Abstract
The use of liquid injectable silicone for soft tissue augmentation is controversial. Proponents of its use consider it safe when highly purified medical-grade product is employed appropriately by well-trained and experienced physicians, whereas opponents believe complications from silicone injections are inherently inevitable and unpredictable and that they outweigh the benefits. One of the feared complications is granuloma formation. In this article, the authors report two cases of granulomatous nodules from silicone injections and present the histological features. These cases highlight the need for continued vigilance among clinicians about this complication and the importance not only of careful selection of filler products, but also of patients knowing the credentials of their injection practitioners.
Liquid injectable silicone (LIS) has been utilized for soft tissue augmentation for more than five decades. Currently, only two LIS products (AdatoSil and Silikon 1000) are United States Food and Drug Administration (FDA) approved and only for treatment of retinal detachment. Therefore, any cosmetic injection of these products is off label. The use of LIS for soft tissue augmentation has been controversial due to the complications associated with it. Promoters of this filler believe that the complications are due to impurities from adulterated or industrial grade products, large volume injections, poor techniques, and use by nonphysicians and/or inexperienced physicians. They believe if highly purified medical-grade silicone is injected by a skilled physician in small volumes via a microdroplet technique, it is a safe and gratifying procedure. Critics argue that the complications associated with LIS, although rare, are frequent enough to make it unacceptable for cosmetic purposes. Furthermore, complications can occur even when purified medical-grade silicone and appropriate techniques are employed.1–3 The most commonly observed complications are granuloma formation and migration. In this case presentation, the authors report two patients who developed granulomatous reactions following silicone injections.
Case 1
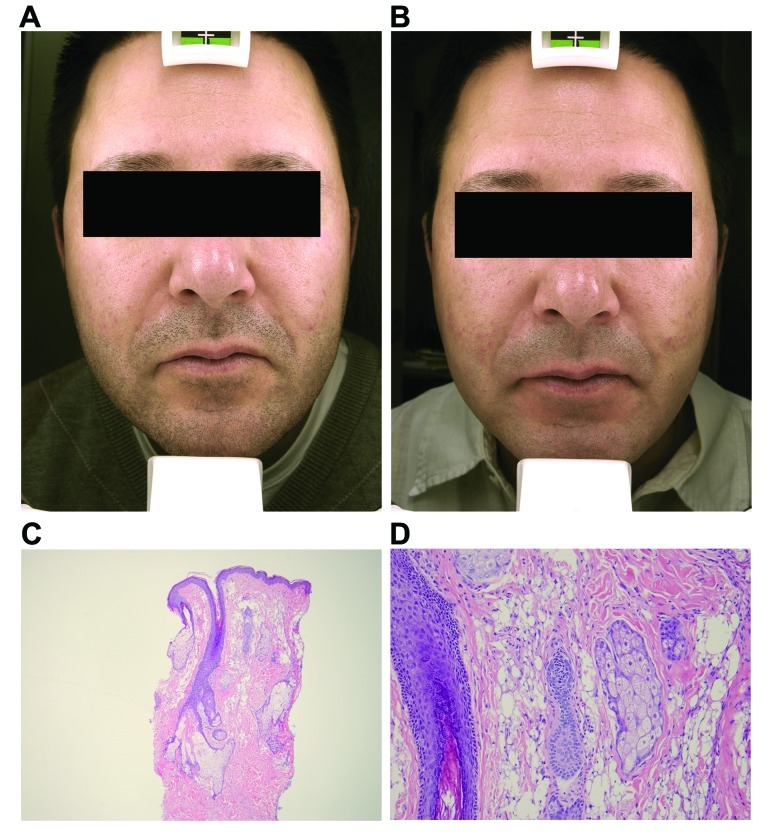
A 39-year-old man presented with subcutaneous nodules along both nasolabial folds (Figure 1A). Five years prior to this photo, he was injected with a filler substance into the nasolabial folds by a nurse injector in a medical day spa. At the time of the injection, he was informed that the specific product injected was Restylane. Within five weeks after the injection, he noticed the development of redness and firm nodules in the areas of these injections. He expressed concern to the injector who then relayed that the product was actually not Restylane at all, but was a silicone product that had been obtained very recently. The injector recommended that he see a dermatologist for evaluation. It was then that the patient originally presented to one of the authors (JLC). Intermittent intralesional steroid (triamcinolone 15–20mg/cc) was performed at approximately two-month intervals. On three occasions, pulse dye laser was also performed to decrease the erythema. After a literature search of other possible treatments, tacrolimus ointment was initiated for two months4 without improvement. Subsequently, imiquimod (Aldara®) was applied for a period of eight weeks,5 but no clinical improvement was noted. A punch biopsy of the right nasolabial fold was performed.
Figure 1.
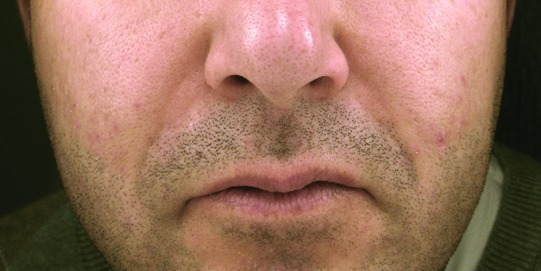
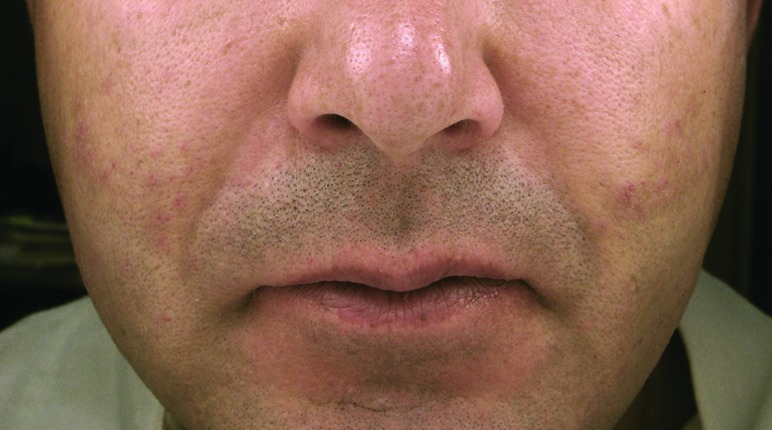
The granulomatous nodules of Patient 1 along the bilateral nasolabial folds prior to (A) and after treatment (B). Histopathological features of the nodular lesions from Patient 1 (C: 50x; D: 200x).
Hematoxylin and eosin evaluation demonstrated a granulomatous process intercalated between and among the collagen bundles in the mid dermis. There were spherioles of washed out artifact consistent with silicone. The overlying epidermis and deeper subcutis were otherwise unremarkable (Figures 1C–1D). These histopathological findings were wholly consistent with the clinical suspicion of a granulomatous reaction to silicone. Following the histological confirmation of silicone granuloma, this patient was again treated with a series of higher dose intralesional steroid injections (30–40mg/cc).6 After three injections, the patient noted that the nodules had significantly decreased in size and softened (Figure 1B). These higher dose intralesional steroid injections have been continued during intervals of flares of erythematous nodules approximately every four months.
Case 2
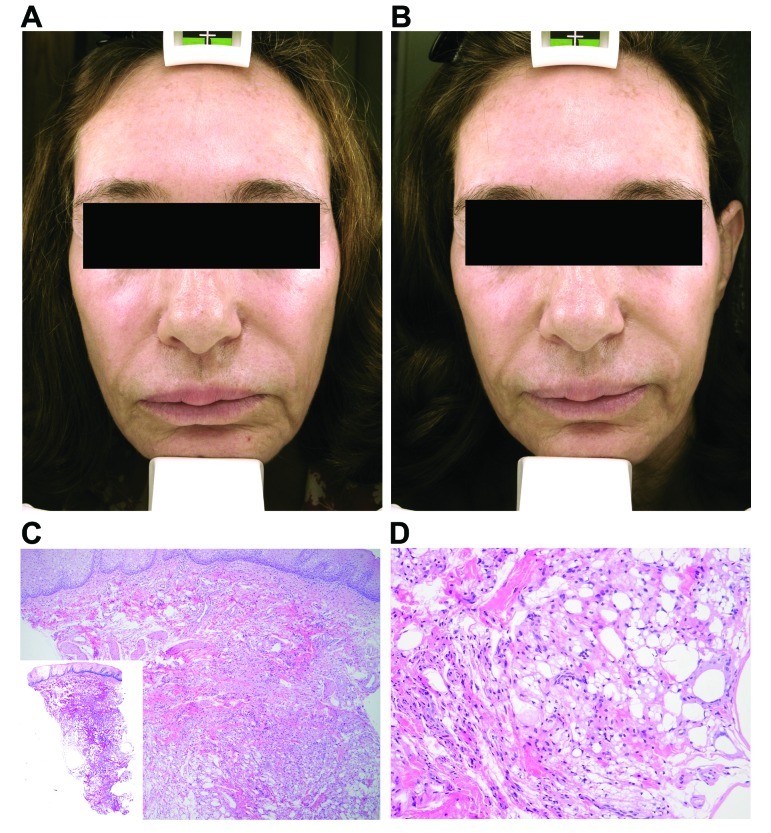
A 57-year-old woman presented with firm nodular swellings involving both the upper and lower lips (Figure 2A). She admitted having had a substance injected into her lips for lip augmentation a few years ago by a nonphysician in California. From reading newspapers and knowing other patients who had been injected by the same person who posed as a physician, she felt comfortable saying it was most likely some type of silicone product.
Figure 2.
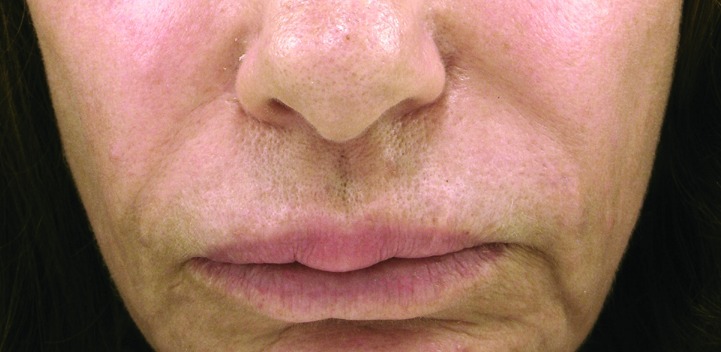
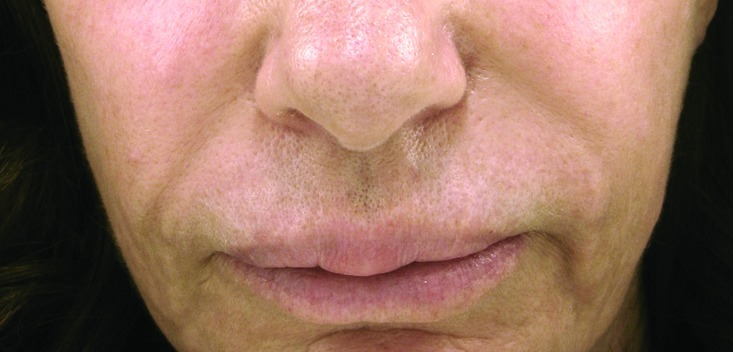
The granulomatous nodules of Patient 2 at the upper and lower lips prior to (A) and after treatment (B). Histopathological features of the nodular lesions from Patient 2 (C: 50x with 12.5x as the inset; D: 200x).
A punch biopsy of the upper lip nodule was performed, and histopathological evaluation demonstrated a submucosal proliferation of histiocytes that nearly entirely replaced the submucosa (Figure 2C–2D). These histiocytes infiltrated around and between collagen bundles and surrounded neurovascular structures and adipocytes. They were also found diffusely throughout the specimen and its edges. The histiocytes displayed delicate, bland ovoid nuclei and abundant cytoplasm. The cytoplasm was clear in areas, while in others it had a frothy to vesicular, pale, amphophilic appearance. A polarized light examination was negative for polarizable material. No mitotic figures were identified. The overlying epidermis was intact and unremarkable. These findings were entirely compatible with the clinical suspicions of a reaction to silicone filler material. Following the histological confirmation, this patient was treated with four sessions of pulse dye laser and topical pimecrolimus cream twice a day over a three-month period. After discussing potential atrophy and local side effects of intralesional steroid, she initially declined injections to the affected areas. After the laser treatments and intervening topical calcineurin inhibitor application, she thought the upper lip had responded with decreased size and firmness of the nodules, but the lower lip swelling continued to bother her. She then consented to have her lips treated with intralesional steroid injections at low doses (10–15mg/cc) with some effect (Figure 2B).
Discussion
Liquid injectable silicone has many individual characteristics that physicians would classify as “ideal” for a soft tissue augmentation agent. It is odorless, colorless, nonvolatile, noncarcinogenic, thermally stable to allow heat sterilization, chemically stable when stored at room temperature for long periods of time, and does not have to be reconstituted prior to use. Furthermore, it is relatively affordable for physicians and patients alike. The controversies around silicone arose, however, because of reported complications associated mainly with impure and non-FDA specific formulations over many years (though the FDA-approved uses of Silikon 1000 and AdatoSil are for retinal detachment and not cosmetic use). While LIS is relatively inert and minimally antigenic, some formulations have been reported to cause granulomatous inflammatory responses as seen in the two patients presented here who were injected with nonapproved formulations unbeknownst to them. Unfortunately, because the scientific literature and mass media have not adequately differentiated silicone products as well as methods of injections, the reputation of LIS has been marred by these reported complications.7
Physicians with many years of experiences with silicone injections have pointed out the importance of using highly purified medical-grade product and adhering to a stringent regimen.8 The injection techniques have varied among injecting physicians, but the serial microdroplet puncture technique proposed by Orentreich is currently the most accepted method of injection.9 This technique injects less than 0.01mL of silicone into the subdermal plane at 2 to 4mm intervals. These injected microdroplets then become surrounded by a capsule of collagenous fibrous tissue, which holds them in place and minimizes migration of these particles. In addition, this gradual fibroplasia will ensure the injected area has the same texture as the adjacent tissue and that the product is not palpable. This technique requires spacing the injections by at least a month to allow adequate fibroplasia to occur. In addition to perfecting the techniques, the purity and consistency/viscosity of LIS have improved over the years to improve the safety and efficacy.
Complications from silicone injections range from minor to serious. Minor complications include erythema, ecchymosis, and edema that occur at the injection site, which are not specific to LIS and are seen with other fillers as well. The more serious complications include granulomas,10–17 product migration,10,11,18,19 cellulitis,3,20 ulcerations and disfiguring scars,3,12 cystic lesions,19 granulomatous hepatitis,11 pneumonitis,21,22 embolism,21,23 and even death.11,21 Most of these serious complications are associated with the use of adulterated or industrial grade silicone, which contains many contaminants, by usually untrained practitioners as in the two cases presented in this report. However, even proponents of highly purified medical-grade silicone injections agree there is approximately a three-percent complication rate even when it is appropriately employed.24,25 This might be due to a mild subclinical microscopic granulomatous reaction to silicone, which becomes clinically apparent in a susceptible individual when infectious or inflammatory processes are present in nearby tissue.24–26 Highly purified medical-grade silicone is not 100-percent safe for injectable cosmetic use, and adverse events can occur, as with any other medication, device, or procedure used in modern medicine. Selecting an appropriate agent and patient, proper training in technique, a thorough evaluation and discussion of risk-benefit ratio, and adequate patient education are essential in its use.7,8
The problem in the field of silicone injection and facial injections for aesthetic purposes in general, is that many of the injectors are not well trained. Many injectors are not even physicians, and injectors clearly are not always using the most purified products or approved formulations of silicone products and/or are not adhering to the microdroplet technique and may not understand the importance of proper skin preparation with an antimicrobial agent. Oftentimes, the patients do not know what products they have received because the practitioner refers to the product as a “filler.” Because facial injections are currently in demand and complications may occur even with the most experienced and well-trained physicians, clinicians should be aware of these issues (patients knowingly or unknowingly receiving various forms of LIS) and be knowledgeable about the treatment of silicone injection complications.
Treatments for silicone granulomas have largely been based on case reports. Intralesional steroids and systemic steroids are the most commonly used approaches. Other treatment modalities that have shown some efficacy include minocycline in conjunction with low-dose prednisone or celecoxib,27–29 imiquimod cream,5 topical tacrolimus or pimecrolimus (the authors’ patients), etanercept,26,30 isotretinoin,31 allopurinol,32 and laser treatments.33 Combination treatments are sometimes necessary and effective as seen in the two patients described in this report. When medical treatments fail, surgical excision can be employed, especially for smaller nodules.34
Conclusion
Although liquid silicone is likely safe if a highly purified product is appropriately employed by skilled physicians in carefully selected patients, it will undoubtedly continue to be misused by untrained injectors, and complications will continue to occur, sometimes decades later. Dermatologists need to be vigilant about the potential complications associated with proper and improper use of silicone fillers and be equipped to treat the complications when they do arise. Equally important, as advocates for patients, dermatologists should continue to educate their patients and the public about being an active coparticipant in their aesthetic care. Patients need to make sure they are seeing reputable physicians who are using FDA-approved, purified products. Patients also need to be more aware of the potential harm if impure substances, including nonpurified silicone, are injected or if any substance is injected using improper techniques.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
References
- 1.Achauer BM. A serious complication following medical-grade silicone injection of the face. Plast Reconstr Surg. 1983;71(2):251–254. doi: 10.1097/00006534-198302000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Wilkie TF. Late development of granuloma after liquid silicone injections. Plast Reconstr Surg. 1977;60(2):179–188. doi: 10.1097/00006534-197708000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Rapaport MJ, Vinnik C, Zarem H. Injectable silicone: cause of facial nodules, cellulitis, ulceration, and migration. Aesthetic Plast Surg. 1996;20(3):267–276. doi: 10.1007/s002669900032. [DOI] [PubMed] [Google Scholar]
- 4.De Boulle K. Management of complications after implantation of fillers. J Cosmet Dermatol. 2004;3(1):2–15. doi: 10.1111/j.1473-2130.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%) Dermatol Surg. 2003;29(4):429–432. doi: 10.1046/j.1524-4725.2003.29102.x. [DOI] [PubMed] [Google Scholar]
- 6.Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26(3):356–364. doi: 10.1016/j.asj.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S–84S. doi: 10.1097/01.prs.0000234919.25096.67. [DOI] [PubMed] [Google Scholar]
- 8.Duffy DM. Liquid silicone for soft tissue augmentation. Dermatol Surg. 2005;31(11 Pt 2):1530–1541. doi: 10.2310/6350.2005.31238. [DOI] [PubMed] [Google Scholar]
- 9.Orentreich DS. Liquid injectable silicone: techniques for soft tissue augmentation. Clin Plast Surg. 2000;27(4):595–612. [PubMed] [Google Scholar]
- 10.Altmeyer MD, Anderson LL, Wang AR. Silicone migration and granuloma formation. J Cosmet Dermatol. 2009;8(2):92–97. doi: 10.1111/j.1473-2165.2009.00436.x. [DOI] [PubMed] [Google Scholar]
- 11.Ellenbogen R, Rubin L. Injectable fluid silicone therapy. Human morbidity and mortality. JAMA. 1975;234(3):308–309. [PubMed] [Google Scholar]
- 12.Mastruserio DN, Pesqueira MJ, Cobb MW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34(5 Pt 1):849–852. doi: 10.1016/s0190-9622(96)90043-2. [DOI] [PubMed] [Google Scholar]
- 13.Schwartzfarb EM, Hametti JM, Romanelli P, Ricotti C. Foreign body granuloma formation secondary to silicone injection. Dermatol Online J. 2008;14(7):20. [PubMed] [Google Scholar]
- 14.Poveda R, Bagan JV, Murillo J, Jimenez Y. Granulomatous facial reaction to injected cosmetic fillers—a presentation of five cases. Med Oral Patol Oral Cir Bucal. 2006;11(1):E1–E5. [PubMed] [Google Scholar]
- 15.Lombardi T, Samson J, Plantier F, Husson C, Kuffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med. 2004;33(2):115–120. doi: 10.1111/j.1600-0714.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 16.Ficarra G, Mosqueda-Taylor A, Carlos R. Silicone granuloma of the facial tissues: a report of seven cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(1):65–73. doi: 10.1067/moe.2002.124459. [DOI] [PubMed] [Google Scholar]
- 17.Schuhmachers R. [Silicone oil granuloma after injection for wrinkles] Arch Klin Exp Dermatol. 1966;227(1):322–325. doi: 10.1007/BF00502848. [DOI] [PubMed] [Google Scholar]
- 18.Mustacchio V, Cabibi D, Minervini MI, Barresi E, Amato S. A diagnostic trap for the dermatopathologist: granulomatous reactions from cutaneous microimplants for cosmetic purposes. J Cutan Pathol. 2007;34(3):281–283. doi: 10.1111/j.1600-0560.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 19.Tirakunwichcha S, Tulvatana W, Kulvichit K. An unusual complication from esthetic injection of liquid silicone into the forehead. Am J Ophthalmol. 2003;136(5):944–945. doi: 10.1016/s0002-9394(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 20.Winer LH, Sternberg TH, Lehman R, Ashley FL. Tissue reactions to injected silicone liquids. A report of three cases. Arch Dermatol. 1964;90:588–593. doi: 10.1001/archderm.1964.01600060054010. [DOI] [PubMed] [Google Scholar]
- 21.Schmid A, Tzur A, Leshko L, Krieger BP. Silicone embolism syndrome: a case report, review of the literature, and comparison with fat embolism syndrome. Chest. 2005;127(6):2276–2281. doi: 10.1378/chest.127.6.2276. [DOI] [PubMed] [Google Scholar]
- 22.Gurvits GE. Silicone pneumonitis after a cosmetic augmentation procedure. N Engl J Med. 2006;354(2):211–212. doi: 10.1056/NEJMc052625. [DOI] [PubMed] [Google Scholar]
- 23.Price EA, Schueler H, Perper JA. Massive systemic silicone embolism: a case report and review of literature. Am J Forensic Med Pathol. 2006;27(2):97–102. doi: 10.1097/01.paf.0000188072.04746.d5. [DOI] [PubMed] [Google Scholar]
- 24.Duffy DM. Commentary. The long-term host response to liquid silicone injected during soft tissue augmentation procedures: a microscopic appraisal. Dermatol Surg. 2007;33(Suppl 2):S192. doi: 10.1111/j.1524-4725.2007.33359.x. [DOI] [PubMed] [Google Scholar]
- 25.Zappi E, Barnett JG, Zappi M, Barnett CR. The long-term host response to liquid silicone injected during soft tissue augmentation procedures: a microscopic appraisal. Dermatol Surg. 2007;33(Suppl 2):S186–S192. doi: 10.1111/j.1524-4725.2007.33359.x. discussion S192. [DOI] [PubMed] [Google Scholar]
- 26.Desai AM, Browning J, Rosen T. Etanercept therapy for silicone granuloma. J Drugs Dermatol. 2006;5(9):894–896. [PubMed] [Google Scholar]
- 27.Arin MJ, Bate J, Krieg T, Hunzelmann N. Silicone granuloma of the face treated with minocycline. J Am Acad Dermatol. 2005;52(2 Suppl 1):53–56. doi: 10.1016/j.jaad.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Beer K. Delayed onset nodules from liquid injectable silicone: report of a case, evaluation of associated histopathology and results of treatment with minocycline and celocoxib. J Drugs Dermatol. 2009;8(10):952–954. [PubMed] [Google Scholar]
- 29.Senet P, Bachelez H, Ollivaud L, Vignon-Pennamen D, Dubertret L. Minocycline for the treatment of cutaneous silicone granulomas. Br J Dermatol. 1999;140(5):985–987. doi: 10.1046/j.1365-2133.1999.02853.x. [DOI] [PubMed] [Google Scholar]
- 30.Pasternack FR, Fox LP, Engler DE. Silicone granulomas treated with etanercept. Arch Dermatol. 2005;141(1):13–15. doi: 10.1001/archderm.141.1.13. [DOI] [PubMed] [Google Scholar]
- 31.Lloret P, Espana A, Leache A, et al. Successful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31(4):486–490. doi: 10.1111/j.1524-4725.2005.31122. [DOI] [PubMed] [Google Scholar]
- 32.Redondo P, Del Olmo J, Alberola I. In situ and distant foreign body granulomas caused by silicone. Treatment with allopurinol. Br J Dermatol. 2005;152(5):1064–1065. doi: 10.1111/j.1365-2133.2005.06525.x. [DOI] [PubMed] [Google Scholar]
- 33.Chui CH, Fong PH. Carbon dioxide laser vaporization of facial siliconomas: flash in the pan or way of the future? Ann Plast Surg. 2008;60(3):272–275. doi: 10.1097/SAP.0b013e318065c4e1. [DOI] [PubMed] [Google Scholar]
- 34.Park TH, Seo SW, Kim JK, Chang CH. The efficacy of perilesional surgical approach for foreign body granuloma. Plast Reconstr Surg. 2011;127(6):121e–123e. doi: 10.1097/PRS.0b013e318213a15c. [DOI] [PubMed] [Google Scholar]