we could apply as having LTBI at baseline. Children who were TST negative at baseline had rates comparable with those of the TST-positive children: BCG vaccination history, previous history of clinical tuberculosis, and history of tuberculosis contacts. Three of the 120 subjects reported direct contact with someone who had a recent diagnosis of tuberculosis, and 2 subjects had received antituberculous therapy in the past.
Change in 25(OH)D concentrations
At baseline, the mean (±SD) 25(OH)D concentration in the whole sample was 7 ± 4 ng/mL (18 ± 10 nmol/L). Before the intervention, all the children in the study had 25(OH)D concentrations <20 ng/mL (50 nmol/L), and 82% of the children had 25(OH)D concentrations <10 ng/mL (25 nmol/L). After 6 mo of intervention, 34 (60%) of the children who received 800 IU vitamin D daily still had 25(OH)D concentrations <20 ng/mL, and 2 (4%) had concentrations <10 ng/mL. The mean serum 25(OH)D concentration rose from 7 ± 4 to 20 ± 5 ng/mL in the vitamin D group. In contrast, 56 (98%) of the children who received placebo still had, 6 mo later, 25(OH)D concentrations <20 ng/mL, and 34 (60%) still had concentrations <10 ng/mL. The mean serum concentrations of 25(OH)D among the placebo group had fallen by January (from 7 ± 4 ng/mL at baseline to 4 ± 1 ng/mL) and had risen only slightly (to 10 ± 6 ng/mL) by May (Table 2). All children in the placebo group were given vitamin D supplements starting in November 2010.
TABLE 2.
Concentrations of serum 25(OH)D before and after the intervention1
| Treatment arm |
||
| Vitamin D | Placebo | |
| Baseline | ||
| No. of subjects | 60 | 59 |
| Concentration (ng/mL) | 7.0 ± 3.62 | 7.3 ± 3.9 |
| Proportion <20 ng/mL | 100 | 100 |
| 3 mo | ||
| No. of subjects | 61 | 59 |
| Concentration (ng/mL) | 18.2 ± 6.73 | 4.1 ± 0.7 |
| Proportion <20 ng/mL | 67 | 100 |
| 6 mo | ||
| No. of subjects | 57 | 55 |
| Concentration (ng/mL) | 19.8 ± 5.03 | 9.6 ± 4.0 |
| Proportion <20 ng/mL | 60 | 98 |
25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
Significantly different from placebo, P < 0.0001 (Student's t test).
Anthropometric measurements
After 6 mo, children in both groups showed increases in height, weight, midupper arm circumference, BMI, and triceps and subscapular skinfold thickness, as expected. However, children randomly assigned to the vitamin D group gained significantly more height (mean ± SD: 2.9 ± 1.6 cm) than did the children in the placebo group (2.0 ± 1.7 cm) (95% CI: 2.16, 2.81; P = 0.0025) (Table 3). All other changes in anthropometric measurements did not differ significantly between the vitamin D and placebo groups.
TABLE 3.
Characteristics of participants after 6 mo of daily supplementation with 800 IU vitamin D or placebo1
| Treatment arm |
||
| Vitamin D (n = 59) | Placebo (n = 58) | |
| Weight (kg) | 44.7 ± 7.7 | 46.6 ± 9.5 |
| Weight change (kg) | 2.0 ± 1.6 | 1.8 ± 1.6 |
| Height (cm) | 154.4 ± 7.8 | 154.5 ± 8.2 |
| Height change (cm) | 2.9 ± 1.62 | 2.0 ± 1.7 |
| BMI (kg/m2) | 18.6 ± 2.2 | 19.4 ± 2.6 |
| BMI change (kg/m2) | 0.2 ± 0.6 | 0.3 ± 0.7 |
| Midupper arm circumference (cm) | 24.3 ± 2.6 | 24.6 ± 3.0 |
| Midupper arm circumference change (cm) | 1.2 ± 1.5 | 1.1 ± 0.8 |
| Triceps skinfold thickness | 12.1 ± 5.4 | 13.2 ± 5.4 |
| Triceps skinfold thickness change | 0.8 ± 2.5 | 1.2 ± 2.0 |
| Subscapular skinfold thickness | 10.5 ± 4.6 | 11.1 ± 4.4 |
| Subscapular skinfold thickness change | 1.0 ± 1.9 | 1.2 ± 1.8 |
All values are means ± SDs.
Significantly different from placebo, P = 0.0025 (Student's t test).
TST conversion and T cell assay (T-SPOT.TB) results
Of the 115 children who underwent repeated TST at the end of the study, 41 (36%) had a TST ≥10 mm at the second measurement done after 6 mo: 17 (29%) in the vitamin D group compared with 24 (42%) in the placebo group. The TST converted from negative to positive in 5 of 45 children (11%) who received vitamin D and in 11 of 41 (27%) children who received placebo. Although not a statistically significant difference, 59% fewer new LTBIs were observed in the vitamin D group than in the placebo group (RR: 0.41; 95% CI: 0.16, 1.09; P = 0.06) (Table 4). Nine of the 34 initially TST-positive subjects reverted to TST negative by the end of the study: 4 of 16 (25%) in the vitamin D group and 5 of 18 (28%) in the control group.
TABLE 4.
TST conversion from baseline after intervention by treatment arm1
| Treatment arm |
Total | ||
| Vitamin D | Placebo | ||
| TST | |||
| Positive at baseline | 16 | 18 | 34 |
| Did not change | 40 | 30 | 70 |
| Converted | 5 | 11 | 16 |
| Total | 61 | 59 | 120 |
P = 0.06 (Fisher exact test). TST, tuberculin skin test.
All initially TST-positive participants, and all those whose TSTs had converted to positive by the end of the study, were given T-SPOT.TB tests. Of the 22 children in the vitamin D group who were initially either TST negative or TST positive but T-SPOT.TB negative at baseline, 3 (14%) tested positive for T-SPOT.TB at end of the study compared with 5 (17%) of the 29 children with that pattern of test results in the placebo group. Seven initially positive subjects reverted to T-SPOT.TB negative at the end of the study: 3 were in the vitamin D group (14%) compared with 4 in the placebo group (14%) (P = 0.9).
To examine the relation between TST conversion and 25(OH)D concentrations achieved by the end of the intervention, we categorized final 25(OH)D concentrations into very severe (≤10 ng/mL), severe (11–15 ng/mL), moderate (16–20 ng/mL), and mild (>20 ng/mL) (Table 5). Only one TST conversion was observed among children with final 25(OH)D concentrations of >20 ng/mL, whereas 8 conversions were observed in the group with concentrations ≤10 ng/mL (ordinal χ2 test for trend = 3.77, P = 0.05).
TABLE 5.
TST conversion rate by serum 25(OH)D concentrations at 6 mo1
| Serum 25(OH)D |
Total | ||||
| ≤10 ng/mL | 11–15 ng/mL | 16–20 ng/mL | >20 ng/mL | ||
| TST converted | 8 | 3 | 4 | 1 | 16 |
| Did not change | 30 | 21 | 26 | 27 | 104 |
| Total | 38 | 24 | 30 | 28 | 120 |
P-trend = 0.05 (chi-square test). TST, tuberculin skin test; 25(OH)D, 25-hydroxyvitamin D.
DISCUSSION
In this small, randomized, feasibility trial among Mongol schoolchildren, vitamin D deficiency was almost universal at baseline; children had a mean 25(OH)D concentration of 7 ng/mL, and 82% of them had 25(OH)D concentrations of <10 ng/mL (25 nmol/L). The children in the placebo group showed a drop in mean 25(OH)D concentrations to 4 ng/mL by January. Daily supplementation with 800 IU/d resulted in mean serum 25(OH)D concentrations increasing from 7 to 20 ng/mL. Nevertheless, 60% of the children receiving daily supplements of 800 IU had concentrations that remained <20 ng/mL after 6 mo, which indicated that higher levels of supplementation may be needed to bring most children into the normal range, ie, >20 ng/mL according to a recent Institute of Medicine Review (http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx).
Vitamin D supplements resulted in small but significantly greater gains in height, but not in body weight. To the best of our knowledge, ours was the first study to show that vitamin D supplementation, as an isolated intervention, can improve growth in healthy children. In an earlier study in Ulaanbaatar, Mongolia (19), we showed that children who drank vitamin D–fortified milk grew significantly taller, but we could not distinguish the effect of vitamin D from other contributions of the milk. This effect of vitamin D may explain the often-repeated observation that children grow more during the spring and summer than in the fall and winter (20–25).
Our study is the first we know of to document the rate of LTBI among Mongol schoolchildren. Use of the T-SPOT.TB assay, which is more specific than the TST and distinguishes M. tuberculosis infection from BCG immunization, is especially important in this commonly BCG vaccinated population; we found 12% of our children to have LTBI. Through use of a TST definition of LTBI, we found that 28% of our children qualified for the diagnosis. In this trial we found a 59% reduction in the TST conversion rate in the group taking 800 IU vitamin D/d for 6 mo compared with the rate of conversion in the placebo group (P = 0.06). In a secondary analysis, there was only one TST conversion among those achieving >20 ng/mL 25(OH)D, whereas there were 8 TST conversions among those not achieving ≥10 ng/mL (P = 0.05). In a somewhat indirect, although parallel, finding that addresses disease progression in initially TST-positive subjects and not TST conversion, Talat et al (26) reported that tuberculosis progression was diminished in the household contacts of patients with tuberculosis who were in the highest tertile of plasma 25(OH)D concentrations compared with those in the lowest tertile (P = 0.002).
In our study, the TST conversion rate over 6 mo is high. This may have been related to a concurrent outbreak of tuberculosis infection among first graders at the school, where we conducted our study. Alternatively, our high TST conversion rate may reflect a “booster phenomenon,” observed after repeated tuberculin tests (27), rather than acquisition of LTBI. On the other hand, there have been reports suggesting that vitamin D deficiency may cause anergy in the delayed hypersensitivity skin test in humans (28). Another study suggested that vitamin D supplementation may be beneficial to individuals with insufficient vitamin D concentrations but may increase the risk of tuberculosis among individuals with normal or high concentrations (29).
What possible explanations are there for the lower TST conversion rate in children who received vitamin D? If vitamin D had increased acquired immunity, one might have expected the opposite result, ie, that vitamin D supplementation would have been associated with a higher rate of skin test conversion. However, if instead, vitamin D increased innate immunity, one might see our result. If innate immunity sufficiently inhibits the growth of the initial bacterial invasion, the number of bacilli to which the host immune system is exposed might remain below the minimum required to stimulate T- cell expansion and acquired immunity. This hypothesis is consistent with the in vitro finding of Liu et al (14) and the in vivo finding of Martineau et al (5). Also consistent with this interpretation is a double-blind randomized controlled trial among 192 healthy tuberculosis contacts, who received a single oral dose of 2.5 mg vitamin D (100,000 IU) or placebo and were followed for 6 wk (5). Efficacy of vitamin D supplementation was determined by comparing the luminescence of samples before and after the dose, based on the assumption that a stronger immune response would suppress growth of bacteria and thus the luminescence observed. According to this measure, vitamin D supplementation suppressed bacterial growth at 24 h, an indicator of innate immunity, by 20.4% more than placebo (P = 0.03) but did not affect luminescence or interferon-γ secretion at 96 h—an indicator of acquired immunity. On the other hand, in a study by Yesudian et al (30), significant change in antimycobacterial immunity (as expressed by the BCG–lux assay) did not occur even though the mean 25(OH)D concentration increased significantly after ultraviolet B treatment (P < 0.01).
None of the participants were tested for HIV. Although Mongols are vulnerable to HIV/AIDS, the number of HIV/AIDS cases reported from Mongolia is still very low. Between 2002 and 2007, the central laboratory of the National Center for Chronic Disease Control had tested 1920 tuberculosis patients for HIV; only one of the tests was positive.
Our reliance on self-reported vitamin D adherence may have caused us to overestimate the degree of supplementation that the children were actually getting, which may have weakened our conclusion that 800 IU/d is not enough to get most children to the Institute of Medicine–recommended serum concentration of 20 ng/mL in this group with low exposure to sunlight. However, the increase in serum 25(OH)D concentrations observed in the children who took the vitamin D supplement is consistent with previous observations that have shown that blood concentrations of 25(OH)D increase by ∼1 ng/mL for every 100 IU vitamin D ingested (31).
The small size of our study and our reliance, for reasons related to resource limitations, on the TST did not allow us to settle the question of whether in this group vitamin D supplementation really reduced the rate of acquisition of LTBI. However, the possible public health importance of the effect size observed should justify a larger and better-funded study to settle the issue.
In conclusion, supplementation with 800 IU vitamin D/d, compared with placebo, for 6 mo in a group of Mongol schoolchildren had favorable and statistically significant effects on serum 25(OH)D concentrations and on growth in stature. We also observed a 59% greater reduction in the TST conversion rate in the group given vitamin D, but this difference was not statistically significant. Yet, if an effect this large could be shown in a larger study, the public health significance would be clear, because improving vitamin D status could increase innate immunity and thus play a role in the primary prevention of tuberculosis infection.
Acknowledgments
We are grateful to the participants and families and the staff of Ulaanbaatar Public School #65; T Lkhagvasuren and other faculty at the Health Science University of Mongolia; (former Mongolian Minister of Health Lambaa, Mongolian Ministry of Education; R Bold former Ambassador to the United States); and S Boldbaatar, N Naranbaatar, E Munkh-Ochir, G Ganzorig, T Munkh-Orgil, and T Maidar for their support. We also thank our dedicated and hard-working research assistants B Oyunbileg, B Ninjin, and B Uyanga; the doctor and nurses from the TB surveillance center (Songino-khairkhan district); and D Enkhmaa, K Pankratz, and Enkhee (the Swanson Foundation) for their assistance.
The authors’ responsibilities were as follows—DG, BRB, and WCW: conception and design of the study; DG: drafting of the manuscript and statistical analyses; DG, WB, and DB: conduct of the study; MFH: vitamin D interpretation; and DG, WCW, EG, WF, NS, and BRB: interpretation of the data. All authors approved the final version of the manuscript. None of the authors had any personal or financial conflicts of interest.
Footnotes
Abbreviations used: BCG, Bacillus Calmette-Guérin; LTBI, latent tuberculosis infection; TST, tuberculin skin test; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1. Global Tuberculosis Control WHO 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf (cited 11 January 2012)
- 2.Bloom BR, Murray CJL. Tuberculosis: commentary on a reemergent killer. Science 1992;257:1055–64 [DOI] [PubMed] [Google Scholar]
- 3.Ganmaa D, Tserendolgor U, Frazier L, Nakamoto E, Jargalsaikhan N, Rich-Edwards J. Effects of vitamin D fortified milk on vitamin D status in Mongolian school age children. Asia Pac J Clin Nutr 2008;17:68–71 [PubMed] [Google Scholar]
- 4.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child 2002;87:111–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martineau AR, Wilkinson R, Wilkinson K, Newton S, Kampmann B, Hall B, Packe G, Davidson R, Eldridge S, Maunsell Z, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 2007;176:208–13 [DOI] [PubMed] [Google Scholar]
- 6.Davies PDO. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle 1985;66:301–6 [DOI] [PubMed] [Google Scholar]
- 7.Chan TY. Seasonal variations in vitamin-D status and the incidence of tuberculosis in different countries. Respiration 1999;66:196. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy R. Identifying genetic susceptibility factors for tuberculosis in Africans: a combined approach using a candidate gene study and a genome-wide screen. Clin Sci (Lond) 2000;98:245–50 [PubMed] [Google Scholar]
- 9.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 2000;355:618–21 [DOI] [PubMed] [Google Scholar]
- 10.Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, Biggs BA. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis 2008;46:443–6 [DOI] [PubMed] [Google Scholar]
- 11.Arnedo-Pena A, Juan-Cerdan JV, Romeu-Gracia A, Garcia-Ferrer D, Holguin-Gomez R, Iborra-Millet J, Herrero-Carot C, Sanchis-Pinana J, Bellido-Blasco J, Ferrero-Vega JA, et al. Latent tuberculosis infection, tuberculin skin test conversion and vitamin D status in contacts of tuberculosis patients: a cross-sectional and case-control study. BMC Infect Dis 2011;11:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-dihydroxyvitamin D3, induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun 1998;66:5314–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 1986;57:159–63 [PMC free article] [PubMed] [Google Scholar]
- 14.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–3 [DOI] [PubMed] [Google Scholar]
- 15.Gibson RS. Principles of nutritional assessment. New York, NY: Oxford University Press, 1990:187–208 [Google Scholar]
- 16.Barnes PF. Weighing gold or counting spots: which is more sensitive to diagnose latent tuberculosis infection? Am J Respir Crit Care Med 2006;174:731–2 [DOI] [PubMed] [Google Scholar]
- 17.Gordon CM, DePeter K, Feldman HA, Grace E, Emans J. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004;158:531–7 [DOI] [PubMed] [Google Scholar]
- 18.Docio S, Riancho J, Perez A, Olmos J, Amado J, Gonzalez-Macias J. Seasonal deficiency of Vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 1998;13:544–8 [DOI] [PubMed] [Google Scholar]
- 19.Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, Willett WC, Frazier AL. Milk consumption and the prepubertal somatotropic axis. Nutr J 2007;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelander L, Karlberg J, Albertsson-Wikland K. Seasonality in lower leg length velocity in prepubertal chidlren. Acta Paediatr 1994;83:1249–54 [DOI] [PubMed] [Google Scholar]
- 21.Mirwald RL, Bailey DA. Seasonal height velocity variation in boys and girls. Am J Hum Biol 1997;9:709–15 [DOI] [PubMed] [Google Scholar]
- 22.Palmer CE. Seasonal variation in average growth in weight of elementary school children. Public Health Rep 1933;48:211–4219315389 [Google Scholar]
- 23.Bogin B. Monthly changes in the gain and loss of growth in weight of children living in Guatemala. Am J Phys Anthropol 1979;51:287–91 [DOI] [PubMed] [Google Scholar]
- 24.Bogin BA. Seasonal pattern in the rate of growth in height of children living in Guatemala. Am J Phys Anthropol 1978;49:205–10 [DOI] [PubMed] [Google Scholar]
- 25.Marshall WA, Swan AV. Seasonal variation in growth rates of normal and blind children. Hum Biol 1971;43:502–16 [PubMed] [Google Scholar]
- 26.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R, Vitamin D. Deficiency and tuberculosis progression. Emerg Infect Dis 2010;16:853–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menzies D. What does tuberculin reactivity after Bacille Calmette-Guérin vaccination tell us? Clin Infect Dis 2000;31(suppl):S71–4 [DOI] [PubMed] [Google Scholar]
- 28.Toss G, Symreng T. Delayed hypersensitivity response and vitamin D deficiency. Int J Vitam Nutr Res 1983;53:27–31 [PubMed] [Google Scholar]
- 29.Nielsen NO, Skifte T, Andersson M, Wohlfahrt J, Søborg B, Koch A, Melbye M, Ladefoged K. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case-control study in Greenland. Br J Nutr 2010;104(10):1487–91 [DOI] [PubMed] [Google Scholar]
- 30.Yesudian PD, Berry JL, Wiles S, Hoyle S, Young DB, Haylett AK, Rhodes LE, Davies P. The effect of ultraviolet B-induced vitamin D levels on host resistance to Mycobacterium tuberculosis: a pilot study in immigrant Asian adults living in the United Kingdom. Photodermatol Photoimmunol Photomed 2008;24:97–8 [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Chen TC, Vitamin D. Deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6S [DOI] [PubMed] [Google Scholar]