Abstract
Over the last decades, the visual-search paradigm has provided a powerful test bed for competing theories of visual selective attention. However, the information required to decide upon the correct motor response differs fundamentally across experimental studies, being based, for example, on the presence, spatial location, or identity of the target item. This variability raises the question as to whether estimates of the time taken for (i) focal-attentional selection, (ii) deciding on the motor response, and (iii) response execution generalize across search studies or are specific to the demands of a particular task set. To examine this issue, we presented physically identical stimulus material in four different search task conditions, requiring target localization, detection, discrimination, or compound responses, and combined mental chronometry with two specific electroencephalographic brain responses that are directly linkable to either preattentive or postselective levels of visual processing. Behaviorally, reactions were fastest for localization, slowest for compound responses, and of intermediate speed for detection and discrimination responses. At the electroencephalographic level, this effect of task type manifested in the timing of the stimulus- and response-locked lateralized readiness potential (indexing motor-response decisions), but not posterior contralateral negativity (indexing focal-attentional selection), component. This result demonstrates that only the stage of preattentive visual coding generalizes across task settings, whereas processes that follow focal target selection are dependent on the nature of the task. Consequently, this task set-specific pattern has fundamental implications for all types of experimental paradigms, within and beyond visual search, that require humans to generate motor responses on the basis of external sensory stimulation.
Keywords: decision making, stimulus–response translation, visual attention
Deciding upon the appropriate motor (e.g., vocal, manual) response is one of the most ubiquitous tasks posed by everyday life. In most instances, such decisions are determined by relevant or salient sensory (e.g., visual, auditory, tactile) information extracted from the multitude of stimuli present in the external world by selective-attention mechanisms (1, 2). Over the last century, a remarkable variety of experimental paradigms (e.g., visual search, dual task, task switching) has been developed to approximate such decision-making processes in the laboratory. One prominent example, which has provided a powerful test bed for competing theories of visual selective attention (3–5), is visual search. In the standard visual-search paradigm, humans or other primates are presented with a display that can contain a target item among a variable number of distractor items, with reaction times (RT) to the target and response accuracy providing the critical performance measures. Interestingly, however, when study designs are compared in terms of their underlying task settings [i.e., stimulus–response (S–R) mappings], it turns out that the information necessary to decide on the correct motor response is highly variable.
Main Categories of Visual-Search Task Settings
In principle, most visual-search studies (as well as nonsearch studies with a single stimulus) can be classified as belonging to one of three categories of task setting: detection, localization, or identification. In detection tasks, participants typically are required to discern the presence versus the absence of a target in a given display (4), while the target occurs only in a certain percentage of trials (e.g., 20, 50, or 80%). This judgment usually is realized by linking two different motor responses (e.g., left/right thumb pressing left/right mouse button) to the two possible target events (target present/absent). In localization tasks, a target item typically is present on each trial, and participants are required to indicate a spatial characteristic of the target (e.g., whether the target is positioned to the left or to the right of the vertical midline of the display). In such tasks, the S–R mapping can be spatially congruent (e.g., left-hand response for the left target position) or incongruent (e.g., left-hand response for right target position), with the latter inducing substantial RT costs (the Simon effect) (6, 7). Note that in detection and localization tasks, it is sufficient to know either whether or where a target appeared in the display. Critically, this information can be obtained without the subject’s needing to be explicitly aware of the target’s exact featural identity (e.g., that the target is a red, vertical bar). In this regard, detection and localization tasks are fundamentally different from identification tasks, in which participants are required to extract a prespecified target attribute or a set of target attributes to select the correct motor response. Identification tasks can be classified further according to (i) the depth of postselective processing (i.e., perceptual analysis of the attentionally selected target item) necessary to decide on the appropriate motor response and (ii) the linkage between target- and response-defining attributes. First, the depth of processing required to reveal the target’s identity can be highly variable across identification tasks; for instance, target identification may require knowledge about the target's dimensional identity [e.g., that the target is color defined (8)] or about its precise featural identity within the defining dimension [e.g., that the target is colored blue (9)]. Second, the search-critical attribute (e.g., the outline shape or surface color) that distinguishes the target from its surround can be dissociated from the response-critical attribute (e.g., vertical or horizontal internal stripes) that determines the motor response [i.e., a compound task (10, 11)]; alternatively, both target- and response-defining attributes can be invariantly linked to each other [i.e., a discrimination task (12)].
The diversity of task settings that have been implemented in visual-search studies raises the question whether estimates of the time taken for (i) attentionally selecting the target, (ii) deciding upon the appropriate motor response, and (iii) executing the response simply generalize across the variety of paradigms used. As detailed above, task settings differ fundamentally with regard to the information (e.g., about the presence, location, or identity of a target) required to initiate the correct motor response. Accordingly, activation patterns that have been revealed for focal-attentional selection and/or motor-response decisions/executions may be valid only for the particular task set required by the paradigm. For instance, it is plausible that when the task requires target (feature) identification rather than just detection or localization, the processes of target selection may be delayed to encode the critical information. Alternatively, the time taken for target selection may not differ for identification versus detection or localization tasks, but postselective processes of stimulus analysis and S–R mapping may be extended in identification tasks. Thus, it cannot be taken for granted that estimates of the time demands for attentional selection, S–R mapping, and response execution generalize automatically from one type of task setting to the others.
Experiment 1
The present study was designed to investigate this issue of generality by presenting physically identical stimulus materials in four different search task conditions, requiring target localization, detection, discrimination, and compound responses (see below), and examining measures of RT performance along with two specific electroencephalographic brain responses that are directly linkable to focal-attentional selection and motor-response decision/execution, respectively. Specifically, we used the simplest variant of visual search, in which the target is defined by a unique feature that singles it out from its surround, such as a red circle among blue circles; phenomenally, such items appear to “pop out” of the display. In the first task, the localization task, participants had to indicate the target’s positioning, left versus right relative to the vertical midline of the search array, with spatially congruent response button assignments (left thumb for left target locations and right thumb for right target locations). In the second task, the detection task, participants were required to discern the presence versus the absence of a target item in the array (targets appeared with a probability of 66.6%) by pressing the assigned response button (such as left thumb for the presence of the target and right thumb for the target’s absence). In the third task, the dimension discrimination task, the specific target-defining dimension was linked to a specific motor response (such as left thumb for the color-defined target and right thumb for the shape-defined target). That is, as elaborated above, participants had to identify the dimensional identity of the selected target item before they could decide on the associated motor response. The fourth task, the compound task, was designed to dissociate this linkage of target- and response-defining attributes. As in the discrimination task, the target was defined by either color or shape; however, this time, the motor response was determined independently of the target-defining dimension by the target’s orientation (such as left thumb for a vertically oriented target and right thumb for a horizontally oriented target).
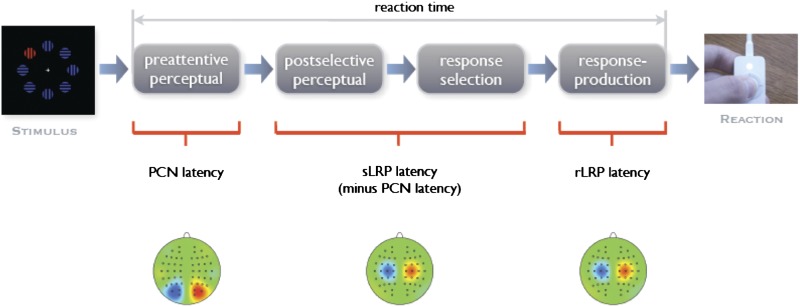
Electroencephalographically, our analyses (Fig. 1) focused first on the posterior contralateral negativity (PCN),* a well-known and extensively studied component generally thought to reflect the allocation of focal attention in visual space (15–17). In more detail, the PCN is a negatively directed deflection most prominent over visual areas contralateral to the location of an attended object, with its maximum occurring approximately in the time window 175–300 ms poststimulus. To extract this component from overlapping visual (target) selection-nonspecific event-related potentials (ERPs), it is recommended to subtract the waveforms recorded ipsilateral to the target location from the contralateral waveforms, resulting in the contralateral-minus-ipsilateral PCN difference wave. The amplitude of this PCN wave typically is interpreted as indicating the amount of attentional-resource allocation required for a task, and its latency has been regarded as marking the transition from preattentive sensory coding to the stage of focal-attentional selection (18, 19). Recently, the timing of the PCN has been shown to depend on a variety of factors, including bottom-up stimulus intensity (20) and saliency (21, 22) as well as top-down featural (23) and dimensional set (24), illustrating the flexibility of human visuocortical processing as a function of external (stimulus) and internal (system) settings.
Fig. 1.
Schematic of the present approach to temporally disentangling perceptual and response-related processing stages of the human information processing chain. In particular, by combining mental chronometry with PCN and LRP activations, the time demands of three distinct processing stages can be electrocortically dissociated: (i) preattentive perception, (ii) postselective perception and response selection, and (iii) response production. Preattentive perceptual processes of feature contrast and salience coding determine focal-attentional target selection. Postselective perceptual processes extract defining attributes of the selected item to ascertain that this item is indeed a target as well as response-critical attributes that then are mapped onto the appropriate response alternative. Finally, the motor response thus selected is executed.
Second, we concentrated on the lateralized readiness potential (LRP), which also is an extensively explored component that has been linked to the activation and execution of effector-specific motor responses (25, 26). The LRP is a negatively directed deflection that is strongest over motor areas contralateral to the side of a unimanual response, with its maximum occurring approximately in the 100-ms time window preresponse. To extract this component from overlapping motor-unspecific ERPs, waveforms recorded ipsilateral to the response side are subtracted from contralateral waveforms, resulting in the contralateral-minus-ipsilateral LRP difference wave. When computed relative to stimulus onset (i.e., stimulus-locked LRP), the LRP onset marks the start of effector-specific motor activation after the completion of response-selection processes (27). By contrast, when computed relative to response onset (i.e., response-locked LRP), the timing of the LRP onset indexes the time required to execute the motor response (14, 28).
In Experiment 1, we analyzed the PCN together with both stimulus- and response-locked LRPs to dissociate preattentive perceptual, postselective perceptual plus response selection, and response execution processes as a function of the visual-search task set (14). Because we used the same physical pop-out stimuli for all four task conditions, we expected the PCN timing and activation, indexing focal-attentional selection, to be immune to task-set manipulations. That is, we expected every task-relevant target to be selected automatically based purely on its salience (22), whether or not the task required explicit knowledge about the target’s identity. Further, we expected the timing of the stimulus-locked LRP, indexing motor-response decisions, to largely depend on the task set used, with latencies generally being increased as the target needs to be categorized more precisely. That is, motor-response decisions, and thus RTs, were predicted to be fastest for localization tasks, followed by detection and discrimination tasks, and slowest for compound tasks. A benefit for localization relative to detection tasks was predicted because the spatially congruent S–R mapping for the localization responses required in our task should speed up S–R translation at the stage of response selection [i.e., Simon effect (6)]. Differences between detection and discrimination tasks were predicted based on the assumption that the latter requires additional postselective processing to extract the target's response-critical dimensional identity. The cost for compound relative to discrimination responses is predicted to originate from the increased amount of recurrent processing required to extract the target’s response-critical featural (which in the compound task is further dissociated from the target-defining attribute), as compared with its dimensional (which, in the discrimination task, directly indicates the appropriate motor response), identity. Finally, we analyzed response-locked LRPs to discern any task-set effects at the levels of response activation and execution.
Materials and Methods.
Participants.
Thirteen observers (four female) took part in this study. Their ages ranged from 20–30 y (median 25 y). All participants had normal or corrected-to-normal vision, and none reported a history of neurological disorders. Observers were paid or received course credit for participating. One observer was excluded because of excessive eye movement artifacts.
Stimuli and study design.
The visual-search displays (Fig. 1) used in the present study consisted of eight colored shape stimuli arranged in a circular array against a black background. Each stimulus was presented equidistantly (visual angle: 3.0°) around a white central fixation point. In one third of all trials in the detection task, no target was presented; that is, displays consisted of eight homogeneous distractor items (yellow circles; CIE 0.389, 0.518, 68; radius: 1.2°). In all other trials (two thirds) of the detection task, and on every trial in the three other task conditions (localization, discrimination, and compound), one of the six lateral locations contained a singleton feature target, equally likely defined in the color (blue circles; CIE 0.213, 0.264, 68; radius: 1.2°) or shape dimension (yellow squares; CIE 0.389, 0.518, 68; 2.4° × 2.4°), together with seven homogeneous distractor items. Each stimulus outline contained a grating composed of three black bars (0.4° × 2.4°) separated by two gaps (0.3° × 2.4°), which were oriented randomly, either vertically or horizontally.
The experiment was performed in a dimly lit, sound-attenuated, and electrically shielded experimental chamber (IAC). Search displays were presented on a 17-in computer screen, mounted at a viewing distance of ∼75 cm. Each experimental session was divided into four parts, one for each of the four different search task conditions. Each task condition consisted of four blocks of 108 trials each, except for the detection task, which consisted of six blocks of 108 trials. This design ensured an equal number of target-present trials for all four task conditions. A trial started with the presentation of a white central fixation point for 500 ms, followed immediately by the search display, which was presented for 200 ms. Trials were terminated by the subject’s response or after a maximum period of 1,000 ms. In case of a response error or if no response was given within the maximum allowed RT window, the word “FEHLER” (German word for “error”) was presented centrally for 1,000 ms, signaling erroneous behavior. The subsequent intertrial interval displayed a white central fixation point for a randomly chosen duration of 950, 1,000, or 1,050 ms. Before the start of each experimental (task-set) condition, at least one block of practice was administered to familiarize subjects with the required S–R mapping. Except for the localization task, assignments of mouse-button responses were reversed halfway through the task condition. After each block, subjects received feedback concerning their mean error rate and reaction time. Each subject performed all four task conditions, with the sequence of task conditions being counterbalanced across subjects.
EEG recording and data analysis.
The EEG was digitized continuously at 1 MHz using Ag/AgCl active electrodes (actiCAP system; Brain Products) from 64 scalp sites; electrodes were placed in accordance with the 10–10 System (29). To monitor blinks and eye movements, the electrooculogram was recorded by electrodes placed, respectively, at the outer canthi of the eyes and the superior and inferior orbits. All electrophysiological signals were amplified using BrainAmp amplifiers (Brain Products) with a bandpass filter (0.1–250 Hz). During data acquisition, all electrodes were referenced to FCz and rereferenced offline to averaged mastoids. All electrode impedances were kept below 5 kΩ.
Before the EEGs were epoched, the raw data were inspected visually to remove nonstereotypical noise manually and then high-pass filtered using a Butterworth infinite impulse response filter at 0.5 Hz (24 dB per octave). Next, an infomax independent component analysis was conducted to identify components representing blinks and/or horizontal eye movements and to remove these artifacts before back-projection of the residual components. For the PCN and stimulus-locked LRP analyses, the continuous EEG then was epoched into 1-s segments relative to a 200-ms prestimulus interval, which was used for baseline correction. For the response-locked LRP analysis, the continuous EEG first was epoched into 2.2-s segments extending from 1 s before to 1.2 s after stimulus onset. Next, a baseline correction was performed based on the prestimulus interval (−200- to 0 ms). Then the signals were re-epoched into response-locked segments extending from 800 ms before to 200 ms after response onset. Only trials with correct responses and without artifacts [defined as any signal exceeding ±60 μV, bursts of electromyographic activity (the maximum voltage step allowed per sampling point was 50 μV), and activity lower than 0.5 μV within intervals of 500 ms (indicating dead channels)] were considered for further analysis on an individual-channel basis before the ERP waveforms were averaged.
The PCN component was quantified by subtracting ERPs measured at lateral parieto-occipital electrodes (PO7/PO8) ipsilateral to the target’s location from contralateral ERPs. The latencies of the PCNs were defined individually as the maximum negatively directed deflection in the time period 150–350 ms poststimulus. The amplitudes of the PCNs were computed by averaging five sample points before and after the maximum deflection. The LRP component was computed by subtracting ERPs measured at medial central electrodes (C3/C4) ipsilateral to the unimanual response side from contralateral ERPs. The onset latencies of the LRPs were determined by the jackknife-based scoring method (30), according to which the LRP onset is indicated when the LRP amplitude meets a specific criterion. As recommended by Miller et al. (30), we used 50 and 90% of the maximum LRP activation as optimal criteria for defining stimulus- and response-locked LRP onset latencies, respectively. The amplitudes of the LRPs were calculated by averaging five sample points before and after the maximum deflection obtained in the time window of 200–600 ms poststimulus for stimulus-locked LRPs and in the period 100–20 ms preresponse for response-locked LRPs.
Differences in behavioral (reaction times, error rates) and electrophysiological measures (PCN latencies/amplitudes; stimulus-locked LRP onset latencies/amplitudes; response-locked LRP onset latencies/amplitudes) were assessed by conducting separate one-way repeated-measure ANOVAs with the factor task set (localization, detection, discrimination, compound). Significant main effects were examined further by post hoc comparisons [Tukey’s honestly significant difference (HSD)].
Results and Discussion.
Behavior.
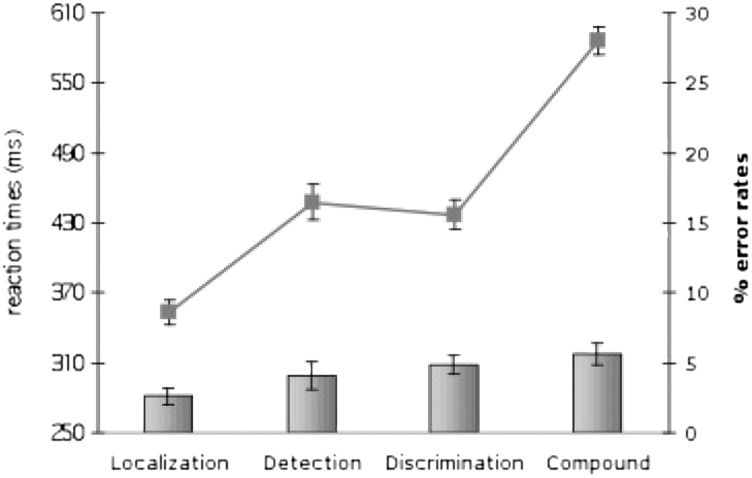
As illustrated in Fig. 2 (bars), participants generally exhibited more error-prone behavior when performing tasks that required feature-based (5.6% errors) or dimension-based (4.8% errors) identification of the target than when performing tasks that did not [localization: 2.6%; detection: 4.1%; (F(3,33) = 6.36, P < 0.001]. Post hoc contrasts confirmed that localization-response errors differed significantly from discrimination-response (P < 0.01) and compound-response errors (P < 0.001) but not from detection-response errors (P > 0.20). All other comparisons were nonsignificant. Furthermore, reaction times were highly dependent on task set [F(3,33) = 125.58, P < 0.001]. As confirmed by post hoc comparisons, RTs were fastest for localization responses (354 ms), slowest for compound responses (589 ms) and were of intermediate speed for detection (461 ms) and discrimination (441 ms) responses. Although the order of the fastest (localization) and slowest (compound) task conditions was as predicted, there was a somewhat counterintuitive, unanticipated pattern of statistically comparable latencies in the detection and discrimination responses (P > 0.34).
Fig. 2.
Reaction times (lines) and error rates (bars) as a function of task set.
When the requirements for the detection and the discrimination responses are compared, there are two main differences that, in combination, can account for this pattern of comparable response times. The first difference is in the S–R mapping: In the discrimination task the two possible target-defining dimensions (color and shape) were linked invariantly to two different motor responses (i.e., left and right thumb, respectively), but in the detection task the two dimensions were mapped onto a single response (i.e., left or right thumb). This differential S–R mapping has profound consequences when interactive stimulus- and response-based intertrial dynamics are taken into consideration. As demonstrated by a recent study (19), the stage of S–R translation is expedited markedly when both the target-defining attribute (i.e., dimension) and the motor response either repeat or change simultaneously across trials, and is prolonged when just one of the two repeats while the other one changes (see Kingstone, ref. 31). Consequently, response selection was faster overall in the present discrimination task in which dimension repetitions/changes were associated invariably with response repetitions/changes, respectively. In the present detection task, by contrast, these invariant associations were the case in only 50% of two consecutive target-present trials. In the other half of the trials, however, dimension changes were associated with response repetitions, which, when averaging all inertrial conditions together, gave rise to prolonged motor-response decisions for detection trials. The second difference is the target prevalence: The likelihood that a target would appear was much lower in the detection task (66.6%) than in the discrimination task (100%). Theoretically, this difference may have affected the perceptual evidence (or threshold) (32) required for attentional target selection (i.e., delaying PCN latencies), the motor threshold for deciding on the correct motor response (i.e., delaying stimulus-locked LRP onset latencies), or both (but see below).
PCN.
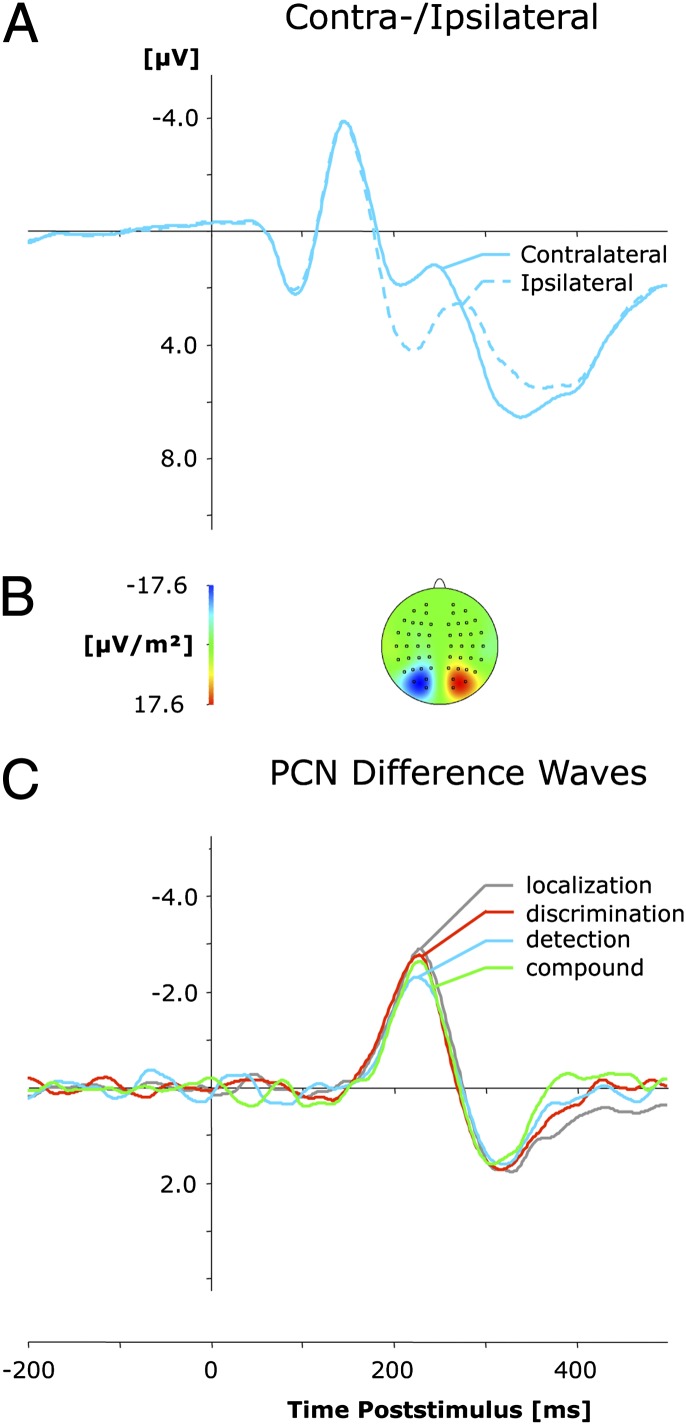
Grand average ERP waveforms are shown separately for contra- and ipsilateral targets, relative to the hemisphere of the recording electrode (PO7/PO8), in Fig. 3A. Fig. 3C presents the corresponding (contralateral-minus-ipsilateral) difference waves as a function of task set. For all four task conditions, a solid PCN was elicited, as can be seen in Fig. 3 as a more negative than positive voltage in the time window ∼180–280 ms poststimulus. As illustrated by Fig. 3C, no task type differences were discernable with regard to PCN timing, as evidenced statistically by the absence of a significant main effect of task set on PCN latencies (F < 1, P > 0.71). In contrast, a PCN of slightly lower amplitude appeared to be elicited when the task required detection (−2.77 μV) than in the other three task conditions (average, −2.97 μV). However, the main effect of task set on PCN activations was far from statistically significant (F < 1, P > 0.70).
Fig. 3.
Grand-averaged event-related brain potentials elicited in the 500-ms interval following stimulus onset, relative to a 200-ms prestimulus baseline, at electrodes PO7/PO8. (A) Waveforms contra- and ipsilateral to the singleton location. (B) Topographical map of the PCN scalp distribution at the point in time at which the difference between contra- and ipsilateral waveforms is maximal. This map was computed by mirroring the contra-/ipsilateral waves to obtain symmetrical values for both hemispheres (using spherical spline interpolation). (C) PCN difference waves obtained by subtracting ipsilateral from contralateral activity for each of the four task set conditions (localization, detection, discrimination, and compound).
In line with our expectations, this pattern confirms that any task-relevant target will be selected automatically for focal attention with the same latency, no matter whether the selected item needs to be analyzed further attentionally to extract the target’s identity (as in identification tasks) or does not (as in localization and detection tasks). Thus, this finding supports interpretations of the PCN (16) that assume automatic engagement of focal attention onto pop-out targets as long as these targets are relevant for the task to be performed and contradicts notions that assume the PCN reflects postselective processes associated with the analysis of target features at attended locations (33, 34). If the latter had been the case, the PCNs elicited in the present localization and detection tasks would have differed from those generated in identification (i.e., discrimination, compound) tasks, simply because responding in the former tasks did not require any deeper attentional analysis of the dimensional or featural identity of the selected item.
Stimulus-locked LRP.
Fig. 4 Left shows the LRP time-locked to the stimulus onset for each of the four task conditions at medial central scalp sites (C3/C4). As can be seen, the rise of the LRP occurred fastest for the localization task (199 ms), with gradually increasing onset latencies for the discrimination (262 ms), detection (306 ms), and compound tasks (374 ms). Furthermore, the strongest LRP was triggered in the localization task (−2.09 μV), followed by the discrimination task (−1.23 μV), and, with comparable signal strength, the detection (−1.03 μV) and compound tasks (−1.04 μV). Both patterns were substantiated by significant main effects of task set, for the stimulus-locked LRP onset latencies [Fc(3, 33) = 52.45, Pc < 0.001]† and amplitudes [F(3,33) = 18.94, P < 0.001]. Post hoc comparisons (Tukey’s HSD) confirmed that all four task conditions differed significantly in the stimulus-locked LRP timing (P < 0.01), but only the localization task differed reliably from the other three task conditions (P < 0.01) in the stimulus-locked LRP amplitudes.
Fig. 4.
Lateralized LRPs for each of the four task set conditions (localization, detection, discrimination, and compound) at electrodes C3/C4. (Left) LRP waves time-locked to the onset of the search array. (Center) Topographical map of the grand-averaged LRP scalp distribution at the point in time at which the difference between the waveforms contra- and ipsilateral to the unimanual motor effector reached its maximum. This map was computed by mirroring the contra-/ipsilateral waves to obtain symmetrical values for both hemispheres (using spherical spline interpolation). (Right) LRP waves time-locked to the onset of the motor action (i.e., button press).
This stimulus-locked LRP amplitude pattern is indicative of more forceful responses when participants perform a binary, congruent localization task (also see Leuthold, ref. 35, for a detailed review of LRP Simon effects). The pattern of stimulus-locked LRP onset latencies, on the other hand, mirrors exactly the “chronological” order of the four tasks (i.e., localization < discrimination < detection < compound) as revealed in the manual RTs. This finding indicates that the RT differences observed among task conditions are driven mainly by differential processing demands on the stages of S–R translation. As outlined above, the faster stimulus-locked LRP timing for localization relative to identification tasks is likely to originate from the recruitment of recurrent visual processing necessary for extracting the response-critical information about the target’s dimensional or featural identity. By contrast, the prolonged stimulus-locked LRP timing for the detection task relative to the discrimination task originates from the particular S–R mappings (affecting intertrial dynamics† and, presumably, differential target probabilities, both of which affecting the thresholds/times for initiating the appropriate motor response (see RT discussion above for more details).
Response-locked LRP.
The effects of task set on the LRPs computed relative to the onset of the response are presented in Fig. 4 Right. As can be seen, the LRP again was activated differently across task settings [F(3,33) = 6.18, P < 0.002], with the most pronounced activations for the localization task (−2.30 μV), intermediate activations for the discrimination task (−1.99 μV), and the smallest deflections for the detection (−1.76 μV) and compound (−1.71 μV) tasks. Furthermore, detection (−87 ms) and localization (−92 ms) responses were executed faster than discrimination (−102 ms) and compound (−115 ms) responses, as evidenced by a statistically significant main effect of task set on the onset latencies of the response-locked LRP [Fc(3, 33) = 4.48, Pc < 0.009]. Crucially, this pattern shows that even the latest stage in the information-processing chain, i.e., the time required simply to execute the motor response, is not readily comparable among the various visual-search tasks. Instead, even the timing at this motor stage contributes to the RT advantages for localization and detection tasks relative to identification tasks.
Experiment 2
Experiment 2 was designed to illuminate further why motor-response decisions (reflected in the stimulus-locked LRP timing) took longer in detection tasks than in discrimination tasks—a somewhat counterintuitive and unanticipated finding of Experiment 1. As elaborated above, one of the main differences between these two tasks that [in addition to differential interactive stimulus- and response-related intertrial dynamics (19)] might account for this reversal in terms of stimulus-locked LRP latencies, is the difference in target probability used in the present detection (66.6%) and discrimination tasks (100%). Recall that the timing of the PCN was statistically unaffected by task set and, thus, by target prevalence. By implication, this stimulus-locked LRP pattern must have been generated at some stage subsequent to focal-attentional target selection. In the Experiment 2, we examined whether the probability with which the target is presented affects the threshold (i.e., criterion) required to initiate the correct motor response. That is, if a target is less likely to occur in a given task, more motor activity (i.e., evidence) might be required to reach the threshold level of motor-response activation. This higher threshold, in turn, could cause a delay in the start of effector-specific motor activation. Accordingly, we expected the timing of the PCN to be unaffected by target prevalence; in contrast, we expected the delay in the stimulus-locked LRP timing would be greater when the probability that the target would appear was lower.
Materials and Methods.
Participants.
Thirteen observers (four female) took part in this study. Their ages ranged from 20–30 y (median, 25 y). All had normal or corrected-to-normal vision and reported no history of neurological disorders. Observers were paid or received course credit for participating. One observer was excluded because of excessive eye movement artifacts.
Stimuli and study design.
The visual-search displays and procedure were identical to those used in the detection task condition of Experiment 1, except that we used three different target probabilities: 20%, 50%, and 80%. To assure a comparable number of target-present trials across all three target frequency conditions, we performed 16 blocks of 72 trials for the 20% condition, six blocks of 72 trials for the 50% condition, and four blocks of 72 trials for the 80% condition, resulting in a total of 1,872 trials. The sequence of target probabilities was counterbalanced across subjects.
EEG recording and data analysis.
The recording and offline analyses of the EEG signals were the same as for the detection task of Experiment 1. Statistically, behavioral measures (reaction times, error rates) and electrophysiological measures (PCN latencies/amplitudes; stimulus-locked LRP onset latencies/amplitudes; response-locked LRP onset latencies/amplitudes) were analyzed by separate one-way repeated-measures ANOVAs with the factor target prevalence (20%, 50%, 80%). Again, significant main effects were examined further using post hoc comparisons (Tukey’s HSD).
Results and Discussion.
Behavior.
As expected, the likelihood with which a target could occur had a remarkable influence (Fig. 5) on reaction times [F(2,22) = 15.55, P < 0.001], but error rates were affected only marginally [F(2,22) = 2.77, P > 0.08]. Specifically, reactions were faster when the target was presented with a relatively high probability (372 ms) than with intermediate (429 ms) or low (419 ms) probability. As revealed by further post hoc comparisons, the reactions to high-prevalence targets were markedly faster than reactions to either intermediate- (P < 0.001) or low-prevalence targets (P < 0.001); no difference was evident between the latter two conditions (P > 0.62). These results are in line with our hypothesis that manipulating the target frequency changes the time required for simple detection responses. Theoretically, such a pattern could originate from modulated target selection times, modulated motor-response decisions, or a combination of both.
Fig. 5.
Reaction times (lines) and error rates (bars) as a function of target prevalence.
PCN.
Fig. 6 shows grand average ERP waveforms obtained at electrodes PO7/PO8 contra- and ipsilateral to the target’s location as a function of target prevalence (high, intermediate, and low) (Fig. 6A), together with the corresponding-difference waves for each of the three experimental conditions (Fig. 6B). A solid PCN was triggered in all three conditions, visible as a more negative (i.e., less positive) deflection in the time range 180–280 ms poststimulus. As can be seen in Fig. 6B, the PCN was the more pronounced when the target was less likely to appear, yielding a significant main effect of target prevalence for PCN activations [F(2,22) = 13.57, P < 0.001]. In particular, the strongest PCN was elicited for low-prevalence targets (−4.79 μV), with monotonically decreasing activations for intermediate-prevalence (−3.98 μV) and high-prevalence (−2.68 μV) targets. Subsequent post hoc comparisons confirmed that all three conditions differed significantly from each other (P < 0.01). Importantly, however, there was no timing difference between the three target-prevalence conditions, as evidenced by the absence of a significant main effect for the PCN latencies [F(2,22) = 0.66, P > 0.526].
Fig. 6.
Grand-averaged event-related brain potentials elicited in the 500-ms interval following stimulus onset, relative to a 200-ms prestimulus baseline, at electrodes PO7/PO8. (A) Waveforms contra- and ipsilateral to the singleton location. (B) Topographical maps of PCN scalp distributions for each of the three target-prevalence conditions (high, middle, and low) at the point in time when the difference between contra- and ipsilateral waveforms was maximal. (C) PCN difference waves obtained by subtracting ipsilateral from contralateral activity for each of the three target-prevalence conditions (high, middle, low).
This activation pattern of the PCN is only partly in line with the view that changing the probability of target presentation also affects the activation threshold (36, 37) that an object must reach at the level of the selection-guiding overall-saliency map to summon focal attention. In this view, one would expect that low-prevalence targets require the accumulation of more perceptual evidence, as reflected by stronger PCN activations, for shifts of focal attention to be initiated. However, at variance with the present results, differential threshold settings also should be associated with differences in the PCN timing, with latencies generally being more delayed when more perceptual evidence is required.
Another mechanism that can account for this data pattern refers to the idea that the visual system copes with changing task demands, such as those posed by differential target probabilities, through cortical amplification of the target signal’s representation (38). In particular, the system might have adapted (see Helson, ref. 39) to target events that occur rarely by “boosting” the identical incoming sensory (target) signal. This signal boost requires some context-sensitive top-down controlled amplifier that keeps track of environmental statistics and adaptively adjusts internal system settings. Thus, by strategically tuning the sensory gain for rare target signals, as indicated by magnified (rather than reduced) PCN deflections, the system can maintain or optimize the detectability of such signals at the same level as for frequent targets.‡
Stimulus-locked LRP.
LRP waveforms computed relative to stimulus onset are presented separately for each of the three (high, intermediate, and low) target-prevalence conditions at C3/C4 in Fig. 7 Left. As can be seen, target prevalence systematically modulated both amplitude [F(2,22) = 5.32, P < 0.01] and timing [Fc(2, 22) = 3.94, Pc < 0.03] of the stimulus-locked LRP: The fastest onset latencies associated with the lowest deflections were evident for high-prevalence targets (265 ms, −1.37 μV), with monotonically increasing onset latencies and activations for intermediate-prevalence (281 ms, −1.73 μV) and low-prevalence (295 ms, 2.01 μV) targets. Even though post hoc comparisons showed that only the high- and low-prevalence conditions differed significantly in terms of timing and magnitude (P < 0.05), the overall pattern clearly demonstrates that the stage of deciding upon the appropriate motor response (i.e., the response-selection stage) (Fig. 8) generally is more prolonged when the likelihood of target occurrence is lower.
Fig. 7.
LRPs for each of the three target-prevalence conditions (high, middle, and low) at electrodes C3/C4. (Left) LRP waves time-locked to the onset of the search array. (Center) Topographical maps of the LRP scalp distribution at the point in time at which the difference between the waveforms contra- and ipsilateral to the unimanual motor effector was maximal. (Right) LRP waves time-locked to the onset of the motor action (i.e., button press).
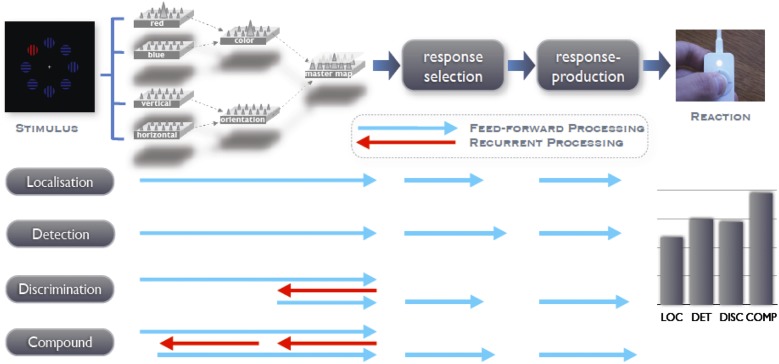
Fig. 8.
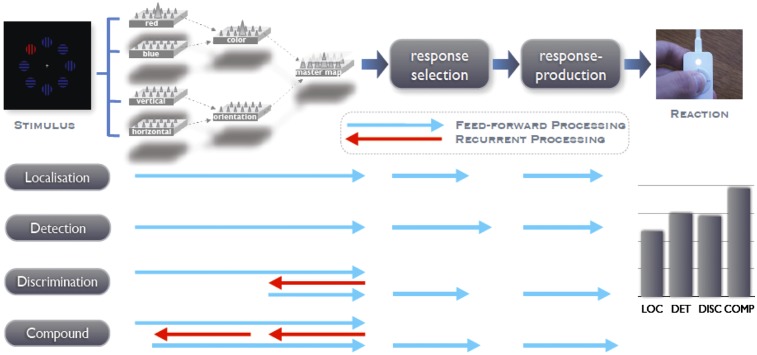
Schematic of target processing in a saliency-based processing architecture, such as assumed by the dimension-weighting account (8), as a function of task set. It is proposed that detection as well as localization of a pop-out target can be accomplished solely on the basis of feedforward processing (blue arrows). In contrast, if precise knowledge of the target’s identity is necessary to select and execute the correct motor response, as in identification tasks, recurrent processes (red arrows) are required to extract the respective information of interest. Specifically, it is suggested that the extraction of a featural target identity is more time consuming than the extraction of a dimensional target identity because two, rather than only one, feedback connections are involved (master map → dimension map → feature map).
These results provide strong support for our hypothesis that the prolonged stimulus-locked LRP latencies obtained for the detection task, compared with those for the discrimination task, in Experiment 1 are attributable—in addition to interactive stimulus- and response-related intertrial dynamics (19)—to the differential target probabilities used in these two types of task. Theoretically, such a pattern is consistent with the idea of a criterion shift (32): The criterion for initiation of a motor response might be set in accordance with the likelihood that a target will occur. In particular, in this view, the enhanced LRP activations would reflect increased demands for motor evidence as participants adopt a more conservative criterion in response to low target prevalence. Crucially, and in line with the notion of criterion shift, the accumulation of motor evidence above the threshold essential to activate the correct response took longer, as evidenced by a delayed stimulus-locked LRP timing, when the target was less likely to appear.§
Response-locked LRP.
Fig. 7 Right presents the LRP time locked to the onset of the motor response. Again the amplitude is reduced significantly [F(2,22) = 3.62, P < 0.04] for high-prevalence targets (−1.75 μV) relative to intermediate-prevalence (−2.21 μV) and low-prevalence (−2.43 μV) targets, mirroring the activation pattern observed for the stimulus-locked LRP. At variance with the stimulus-locked LRP activation pattern, however, there was no amplitude difference between intermediate- and low-prevalence targets (P > 0.05). This result indicates that the gradual decrease in stimulus-locked LRP amplitudes in response to intermediate- relative to low- prevalence targets originated from increased cross-trial variability in the intermediate condition in which both alternative responses (target-present and -absent) were equally probable. This notion is supported further by the observation that the onset latencies of the response-locked LRP were increased statistically [Fc (2, 22) = 3.48, Pc > 0.048] for intermediate-prevalence targets (−78 ms), compared with high-prevalence (−62 ms) and low-prevalence (−72 ms) targets.
These response-locked LRP activations indicate that when one of the two responses is carried out more frequently than the other (e.g., 80% versus 20%, or vice versa), then both responses will be executed more consistently, yielding overall faster response execution times. For high-prevalence targets, faster response execution results from response-based intertrial dynamics; that is, most target-present responses already are weighted, or preactivated, because of residual motor activation carried over from the previous trial (19). In contrast, for low-prevalence targets, reduced cross-trial variability in the effector-specific negativity results from the preceding positivity, which, according to the response-weighting account (RWA; ref. 21), can be taken to reflect residual activations of the overall four-times-more-prevalent, contralateral target-absent response of the preceding trial. In accordance with the RWA, this biasing of the (incorrect) contralateral effector would require a shift of motor activation across hemispheres to activate the correct target-present response above threshold, which then—once a decision to initiate this response has been reached— is carried out with less cross-trial variability.
In summary, the findings of Experiment 2 confirm that target prevalence plays a crucial role in the time required to perform a singleton detection task. In particular, we found response selection processes were prolonged by up to 30 ms when the target occurs with low, as opposed to high, target prevalence. Importantly, there was no discernible influence on the preattentive time demands to select the target focally, indicating that the effect of target prevalence is purely postselective in nature. This pattern demonstrates that reduced target probability, in addition to interactive stimulus- and response-based intertrial dynamics (19), gives rise to delayed motor-response decisions in detection, as compared with discrimination, trials.
General Discussion
By examining two specific electroencephalographic brain responses, along with mental chronometry data, the present study was designed to investigate whether the time demands for (i) selecting a target, (ii) deciding upon the appropriate motor response, and (iii) executing the response are comparable across different types of visual-search tasks or, alternatively, whether they depend on the nature of the respective task set to be implemented. These questions were approached in Experiment 1 by using the same physical stimuli in four different search conditions requiring the localization, detection, dimensional discrimination, or featural identification (compound condition) of a pop-out target. At the behavioral level, reactions were fastest for localization responses, slowest for compound responses, and of intermediate speed for detection and discrimination responses. Electroencephalographically, this effect of task type manifested in the timing of the stimulus- and response-locked LRP but not PCN component. Experiment 2 explored the impact of target prevalence on simple detection responses, revealing stronger PCN activations along with delayed stimulus-locked LRPs when target prevalence was lower, relative to higher, in a block of trials.
A Processing Architecture for the Task Set-Dependent Recruitment of Feed-Forward and Recurrent Processing in Visual Search.
This electroencephalographic dissociation clearly demonstrates that only the initial stage of preattentive visual coding, which mediates focal-attentional selection of the target object, is invariant across visual-search task settings. By contrast, further target-directed processes that occur after focal-attentional selection are highly dependent on the specific demands of the task at hand. The present set of findings is indicative of a visual processing architecture (Fig. 8) that accounts for this behavioral and electroencephalographic signature based on the task-specific recruitment of feedforward and recurrent processing (42) before the target signal enters the response-related stages of the information-processing chain.
The suggested processing architecture is derived from Guided-Search type of models (3, 43) in which the visual scene is registered initially by a set of retinotopically organized feature maps (e.g., for red, vertical) that compute the presence of feature contrast for all locations across the field. These feature-contrast signals then are combined (summed up) in dimension-specific maps (e.g., for color, shape) before being integrated by an overall-saliency, or master, map of activations. It is assumed that the activation landscape on this map guides the deployment of focal attention to the most active (map) location, which is determined in a competitive, winner-take-all process. Crucially, in these models, an active master map unit indicates, or “knows”, only that there is a feature difference at the location it represents relative to its neighboring locations but not the exact dimension or feature that gives rise to this difference. Accordingly, activity at the level of the attention-guiding master map is sufficient to indicate the presence (vs. absence) or location (e.g., left vs. right) of a feature singleton target, and the target’s signal can be transferred directly to the stages of response selection and production (as in the present localization and detection tasks).
Note that this view is substantiated by a recent behavioral study of Müller and colleagues (44), who asked their participants to report the dimensional (or, alternatively, featural) identity of the target on the very last trial of their visual-search detection experiment, that is, just after they had responded “present” to a color- or orientation-defined target. Crucially, participants could report the identity of the target only at chance level (i.e., only some 50% of the color/orientation responses were correct), supporting the idea that observers do not explicitly encode (via the engagement of recurrent processes) the target’s dimensional (or featural) identity when required only to detect its presence. However, as illustrated in Fig. 8, independently of the particular settings required by the task, any task-relevant target will be selected automatically with the same latency, a pattern clearly demonstrated by our PCN timing results (see Experiment 1).
In contrast, if more precise knowledge concerning the target’s identity is required to initiate the correct motor response, as in the present discrimination and compound tasks, recurrent processes are engaged, feeding back from the master map to hierarchically lower levels to extract the relevant stimulus attributes. According to our proposed processing model (Fig. 8), this extraction requires the operation of one feedback connection, from the master map to the dimensional level, to reveal the dimensional identity of the target item, and two even more time-consuming feedback connections, from the master map (via the dimensional level) to the featural level, to reveal the target’s featural identity before the target signal can enter the motor response-related processing stages. [See Lamme and Roelfsema (42) for a detailed review of feed-forward and recurrent connections within the visual modality.]
In brief, following the completion of search processes, we propose that the speed of deciding upon the appropriate motor response is determined by at least three factors that may differ markedly among the various task settings: first, whether (and, if so, to what depth) recurrent processing must be engaged to extract the response-critical target attribute; second, the probability with which a task-relevant target signal may occur (see Experiment 2); and third, interactive stimulus- and response-based intertrial dynamics (19).
Task Set-Dependent Motor-Response Decisions Are Not Specific to Visual Search.
In addition to their implications for the functional architecture of visual search, our findings also advance our understanding of the generality of target processing across a variety of task settings, both within and beyond the domain of visual search. In visual search, the most prominent models of search performance—Feature-Integration Theory (45); Guided-Search Model, (3) and Dimension-Weighting Account (46)—are built on reaction-time experiments, limiting their potential for unequivocally attributing observed timing effects to distinct substages of processing. As demonstrated by a recent EEG study of compound-search performance (19), experimental conditions that differ markedly with respect to the time course of distinct internal (perceptual versus response-related) processing stages can produce equivalent latencies in, and thus remain invisible to, traditional measures of reaction time. In fact, there have been several long-lasting debates concerning the origins of top-down effects [e.g., dimension-cueing effect (47, 48)], bottom-up effects [e.g., redundant-signals effect (49, 50)], and intertrial-history effects [e.g., dimension-switch effect: 8, 51)], typically without acknowledging that different processing stages might be affected differentially by varying task settings. Here we provide an unequivocal demonstration that only the initial stage of preattentive visual coding, as indexed by the timing of the PCN component, generalizes across the variety of task settings used in studies of visual (pop-out) search. In contrast, the time demands of processes that occur subsequent to focal-attentional target selection, such as deeper perceptual stimulus analysis, motor-response decision making, and response execution, are highly dependent on the demands posed by the task at hand and thus not readily comparable across task settings.
Our results also are of fundamental importance for paradigms beyond the domain of visual search: All task-dependent modulations revealed in the present study occurred after the completion of the search process (i.e., focal-attentional selection of the target object), indicating that the critical effects of task type are, in fact, independent of whether the response-defining stimulus attribute had to be searched for initially. Consequently, the revealed dynamics of task-specific, postselective processing would generalize to, and have fundamental implications for, all types of experimental paradigms [e.g., dual task (52, 53) attentional blink (54, 55), or task switching (56, 57)] that require humans, or other primates, to generate motor responses (e.g., vocal, manual) on the basis of external sensory stimulation. For instance, in the psychological-refractory-period (PRP) type of dual-task paradigms, observers are presented with two stimuli separated by a given stimulus-onset asynchrony (SOA), including SOAs in the short time range (e.g., around 100 ms). However, for paradigms such as these, the present findings imply that the processing of the second stimulus would be more delayed with deeper postselective processing demands (specified in the task set) for categorizing the first stimulus. Despite the power of behavioral paradigms devised to investigate such PRP and other effects, it could be argued that systematic analysis of multiple electroencephalographic brain responses along with RT measures, as demonstrated in the present study, would help reveal the loci of bottlenecks in human cognition.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 11086 (volume 109, number 28).
*This component traditionally has been referred to as “N2-posterior-contralateral” (N2pc). However, based on recent evidence (13, 14) that underscores the independence of this component in both timing and activation from the nonlateralized N2, we prefer the term “PCN” (instead of “N2pc”) to avoid misleading associations or interpretations.
†To corroborate that interactive stimulus- and response-based intertrial dynamics, as observed in a previous study (19), did contribute to delayed stimulus-locked LRP latencies for detection responses, relative to discrimination responses, in the present data set, we also analyzed both types of task as a function of whether the target-defining dimension or the required response, respectively, had changed across trials. As expected, no difference in stimulus-locked LRP latencies was evident for discrimination trials (267 vs. 260 ms; P > 0.92) in which dimension repetitions/changes were automatically associated with response repetitions/changes. In contrast, for consecutive target-present trials, which inherently require a response repetition in the detection task, the stimulus-locked LRP timing slowed down markedly when the target-defining dimension was changed, rather than repeated, across trials (338 vs. 267 ms; P < 0.001). This result provides evidence that intertrial dynamics did contribute to the delayed stimulus-locked LRP timing for detection trials relative to discrimination trials, as shown by the analysis of the data averaged across the different intertrial conditions for the same task setting.
‡Theoretically, refractoriness effects (40) also could have played a role in generating this PCN activation pattern. That is, neural populations involved in the selection of target objects might have been more affected in conditions of 80% (average interval, ∼2.5 s) as opposed to 20% (average interval, ∼10 s) target prevalence. However, because refractoriness effects are most pronounced for interevent intervals of 0.5–2 s and decrease only gradually thereafter [see Loveless (41)], they would have played a minor role, at best, in the present experiment.
§Note that the offset of the stimulus-locked LRP appears to occur earlier for low- than for intermediate- and high-prevalence targets (Fig. 7). To examine for this possibility, we additionally assessed stimulus-locked LRP offset latencies based on the jackknife-based scoring method (30), using 50% of the maximum LRP deflection. This analysis revealed the fastest offset latencies [Fc(2, 22) = 21.17, Pc < 0.001] for low-prevalence targets (452 ms), with monotonically increasing offsets for intermediate-prevalence (486 ms) and high-prevalence (516 ms) targets. Post hoc comparisons confirmed that all prevalence conditions differed significantly from each other (P < 0.05). Accordingly, the present stimulus-locked LRP activation effect also might originate from variance differences across conditions, leading to a somewhat more spread-out ERP wave associated with a lower peak amplitude for high target prevalence.
References
- 1.James W. The Principles of Psychology. Vol 2. New York: Holt; 1890. [Google Scholar]
- 2.Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe JM. Guided Search 2.0: A revised model of visual search. Psychon Bull Rev. 1994;1(2):202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe JM. What Can 1,000,000 Trials Tell Us About Visual Search? Psychol Sci. 1998;9(1):33–39. [Google Scholar]
- 5.Müller HJ, Krummenacher J. Locus of dimension weighting: Preattentive or post-selective? Vis Cogn. 2006;14:490–513. [Google Scholar]
- 6.Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81(1):174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- 7.Hommel B. The role of attention for the Simon effect. Psychol Res. 1993;55:208–222. doi: 10.1007/BF00419608. [DOI] [PubMed] [Google Scholar]
- 8.Found A, Müller HJ. Searching for unknown feature targets on more than one dimension: Investigating a “dimension-weighting” account. Percept Psychophys. 1996;58:88–101. doi: 10.3758/bf03205479. [DOI] [PubMed] [Google Scholar]
- 9.Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- 10.Duncan J. Visual search and visual attention. In: Posner MI, Marin OS, editors. Attention and Performance XI. Hillsdale, NJ: Erlbaum; 1985. pp. 85–106. [Google Scholar]
- 11.Pollmann S, Weidner R, Müller HJ, von Cramon DY. Neural correlates of dimension weighting. Vis Cogn. 2006;14:877–897. [Google Scholar]
- 12.Gramann K, Töllner T, Müller HJ. Dimension-based attention modulates early visual processing. Psychophysiology. 2010;47:968–978. doi: 10.1111/j.1469-8986.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 13.Shedden JM, Nordgaard CL. ERP time course of perceptual and post-perceptual mechanisms of spatial selection. Brain Res Cogn Brain Res. 2001;11(1):59–75. doi: 10.1016/s0926-6410(00)00064-1. [DOI] [PubMed] [Google Scholar]
- 14.Töllner T, Zehetleitner M, Krummenacher J, Müller HJ. Perceptual basis of redundancy gains in visual pop-out search. J Cogn Neurosci. 2011;23(1):137–150. doi: 10.1162/jocn.2010.21422. [DOI] [PubMed] [Google Scholar]
- 15.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 16.Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- 17.Hopf JM, et al. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luck SJ, et al. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Töllner T, Gramann K, Müller HJ, Kiss M, Eimer M. Electrophysiological markers of visual dimension changes and response changes. J Exp Psychol Hum Percept Perform. 2008;34:531–542. doi: 10.1037/0096-1523.34.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisson B, Robitaille N, Jolicoeur P. Stimulus intensity affects the latency but not the amplitude of the N2pc. Neuroreport. 2007;18:1627–1630. doi: 10.1097/WNR.0b013e3282f0b559. [DOI] [PubMed] [Google Scholar]
- 21.Töllner T, Zehetleitner M, Gramann K, Müller HJ. Top-down weighting of visual dimensions: Behavioral and electrophysiological evidence. Vision Res. 2010;50:1372–1381. doi: 10.1016/j.visres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Töllner T, Zehetleitner M, Gramann K, Müller HJ. Stimulus saliency modulates pre-attentive processing speed in human visual cortex. PLoS ONE. 2011;6:e16276. doi: 10.1371/journal.pone.0016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eimer M, Kiss M. Involuntary attentional capture is determined by task set: Evidence from event-related brain potentials. J Cogn Neurosci. 2008;20:1423–1433. doi: 10.1162/jocn.2008.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Töllner T, Müller HJ, Zehetleitner M. Top-down dimensional weight set determines the capture of visual attention: Evidence from the PCN component. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr231. [DOI] [PubMed] [Google Scholar]
- 25.Coles MGH. Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 26.Hackley SA, Valle-Inclán F. Which stages of processing are speeded by a warning signal? Biol Psychol. 2003;64:27–45. doi: 10.1016/s0301-0511(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 27.Leuthold H. Programming of expected and unexpected movements: Effects on the onset of the lateralized readiness potential. Acta Psychol (Amst) 2003;114:83–100. doi: 10.1016/s0001-6918(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 28.Miller J. Contralateral and ipsilateral motor activation in visual simple reaction time: A test of the hemispheric coactivation model. Exp Brain Res. 2007;176:539–558. doi: 10.1007/s00221-006-0641-1. [DOI] [PubMed] [Google Scholar]
- 29.American Electroencephalographic Society American Electroencephalographic Society. Guideline thirteen: Guideline for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- 30.Miller J, Patterson T, Ulrich R. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35:99–115. [PubMed] [Google Scholar]
- 31.Kingstone A. Combining expectancies. Q J Exp Psychol A. 1992;44:69–104. [Google Scholar]
- 32.Chun MM, Wolfe JM. Just say no: How are visual searches terminated when there is no target present? Cognit Psychol. 1996;30:39–78. doi: 10.1006/cogp.1996.0002. [DOI] [PubMed] [Google Scholar]
- 33.Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Kiss M, Van Velzen J, Eimer M. The N2pc component and its links to attention shifts and spatially selective visual processing. Psychophysiology. 2008;45:240–249. doi: 10.1111/j.1469-8986.2007.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leuthold H. The Simon effect in cognitive electrophysiology: A short review. Acta Psychol (Amst) 2011;136:203–211. doi: 10.1016/j.actpsy.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 37.Lee DK, Itti L, Koch C, Braun J. Attention activates winner-take-all competition among visual filters. Nat Neurosci. 1999;2:375–381. doi: 10.1038/7286. [DOI] [PubMed] [Google Scholar]
- 38.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 39.Helson H. Adaptation-level as frame of reference for prediction of psychophysical data. Am J Psychol. 1947;60(1):1–29. [PubMed] [Google Scholar]
- 40.Woods DL, Courchesne E, Hillyard SA, Galambos R. Recovery cycles of event-related potentials in multiple detection tasks. Electroencephalogr Clin Neurophysiol. 1980;50:335–347. doi: 10.1016/0013-4694(80)90001-2. [DOI] [PubMed] [Google Scholar]
- 41.Loveless NE. The orienting response and evoked potentials in man. In: Siddle D, editor. Orienting and Habituation: Perspectives in Human Research. London: John Wiley; 1983. pp. 71–101. [Google Scholar]
- 42.Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 43.Töllner T, Gramann K, Müller HJ, Eimer M. The anterior N1 component as an index of modality shifting. J Cogn Neurosci. 2009;21:1653–1669. doi: 10.1162/jocn.2009.21108. [DOI] [PubMed] [Google Scholar]
- 44.Müller HJ, Krummenacher J, Heller D. Dimension-specific intertrial facilitation in visual search for pop-out targets: Evidence for a top-down modulable visual short-term memory effects. Vis Cogn. 2004;11:577–602. [Google Scholar]
- 45.Treisman AM, Gelade G. A feature-integration theory of attention. Cognit Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- 46.Müller HJ, et al. Dimension-based attention modulates feed-forward visual processing. Acta Psychol. 2010;135:117–122. doi: 10.1016/j.actpsy.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Müller HJ, Reimann B, Krummenacher J. Visual search for singleton feature targets across dimensions: Stimulus- and expectancy-driven effects in dimensional weighting. J Exp Psychol Hum Percept Perform. 2003;29:1021–1035. doi: 10.1037/0096-1523.29.5.1021. [DOI] [PubMed] [Google Scholar]
- 48.Theeuwes J, Reimann B, Mortier K. Visual search for feature singletons: No top-down modulation, only bottom-up priming. Vis Cogn. 2006;14:466–489. [Google Scholar]
- 49.Feintuch U, Cohen A. Visual attention and coactivation of response decisions for features from different dimensions. Psychol Sci. 2002;13:361–369. doi: 10.1111/1467-9280.00465. [DOI] [PubMed] [Google Scholar]
- 50.Krummenacher J, Müller HJ, Heller D. Visual search for dimensionally redundant pop-out targets: Parallel-coactive processing of dimensions is location specific. J Exp Psychol Hum Percept Perform. 2002;28:1303–1322. [PubMed] [Google Scholar]
- 51.Mortier K, Theeuwes J, Starreveld P. Response selection modulates visual search within and across dimensions. J Exp Psychol Hum Percept Perform. 2005;31:542–557. doi: 10.1037/0096-1523.31.3.542. [DOI] [PubMed] [Google Scholar]
- 52.Welford AT. The 'psychological refractory period' and the timing of high-speed performance - A review and a theory. Br J Psychol. 1952;43(1):2–19. [Google Scholar]
- 53.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994;116(1):220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 54.Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- 55.Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- 56.Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cogn Psychol. 2000;41(1):211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- 57.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]