Abstract
Bile salt synthesis is a specialized liver function in vertebrates. Bile salts play diverse roles in digestion and signaling, and their homeostasis is maintained by controlling input (biosynthesis) and intestinal conservation. Patients with biliary atresia (i.e., obliteration of the biliary tree) suffer liver fibrosis and cirrhosis. In contrast, sea lamprey thrives despite developmental biliary atresia. We discovered that the sea lamprey adapts to biliary atresia through a unique mechanism of de novo synthesis and secretion of bile salts in intestine after developmental biliary atresia, in addition to known mechanisms, such as the reduction of bile salt synthesis in liver. During and after developmental biliary atresia, expression of cyp7a1 in intestine increased by more than 100-fold (P < 0.001), whereas in liver it decreased by the same magnitude (P < 0.001). Concurrently, bile salt pools changed in similar patterns and magnitudes in these two organs and the composition shifted from C24 bile alcohol sulfates to taurine-conjugated C24 bile acids. In addition, both in vivo and ex vivo experiments showed that aductular sea lamprey secreted taurocholic acid into its intestinal lumen. Our results indicate that the sea lamprey, a jawless vertebrate, may be in an evolutionarily transitional state where bile salt synthesis occurs in both liver and intestine. Understanding the molecular basis of these mechanisms may shed light on the evolution of bile salt synthesis and possible therapy for infant biliary atresia.
Keywords: metamorphosis, Petromyzon marinus, taurine conjugation, intestine sac, cholestasis
Bile salt synthesis is a unique and vital function of the liver for all vertebrates. Bile salts are excreted into the intestinal lumen, where they solubilize fatty acids in mixed micelles and thus facilitate the absorption of lipid-soluble vitamins (1). Recently, bile salts have been implicated in a wide array of signaling functions, from their own homeostasis to glucose metabolism and cardiovascular functions (2, 3), by interacting with nuclear and G protein-coupled receptors that regulate gene expressions (4–10). However, bile salts also have several pathologic effects, such as carcinogenicity and cellular toxicity (11). Through enterohepatic metabolism and circulation, vertebrates maintain bile salt homeostasis essential for physiological functions and detoxification (12–14).
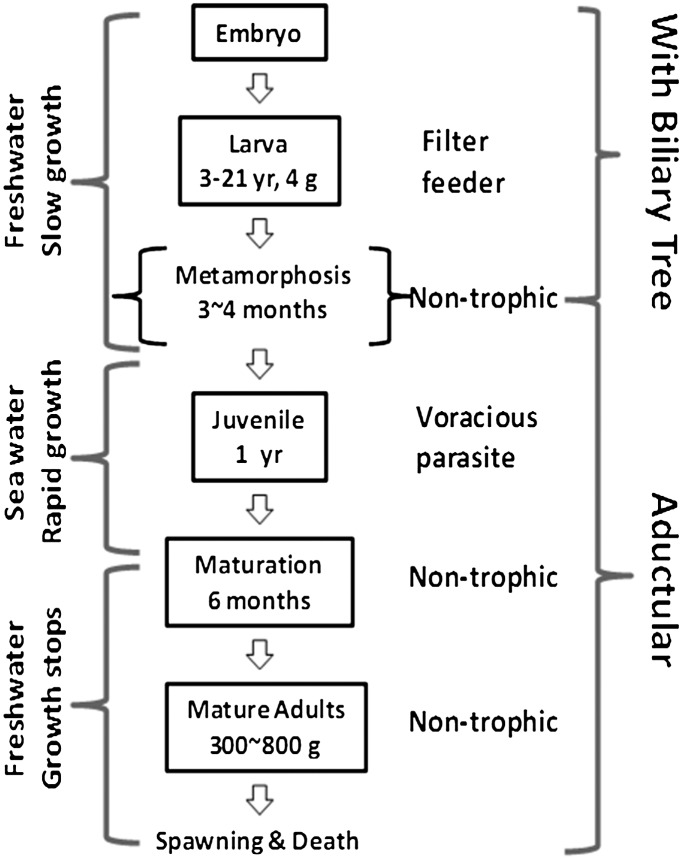
Disruption of bile secretion into the intestine results in liver dysfunctions. Obstruction of bile ducts often causes cholestasis or disturbance of bile formation. Without clinical intervention, cholestasis results in liver toxicity, cirrhosis, and eventually liver failure. In infant biliary atresia, a rare disease in newborns, patients often die within the first few years without treatment (15). A striking contrast is found in lampreys, a group of extant jawless vertebrates that go through developmental biliary atresia during metamorphosis (16). The sea lamprey (Petromyzon marinus), the largest and most widely distributed lamprey species, loses the entire biliary tree when their larvae metamorphose into parasitic juveniles (17). The aductular juveniles feed ferociously and grow exponentially (up to 500-fold increase in body mass within 2 y) into fecund adults (Fig. 1) without complications or liver failure that afflict patients with biliary atresia or other forms of cholestasis.
Fig. 1.
Sea lamprey life history characterized by a dramatic metamorphosis and subsequent migration between fresh and oceanic waters. Sea lamprey larvae filter feed in freshwater until metamorphosis. Biliary atresia occurs during metamorphosis. The postmetamorphic juveniles migrate downstream to the ocean or the Great Lakes (if landlocked) to feed on fish until they reach reproductive stage. The nontrophic adults migrate back to freshwater to spawn.
We reasoned that unique hepatic or extrahepatic mechanisms (or both) have evolved in sea lamprey to promote physiological functions yet minimize cytotoxic effects of bile salts in an aductular life. In particular, we hypothesized that sea lamprey bile salts are mainly synthesized in different digestive organ at different life stage. Here we present evidence for de novo synthesis and secretion of bile salts in sea lamprey intestine.
Results and Discussion
Bile Salt Production During Sea Lamprey Developmental Biliary Atresia.
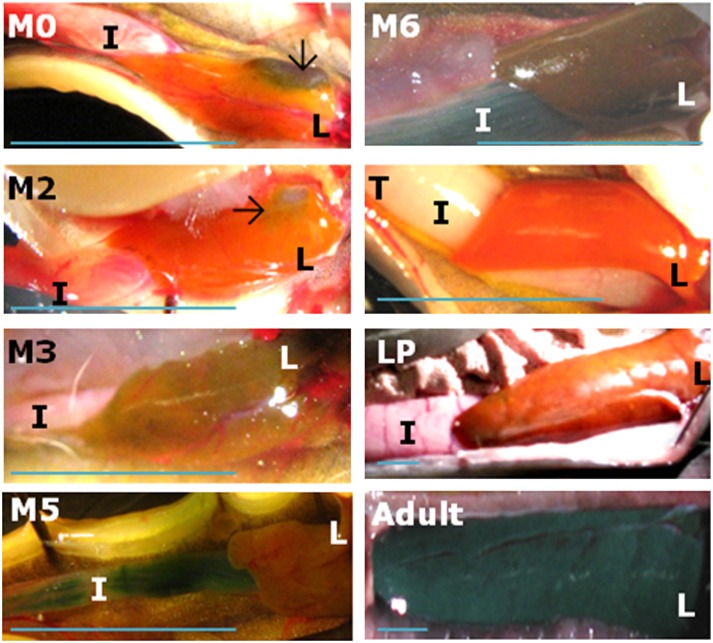
Sea lamprey and human infant biliary atresia share similarities in cellular and histological morphology (18). The main event during biliary atresia is the apoptotic obliteration of bile ducts in both human (19) and sea lamprey (20). However, the cholestatic features commonly found in human liver with biliary atresia have not been documented in sea lamprey. We examined possible signs of cholestasis in sea lamprey liver during metamorphosis, which is divided into seven metamorphic stages, namely M1–M7 (21). We noticed that liver color altered from orange to green at stages M3 to M6, returned to orange when sea lamprey became trophic and parasitic, and changed again to green when adults reach final maturation (Figs. 1 and 2). Green liver is often caused by the accumulation of the bile pigment biliverdin, and is indicative of cholestasis in human biliary atresia and other cholestatic diseases (22). Therefore, sea lamprey seems to experience transient cholestasis during biliary atresia, but recover from this pathologic condition at the end of metamorphosis.
Fig. 2.
Sea lamprey liver and intestine in selected life stages. Selected metamorphic stages (M2, M3, M5, and M6) are shown with larval (M0), newly transformed (T), large parasite (LP), and adult stages. Liver in M5 is not shown in full-size for the purpose of presenting more proportion of the intestine. L, liver; I, intestine. Black arrows indicate the gallbladder in M0 and M2. (Scale bar, 1 cm.)
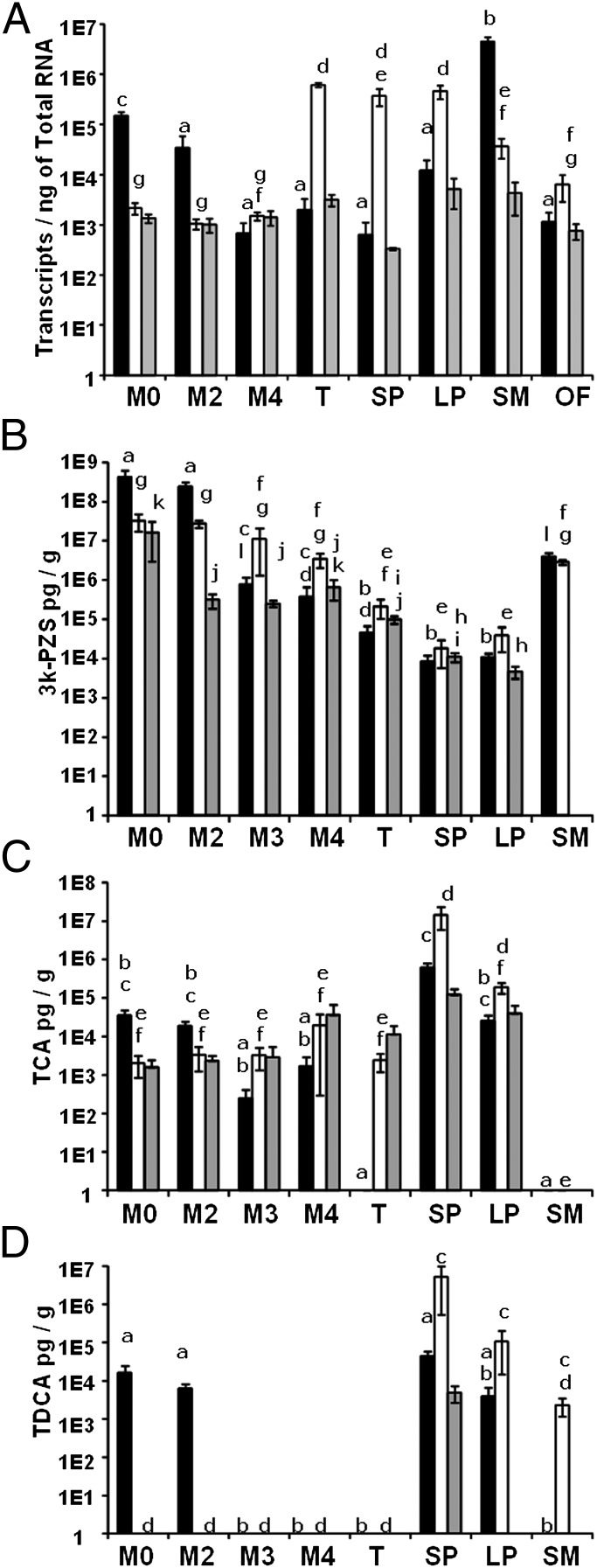
We further hypothesized that bile salt production in liver was reduced during and after developmental biliary atresia in sea lamprey. To test this hypothesis, we examined the bile salt profile and mRNA expressions of cyp7a1 and cyp27a1, which encode the initial enzymes in the classic and alternate pathways, respectively (23, 24). The Cyp27a1 expression was not changed in Cyp7a1 knockout mice (25). We found that hepatic cyp7a1 mRNA and bile salt concentrations decreased dramatically during early developmental biliary atresia (Fig. 3). As metamorphosis progressed, the gallbladder shrank at M2 (Fig. 2) and liver cyp7a1 mRNA level decreased by fivefold compared with M0 (P = 0.01) (Fig. 3A). By M4, the gallbladder and most bile ducts disappeared (Fig. 2 and Fig. S1), and liver cyp7a1 expression reached the lowest level, about 100-fold less than M0 (P < 0.001) (Fig. 3A). Cyp27a1 expression, although not as dramatic, showed similar patterns with a decrease of about 10-fold from M0 to newly transformed (T) in liver (P < 0.001) (see Fig. S3A). Cyp7a1 expression levels in kidneys remained the same across all stages (P > 0.05) (Fig. 3A). These results implied that both bile salt synthetic pathways were suppressed in liver during developmental biliary atresia.
Fig. 3.
Bile salt and cyp7a1 in sea lamprey liver and intestine during and after biliary atresia. Concentrations of (A) cyp7a1 mRNA, (B) 3k-PZS, (C) TCA, (D) TDCA. M0, larval; M2–M4, metamorphic stages 2–4; T, newly transformed; SP, small parasite; LP, large parasite; SM, spermiating male; OF, ovulatory female stages. The y axes are in log scale. Solid bars: liver; open bars: intestine; gray bars: kidney. Vertical lines represent mean ± 1 SEM (n ≥ 3 for all datapoints). Two-way ANOVA showed that both stage and tissue type had effects in A, B, and D (P < 0.001), and an interaction between stage and tissue-type in A (P < 0.001), B (P < 0.001), C (P = 0.017), and D (P < 0.001); Stage, but not tissue type, had effects in C (P < 0.001). Within each panel, bars with same lowercase labels indicate P > 0.05.
Liver bile salt concentrations decreased in similar patterns as cyp7a1 and cyp27a1 expressions during metamorphosis (Fig. 3). Sea lamprey is known to synthesize unique 5α-bile acids and 5α-bile alcohols: allocholic acid (ACA) (26) and its derivative 3-dehydro-ACA (3k-ACA) (27), petromyzonol sulfate (PZS) (28) and its derivative 3-dehydro-PZS (3k-PZS) (29), petromyzonsterol disulfate (PSDS), and petromyzonamine disulfate (PADS) (30). We analyzed their concentrations throughout life stages. C24 bile alcohols, PZS and 3k-PZS, have been found in nature only in lamprey species. Notably, 3k-PZS was one of the major bile salts in larval liver, and its concentration in liver decreased by more than 10,000-fold from M0 to T and remained low throughout the parasitic stage (Fig. 3B). To our surprise, taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), and taurodeoxycholic acid (TDCA) were detected in larval and metamorphic livers (Fig. 3 C and D). TDCA had not been identified in fish or lamprey. Here its identification was based on the retention time of liquid chromatography and fragmentation patterns of tandem mass spectrometry (Fig. S2). TCDCA concentrations did not vary among tissues and stages. These data demonstrate that hepatic production and concentrations of bile salts were minimized during developmental biliary atresia.
Cyp7a1 down-regulation in sea lamprey biliary atresia is strikingly similar to the phenomenon in human with biliary atresia or other cholestatic diseases. In particular, cyp7a1 transcripts decreased by more than 99% in sea lamprey (Fig. 3A), similar to the decrease seen in human biliary atresia (31, 32). However, cyp7a1 down-regulation does not prevent cholestatic injuries to the liver in many mammalian models and human biliary atresia.
De Novo Synthesis of Bile Salts in Sea Lamprey Intestine.
After developmental biliary atresia, sea lamprey no longer maintains direct bile flow from the liver to intestine. Secretion into the systemic circulation is the only alternative route to transport hepatic bile salts. Although bile salts are often sulfated before urinary excretion in mammals with biliary atresia and other forms of cholestasis (33), renal excretion of bile salts in parasitic sea lamprey is highly unlikely because it must minimize or stop urine production to retain water in the hyperosmotic ocean. We found that bile salt production in liver remained minimal during parasitic stage (Figs. 1 and 3 A and B), prompting the question whether extrahepatic bile salt synthetic mechanisms exist in parasitic sea lamprey.
We focused our search on the intestine, where bile salts exert their digestive function. We determined bile salt profiles and cyp7a1 and cyp27a1 mRNA levels in the intestines of larvae before, during, and after biliary atresia, and of feeding parasites. Interestingly, the intestine of parasites showed increase in taurine-conjugated bile salts (Fig. 3 C and D). Intestinal cyp7a1 expression increased more than 100-fold from pre- (M0, M2, and M4) to postdevelopmental biliary atresia stages [T, small parasite (SP), and large parasite (LP)] (Fig. 3A). The increase in cyp7a1 expression also correlated with the increase in TCA and TDCA in the intestine of parasites (Fig. 3 A, C, and D). In contrast, cyp27a1 mRNA levels among M0, T, and SP were not different (P > 0.05), and increased by two- to fivefold from M2 and M4 to T (Fig. S3A). In addition, bile salt synthetic intermediates (7α-hydroxycholesterol, 7α-hydroxy-4-cholesten-3-one, 7α-hydroxy-5β-cholestan-3-one, 5β-cholestane-3α, 7α, 12α, 27α-tetrol, and 3α, 7α, 12α-trihydroxy-5β-cholestanoic acid) were also detected in the intestine of parasites (Table S1). Furthermore, the slower enterocyte turnover rate (9–14 d) in parasitic sea lamprey (34) compared with that of mammals (2–3 d) may favor bile salt synthesis in intestine. These data indicate that bile salt de novo synthesis occurs in the intestine of parasitic sea lamprey.
We also examined whether the increase in intestinal bile salt pool is corroborated with de novo synthesis of cholesterol in intestine. We measured mRNA concentrations of HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthetic pathway. Although the decrease in HMG-CoA reductase mRNA concentrations corresponded to the decrease in cyp7a1 and cyp27a1 mRNA concentrations from M0 to SP and LP in liver (Fig. 3A and Fig. S3 A and B), no difference was found in intestine from M0 to LP (P > 0.05) (Fig. S3B). The cholesterol precursor for bile salt synthesis in intestine may be from either the de novo synthesis in tissues or diet, or both. Notably, in the nontrophic adults, their hepatic or intestinal (or both) HMG-CoA reductase mRNA levels increased dramatically (P < 0.05) (Fig. S3B). This increase in spermiating males may facilitate the synthesis of a bile acid pheromone with secretion rate about 0.5 mg/h (35). Interestingly, lipolysis and fatty acid synthesis in liver and intestine vary significantly among life stages (36). Further examination of cholesterol homeostasis may elucidate the adaptive value of bile salt de novo synthesis in lamprey intestine.
Bile salt de novo synthesis had been recognized as a crucial function limited to the liver, a specialized digestive organ in vertebrates although some hepato-pancreas–like functions have been found in invertebrates, such as crabs (37). In two invertebrate species, Sollasella moretonensis (38) and Ciona intestinalis (39), where intestine is the most developed digestive organ, bile salt-like compounds have been discovered. C. intestinalis does not have a liver but contains a cyp7a1 ortholog (E-values of 1E-64 and 2E-64 compared with mice and human sequences, respectively, in a tBLASTn search; Basic Local Alignment Search Tool, National Center for Biotechnology Information). The notion that evolution of bile salt synthesis may have preceded that of liver is similar to the example where evolution of insulin is believed to have preceded that of the pancreas (40–42). The de novo bile salt synthesis in sea lamprey intestine suggests that bile salt synthesis is a function less specialized in jawless vertebrates compared with jawed vertebrates. Sea lamprey is one of the most basal vertebrates to contain both intestine and liver, both of which support bile salt synthesis. It seems that the site of bile salt synthesis has shifted from the digestive tract to liver as the digestive system became more complex during animal evolution.
Another surprising, but likely adaptive, change in lamprey bile salt synthesis after metamorphosis is the shift in bile salt conjugation. Larval and adult lamprey are known to produce bile alcohol sulfates (26, 28, 43). Sulfation renders bile alcohols soluble, and enables their secretion. Sulfation of taurine-conjugated bile acids is considered an adaptive detoxification mechanism (44). In larval and adult lamprey, bile alcohol sulfates are released into water in large quantities as putative pheromones (29, 30) and sulfation facilitates this process (29). In contrast to the dominance of bile alcohol sulfates in larvae and mature adults, taurine-conjugated bile salts were prominent in parasites (Fig. 3 C and D, and Fig. S4). These taurine-conjugated bile salts may facilitate lipid digestion in the absence of a biliary tree. Alternatively, synthesis of these bile salts may simply serve to eliminate cholesterol. The dramatic changes in the location and the type of bile salt synthesis throughout sea lamprey life history represent a striking example for the adaptation of bile salts to specific functions in particular life stages.
Intestinal Secretion of Taurine-Conjugated Bile Salts.
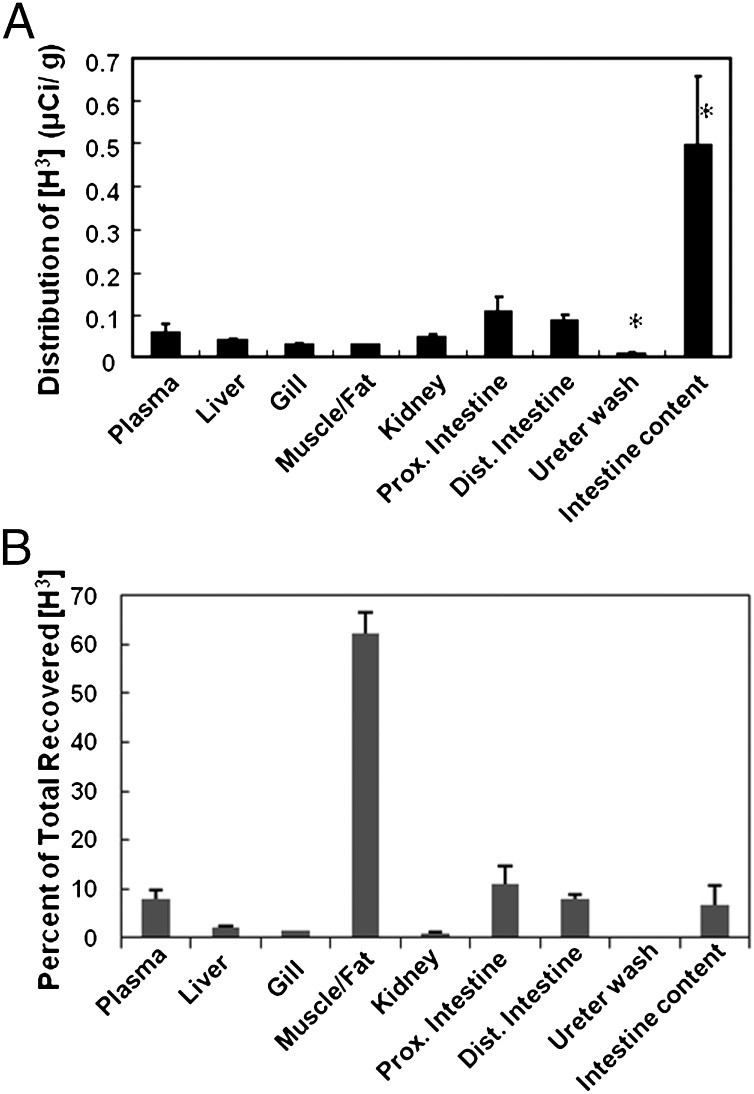
Because TCA was the most abundant bile salt (Fig. 3C) and cyp7a1 mRNA level was high (Fig. 3A) in the intestine of parasites, we predicted that TCA was secreted through the intestine. To test this hypothesis, we designed both in vivo and ex vivo transport assays to measure [H3]TCA transport in the intestine. In vivo tissue distribution of radioactivity after intravenous injection of [H3]TCA showed that intestinal lumen content had 10-times higher tritium concentrations than the plasma in parasites (P < 0.001) after a 12-h incubation (Fig. 4A). Total tritium activity in intestine was at least two-times higher than in plasma (Fig. 4B). Most tritium activity was trapped in the mixture of muscle and fat because of their mass. A minimal amount of tritium was found in kidneys and ureter washings, indicating that kidneys were neither the major storage nor excretion organ for TCA in parasitic lamprey. Whether this is the case in adults when they stop feeding and start their spawning migration remains to be determined.
Fig. 4.
In vivo whole animal perfusion of [H3]TCA showing intestine as a major bile salt secretory organ. Animals used in this experiment were actively feeding parasites. (A) A distribution of tritium concentration in each tissue at 12 h after intravenous injection of [H3]TCA. (B) A percent distribution of the total recovered [H3]TCA in each tissue. *P value < 0.001 compared with any other tissue. Dist., distal; Prox., proximal. Vertical bars represent mean + 1 SEM.
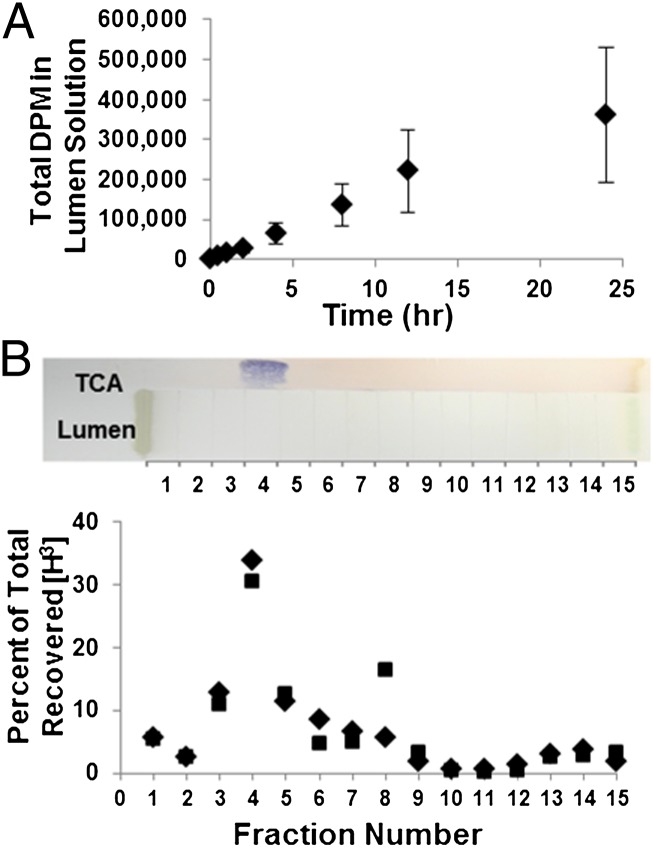
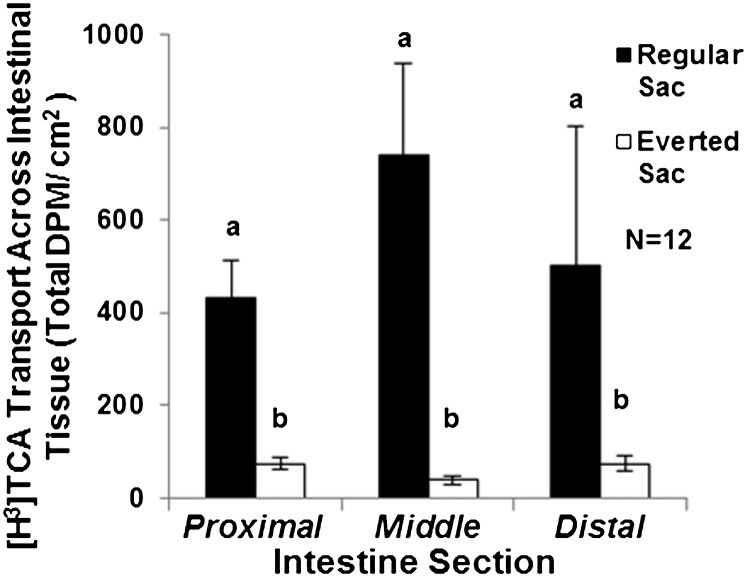
To further elucidate the dynamics of TCA secretion by intestine, we examined TCA transport by ex vivo intestinal sacs over a 24-h time course (Fig. 5). The intestinal tissue did not degenerate after 24-h incubation (Fig. S5) and the tritium counts from lumen solution were contributed by [H3]TCA (Fig. 5B). Intestinal transport of [H3]TCA was much higher in secretion than in absorption (P = 0.0001) (Fig. 6), indicating that TCA transport is a directional and active process. A three-way ANOVA showed no interaction among intestinal section, intestinal orientation, and the sex of animals. The sac orientation is the only factor that affected the transport activity (P = 0.0002, power = 0.988). Taken together, our data implicate an active transport mechanism in the intestine that secretes taurine-conjugated bile salts into the lumen in parasitic sea lamprey. This mechanism is distinct from the enterohepatic circulation where bile salts are reabsorbed from the intestinal lumen to circulation in mammals.
Fig. 5.
Ex vivo time course of TCA transport assay in whole intestinal sac. (A) A 24-h time course of parasitic intestinal sacs incubated in [H3]TCA in Ringer’s solution; n = 4; vertical lines indicate mean ± 1 SEM. (B) PTLC separation and identification of TCA. A PTLC plate was loaded with samples (Upper); TCA, TCA loaded; 5% sulfuric acid solution was used to detect TCA (blue staining); lumen, extracts of intestinal contents from lumen after incubation; incubation solution containing sea lamprey Ringer’s solution and [H3]TCA was loaded in parallel with lumen sample; both lumen sample and incubation solution were scraped into MeOH based on grids 1–15 for scintillation counting; diamonds show counts from intestinal lumen solution after incubation in [H3]TCA Ringer’s solution; squares show counts from incubation solution.
Fig. 6.
Serosal to mucosal transport of [H3]TCA in the intestinal sac. Both regular and everted sacs from three (proximal, middle, and distal) sections of the intestine of each lamprey (six sacs per animal). All six sacs were incubated in sea lamprey Ringer’s solution containing [H3]TCA in an ice-water bath for 4 h. Total DPM was reported after normalized to the surface area of the connective tissue. Closed bars: regular sac readings represent [H3]TCA transport from serosa to mucosa. Open bars: everted sac readings represent [H3]TCA transport from mucosa to serosa. A three-way ANOVA shows that only the sac orientation had an effect on DPM (P = 0.0002, power = 0.988) but not the sex or the sections of the intestine. A one-tailed paired t test showed that regular sacs had greater effect on DPM than everted sacs (P = 0.0001). Vertical lines represent mean ± 1 SEM (n = 12, seven females and five males).
In patients with biliary atresia, bile pigments are not delivered to the intestine, resulting in pale or white stool and green liver. In contrast, intestinal secretion of bile pigments may exist in metamorphic and parasitic sea lamprey where bile salt synthesis occurred. Lamprey intestinal color altered from pink to green during metamorphosis (M2–M6 in Fig. 2). Biliverdin, a bile pigment (from hemoglobin) responsible for this color change, was highly concentrated in intestine at M6, the last stage of metamorphosis. The concentration of biliverdin in intestine at M6 was about 10,000-times higher than M2 (478 ±109 μg/g in M6, 51.3 ±18.7 ng/g in M2). Biliverdin was also concentrated in the mucosal content collected from the intestine at M6. By M4 (Fig. S1), most bile ducts were obliterated and no longer available for bile flow from liver to intestine. Although the liver began to turn green at M3, a sign of biliverdin accumulation, the intestine had a delayed color change to green at M5, and this phenomenon lasted until M6 in both organs (Fig. 2). The corresponding high concentrations of biliverdin (478 ±109 μg/g) and 3k-PZS (6.65 ± 4.01 μg/g) in the intestine at M6 indicated an alternative secretory route for bile products, and most likely occurred through systemic circulation during nontrophic metamorphosis. Contrary to the absence of bile pigments or bile salts in the intestine of patients with biliary atresia, both bile products were secreted into sea lamprey intestine after developmental biliary atresia.
In contrast to the increase in liver bile salts in patients with biliary atresia (31), bile salt levels in sea lamprey liver decreased and intestinal bile salt levels remained the same during developmental biliary atresia (Fig. 3). The disappearance of tight junctions (45) during and after metamorphosis in sea lamprey liver may promote regurgitation into sinusoids followed by secretion through the intestinal epithelium. The clearance of 3k-PZS and biliverdin in intestine was not apparent until postbiliary atresia in T (Figs. 2 and 3B). The alternative excretion through kidneys, adopted by mammals under cholestatic conditions (46), may be less important in sea lamprey as their kidneys completely degenerate and new kidneys regenerate during metamorphosis (17). Taken together, our data show that intestinal secretion of bile salts in sea lamprey is a unique pathway that is likely evolutionarily adaptive, and differs from the pathologically adaptive mechanisms in human biliary atresia.
During evolution, natural selection or genetic drift has resulted in mutant species with phenotypes that mimic human diseases, but are nevertheless adapted to specific environments (47). Sea lamprey biliary atresia is a developmental process that resembles human pathogenic biliary atresia in many cellular features. Aductular parasites secrete bile salts during their rapid growth but slow-growing larvae and atrophic mature adults use bile alcohol sulfates as pheromones (29, 30, 48). The complex lifestyle and bile salt utilization are facilitated by two organs, the liver and the intestine, synthesizing different conjugated bile salts throughout the sea lamprey life cycle.
In summary, our data suggest that sea lamprey has evolved adaptive mechanisms to survive developmental biliary atresia through minimizing bile salt synthesis in liver and relocating bile salt synthesis and secretion to the intestine, but other cholestatic models and humans with biliary atresia suffer from cholestatic liver injuries. Further investigations of the molecular mechanisms underlying these observations may lead to therapies for human biliary atresia.
Materials and Methods
Animals and Tissue Collection.
Experimental procedures were approved by the Michigan State University (MSU) Institutional Animal Use and Care Committee. Animals were held at the US Geological Survey Hammond Bay Biological Station (Millersburg, MI) until shipping to MSU or sampling. Parasites were used in whole-animal perfusion of [H3]taurocholic acid and isolated intestine experiments within 2 mo after detached from hosts. Large larvae (>12 cm) were screened in 2009–2011 to obtain metamorphic animals (21). Randomly selected metamorphic livers were fixed in 4% paraformaldehyde (PFA) for histological examination (Fig. S1). Details of animal sources and handling are listed in the SI Text.
Real-Time Quantitative PCR.
Real-time quantitative PCR was performed using the TaqMan MGB system (Applied Biosystems) as described previously (49). Synthetic oligos were used as standards. The amplicon sequence of cyp7a1 was chosen based on a 771-bp fragment PCR amplified from total cDNAs of sea lamprey tissues. This fragment contained a reading frame of 257 amino acid residues and was verified by tBLASTn (National Center for Biotechnology Information) with an E value of 1E-97 compared with human CYP7A1. Full-length sequences of cyp27a1 and HMG-CoA reductase were obtained from Sea Lamprey Draft Genome 6.0, and both sequences had E values < 1E-150 compared with multiple species including Xenopus laevis, Homo sapiens, and Mus musculus. Sequence information used for real-time quantitative PCR of cyp7a1, cyp27a1, and HMG-CoA reductase is listed in Table S2.
Bile Salt Distribution Measured by LC-MS/MS.
Frozen tissues were homogenized in 75% (vol/vol) ethanol containing [2H5]3k-PZS and [2H4]TCA as internal standards and centrifuged at 13,000 × g for 10 min. Supernatants were freeze-dried and reconstituted in 50% methanol in water (vol/vol) and subjected to LC-MS/MS. Mass spectra were acquired using electrospray ionization in negative or positive (ES+ or ES−) ion mode with multiple reaction monitoring. Data were acquired with MassLynx 4.1 and calibrated and quantified with Quanlynx. Conditions for LC-MS/MS are listed in Table S3.
In Vivo Bile Acid Uptake and Distribution.
Parasites were injected with 25 μCi of [H3]TCA in 400 μL PBS via caudal vein and placed in a bisected aquarium (35) filled with aerated water. About 12 h after the injection, animals were anesthetized with 0.02% MS-222 for blood drawing, and then killed with 0.1% MS-222. The ureter was cannulated and washed with PBS to collect washings. Intestinal content was collected directly after opening the abdomen. Liver, intestine, kidneys, gills, and muscle were collected and weighed. Mass of muscle with fat was estimated by total body mass after subtractions of major organs described above. Blood was centrifuged at 1,000 × g with EDTA for 10 min to obtain plasma. All tissues were homogenized in PBS, and then added with triton X-100 (1%), incubated at room temperature for 20 min, and centrifuged at 13,000 × g for 10 min. Tritium activity of the supernatant was measured as disintegrations per minute (DPM). Amount of [H3]TCA was calculated from DPM detected based on a standard curve. Tritium level was normalized to tissue mass or collected volume.
Whole Intestinal Sac Time Course.
Whole intestine was isolated from parasitic lamprey and sutured into a sac containing 10 mL of lamprey’s Ringer solution (130 mM NaCl, 2.1 mM KCl, 1.8 mM MgCl2, 4 mM Hepes, 4 mM Dextrose, 1 mM NaHCO3, 2.6 mM CaCl2). Sacs were incubated in 700 mL aerated ice cold Ringer containing 20 μCi of [H3]TCA in ice-water bath (0–4 °C). At 30 min, 1, 2, 4, 8, 12, and 24 h, about 100 µL of lumen fluid was collected by a syringe. The incubation solution was also measured simultaneously and the tritium count decreased by less than 10% at 24 h. Intestine was fixed in 4% PFA, examined by H&E staining, and no morphological difference was found from intestinal tissues fixed directly from killed parasites.
Preparative Thin-Layer Chromatagraphy Analysis.
Intestinal solution recovered from lumen and incubation solution were freeze-dried and reconstituted in methanol. Samples were loaded on a preparative thin-layer chromatagraphy (PTLC) plate (20 × 20 cm; Analtech) along with unlabeled TCA. PTLC plate was developed in 2:1 of CHCl3/MeOH. Lanes loaded with TCA were sprayed with 5% sulfuric acid and heated to 110 °C to develop a blue color on TCA. Sample lanes were divided into 15 individual zones and each zone was scraped separately into MeOH. All scraped samples were counted by scintillation. Each measurement was normalized to the total recovered count of the corresponding lane.
Regular and Everted Intestinal Sac Transport Assay.
The intestine of each parasite was isolated and cut into three sections (proximal, middle, and distal) and placed in aerated sea lamprey Ringer’s solution at 4 °C. Each intestinal section was cut into halves and randomly sutured into a regular and an everted sac. One segment of each section was everted with a p-1000 pipette tip. Each intestinal sac was injected with 1–2 mL aerated cold lamprey Ringer’s solution. Sacs from each animal were incubated in 500 mL aerated Ringer’s solution containing 15 μCi of [H3]TCA at 4 °C for 4 h. The initial background [H3] reading of intestinal sacs was ∼40 DPM. The solution in each intestinal sac was collected by a syringe and its H3 activity was counted after 4 h. The effects of intestinal section (proximal, middle, and distal), intestinal sac orientation, and the sex of animals on DPM were analyzed with a three-way ANOVA. To determine the effect of sac orientation when no effect of other two factors was found, a paired t test was used to compare DPM between the regular and the everted sacs within each section of each animal.
Supplementary Material
Acknowledgments
We thank Drs. J. L. Boyer and S. Y. Cai for comments on whole-animal perfusion and intestinal sac experiments; Dr. J. H. Youson for his expertise in identifying metamorphic stages; the Hammond Bay Biological Station staff for space and holding animals; the staff of the US Geological Survey, the US Fish and Wildlife Service, and the Canada Department of Fisheries and Oceans for providing animals; Dr. N. S. Johnson and C. O. Brant for animal and material supply; Dr. S. Yuge for advice in conducting intestinal sac experiment; and V. Rilington and K. Chen for their technical support. This study was supported by the Great Lakes Fishery Commission, National Institute of General Medical Sciences Grant 5R24GM83982, and National Science Foundation Grant IOB 0517491 (to W.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203008109/-/DCSupplemental.
References
- 1.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 2.Khurana S, Raufman JP, Pallone TL. Bile acids regulate cardiovascular function. Clin Transl Sci. 2011;4:210–218. doi: 10.1111/j.1752-8062.2011.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prawitt J, Staels B. Bile acid sequestrants: Glucose-lowering mechanisms. Metab Syndr Relat Disord. 2010;8(Suppl 1):S3–S8. doi: 10.1089/met.2010.0096. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang HB, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 6.Staudinger JL, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie W, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makishima M, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 9.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 10.Parks DJ, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 11.Wang RX, Sheps JA, Ling V. ABC transporters, bile acids, and inflammatory stress in liver cancer. Curr Pharm Biotechnol. 2011;12:636–646. doi: 10.2174/138920111795163986. [DOI] [PubMed] [Google Scholar]
- 12.Houten SM, Auwerx J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann Med. 2004;36:482–491. doi: 10.1080/07853890410018790. [DOI] [PubMed] [Google Scholar]
- 13.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Chardot C. Biliary atresia. Orphanet J Rare Dis. 2006;1:28. doi: 10.1186/1750-1172-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youson JH. Biliary atresia in lampreys. Adv Vet Sci Comp Med. 1993;37:197–255. [PubMed] [Google Scholar]
- 17.Youson JH. Morphology and physiology of lamprey metamorphosis. Can J Fish Aquat Sci. 1980;37:1687–1710. [Google Scholar]
- 18.Youson JH, Sidon EW. Lamprey biliary atresia: First model system for the human condition? Experientia. 1978;34:1084–1086. doi: 10.1007/BF01915363. [DOI] [PubMed] [Google Scholar]
- 19.Funaki N, et al. Apoptosis and cell proliferation in biliary atresia. J Pathol. 1998;186:429–433. doi: 10.1002/(SICI)1096-9896(199812)186:4<429::AID-PATH195>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Boomer LA, et al. Cholangiocyte apoptosis is an early event during induced metamorphosis in the sea lamprey, Petromyzon marinus L. J Pediatr Surg. 2010;45:114–120. doi: 10.1016/j.jpedsurg.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Youson JH, Potter IC. Description of the stages in the metamorphosis of the anadromous sea lamprey, Petromyzon marinus L. Can J Zool-Rev Can Zool. 1979;57:1808–1817. [Google Scholar]
- 22.Nytofte NS, et al. A homozygous nonsense mutation (c.214C->A) in the biliverdin reductase alpha gene (BLVRA) results in accumulation of biliverdin during episodes of cholestasis. J Med Genet. 2011;48:219–225. doi: 10.1136/jmg.2009.074567. [DOI] [PubMed] [Google Scholar]
- 23.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang JY. Bile acids: Regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz M, Russell DW, Dietschy JM, Turley SD. Alternate pathways of bile acid synthesis in the cholesterol 7alpha-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res. 2001;42:1594–1603. [PubMed] [Google Scholar]
- 26.Li WM, Sorensen PW, Gallaher DD. The olfactory system of migratory adult sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile acids released by conspecific larvae. J Gen Physiol. 1995;105:569–587. doi: 10.1085/jgp.105.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun SS, Scott AP, Li WM. Pheromones of the male sea lamprey, Petromyzon marinus L.: Structural studies on a new compound, 3-keto allocholic acid, and 3-keto petromyzonol sulfate. Steroids. 2003;68:297–304. doi: 10.1016/s0039-128x(02)00178-2. [DOI] [PubMed] [Google Scholar]
- 28.Haslewood GAD, Tökés L. Comparative studies of bile salts. Bile salts of the lamprey Petromyzon marinus L. Biochem J. 1969;114:179–184. doi: 10.1042/bj1140179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WM, et al. Bile Acid secreted by male sea lamprey that acts as a sex pheromone. Science. 2002;296:138–141. doi: 10.1126/science.1067797. [DOI] [PubMed] [Google Scholar]
- 30.Hoye TR, et al. Details of the structure determination of the sulfated steroids PSDS and PADS: New components of the sea lamprey (Petromyzon marinus) migratory pheromone. J Org Chem. 2007;72:7544–7550. doi: 10.1021/jo070957l. [DOI] [PubMed] [Google Scholar]
- 31.Chen HL, et al. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia. Pediatr Res. 2008;63:667–673. doi: 10.1203/PDR.0b013e318170a6b5. [DOI] [PubMed] [Google Scholar]
- 32.Demeilliers C, et al. Altered hepatobiliary gene expressions in PFIC1: ATP8B1 gene defect is associated with CFTR downregulation. Hepatology. 2006;43:1125–1134. doi: 10.1002/hep.21160. [DOI] [PubMed] [Google Scholar]
- 33.Alnouti Y. Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 34.Youson JH, Langille RM. Proliferation and renewal of the epithelium in the intestine of young-adult anadromous sea lampreys, Petromyzon marinus L. Can J Zool. 1981;59:2341–2349. [Google Scholar]
- 35.Siefkes MJ, Scott AP, Zielinski B, Yun SS, Li WM. Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biol Reprod. 2003;69:125–132. doi: 10.1095/biolreprod.102.014472. [DOI] [PubMed] [Google Scholar]
- 36.Kao Y-H, Youson JH, Holmes JA, Sheridan MA. Changes in lipolysis and lipogenesis in selected tissues of the landlocked lamprey, Petromyzon marinus, during metamorphosis. J Exp Zool. 1997;277:301–312. [Google Scholar]
- 37.Cherif S, et al. Crab digestive phospholipase: A new invertebrate member. Bioresour Technol. 2010;101:366–371. doi: 10.1016/j.biortech.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Lu ZY, Van Wagoner RM, Harper MK, Hooper JNA, Ireland CM. Two ring-A-aromatized bile acids from the marine sponge Sollasella moretonensis. Nat Prod Commun. 2010;5:1571–1574. [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida M, Murata M, Inaba K, Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc Natl Acad Sci USA. 2002;99:14831–14836. doi: 10.1073/pnas.242470599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan SJ, Steiner DF. Insulin through the ages: Phylogeny of a growth promoting and metabolic regulatory hormone. Am Zool. 2000;40:213–222. [Google Scholar]
- 41.Hernández-Sánchez C, Mansilla A, de la Rosa EJ, de Pablo F. Proinsulin in development: New roles for an ancient prohormone. Diabetologia. 2006;49:1142–1150. doi: 10.1007/s00125-006-0232-5. [DOI] [PubMed] [Google Scholar]
- 42.LeRoith D, Lesniak MA, Roth J. Insulin in insects and annelids. Diabetes. 1981;30:70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- 43.Hagey LR, Møller PR, Hofmann AF, Krasowski MD. Diversity of bile salts in fish and amphibians: Evolution of a complex biochemical pathway. Physiol Biochem Zool. 2010;83:308–321. doi: 10.1086/649966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miura R, Tanaka A, Takikawa H. Urinary bile acid sulfate levels in patients with primary biliary cirrhosis. Hepatol Res. 2011;41:358–363. doi: 10.1111/j.1872-034X.2011.00779.x. [DOI] [PubMed] [Google Scholar]
- 45.Youson JH, Ellis LC, Ogilvie D, Shivers RR. Gap junctions and zonulae occludentes of hepatocytes during biliary atresia in the lamprey. Tissue Cell. 1987;19:531–548. doi: 10.1016/0040-8166(87)90046-2. [DOI] [PubMed] [Google Scholar]
- 46.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: Pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 47.Albertson RC, Cresko W, Detrich HW, 3rd, Postlethwait JH. Evolutionary mutant models for human disease. Trends Genet. 2009;25:74–81. doi: 10.1016/j.tig.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson NS, Siefkes MJ, Li WM. Capture of ovulating female sea lampreys in traps baited with spermiating male sea lampreys. N Am J Fish Manage. 2005;25:67–72. [Google Scholar]
- 49.Chung-Davidson YW, Rees CB, Bryan MB, Li WM. Neurogenic and neuroendocrine effects of goldfish pheromones. J Neurosci. 2008;28:14492–14499. doi: 10.1523/JNEUROSCI.3589-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.