Abstract
Mutations in Wnt receptor LRP5/6 and polymorphism in Wnt-regulated transcription factor TCF7L2 are associated with dysregulation of glucose metabolism. However, it is not clear whether Wnt antagonist Dickkopf (Dkk) has a significant role in the regulation of glucose metabolism. Here, we identified small-molecule inhibitors of Wnt antagonist Dkk through molecular modeling, computation-based virtual screens, and biological assays. One of the Dkk inhibitors reduced basal blood-glucose concentrations and improved glucose tolerance in mice. This Dkk inhibitor appeared to act through DKK2 because the inhibitor exerted no additional effects on glucose metabolism in the Dkk2−/− mice. Our study of Dkk2−/− mice showed that DKK2 deficiency was associated with increased hepatic glycogen accumulation and decreased hepatic glucose output. DKK2 deficiency did not cause in increase in insulin production but resulted in increased Wnt activity and GLP1 production in the intestines. Given that the Dkk inhibitor improved glucose tolerance in a murine model of type 2 diabetes (db/db), we suggest that DKK2 may be a potential therapeutic target for treating type 2 diabetes.
Keywords: signal transduction
The Wnt family of secretory glycoproteins plays important roles in a wide range of biological and pathophysiological processes. The canonical Wnt signaling pathway regulates gene transcription by stabilizing β-catenin, which is otherwise degraded by a proteasome-mediated mechanism. This canonical Wnt-β-catenin signaling pathway is initiated by the binding of Wnt proteins to their two coreceptors, low density lipoprotein (LDL)-related protein (LRP) 5/6 and frizzled proteins. LRP5 and -6 are single transmembrane proteins that contain four YWTD-EGF repeat domains and three LDL receptor repeat domains in their extracellular domains. The frizzled proteins are seven transmembrane proteins (1–4).
Wnt signaling is regulated by a number of naturally occurring antagonists that include four Dickkopf (Dkk) molecules (5, 6). Among these four Dkk molecules, DKK1, -2, and -4 have been demonstrated to be effective antagonists of canonical Wnt signaling (7–10) by directly binding to Wnt coreceptor LRP 5/6 with high affinities (7–9). The Dkk molecules contain two conserved cysteine-rich domains (10). Previously, we and others had demonstrated that the second Cys-rich domains of DKK1 and DKK2 played a more important role in the inhibition of canonical Wnt signaling (11, 12). More recently, we solved the structure of the second Cys-rich domain of DKK2 and delineated LRP5/6-binding sites on the domain (13, 14). By Ala scan mutagenesis, we identified several amino acid residues on the third YWTD repeat domain of LRP5 as being important for binding to DKK1 and DKK2 (15). YWTD repeat domains are found in many proteins, including other lipoprotein receptors, integrins, EGF precursor, and the β-subunits of heterotrimeric G proteins (16). These domains were predicted and shown to form barrel-like structures made of six β-propellers (17–22). These DKK-interacting residues are predicted to be located at the large opening of the barrel-like structure of this YWTD repeat domain and have been confirmed by the structural studies of a DKK1/LRP6 third and fourth YWTD repeat domain complex (20–22). The complex structure also provides additional insights into the interaction of DKK with LRP6 and possible mechanisms for DKK-mediated antagonism of Wnt signaling.
Several studies have suggested the possible involvement of Wnt signaling in the regulation of glucose metabolism (23). One of the β-catenin–associated transcription factors, TCF7L2, was identified as a type 2 diabetes susceptibility gene by genome-wide association studies (24, 25). Wnt signaling stimulates proglucagon and glucagon-like peptide 1 (GLP-1) expression via TCF7L2 in enteroendocrine cells (26). In addition, a putative hypomorphic mutation of LRP6 (R611C) is associated with early onset of type 2 diabetes as well as other pathological conditions, including dyslipidemia, coronary heart disease, and osteoporosis (27). There is also mouse genetic evidence for possible involvement of Wnt signaling in glucose metabolism. The Lrp5−/− mice show impaired glucose tolerance at ages more than 6 mo, presumably because of impaired insulin production from β-cells in the pancreas (28, 29). However, given that LRP5 and -6 also bind to lipoproteins and mutations in these genes are associated with lipid phenotypes (27, 28), it is still not clear whether the effects of LRP5/6 mutations on glucose metabolism are the result of alteration in Wnt signaling.
In this article, we used a combination of chemical biological and mouse genetic approaches to demonstrate that inhibition of Wnt antagonist DKK2 reduces basal blood glucose concentrations and improves glucose tolerance in a mechanism independently of insulin production or peripheral sensitivity. DKK2 deficiency reduced hepatic glucose output and increased hepatic glycogen, which are correlated with increased Wnt activity and GLP-1 production in the intestines. In addition, treatment of the db/db mice, a murine model of type 2 diabetes, with the DKK inhibitor improved glucose tolerance. Thus, our study reveals a potential target for treating type 2 diabetes.
Results
Screen for Small-Molecule DKK Inhibitors.
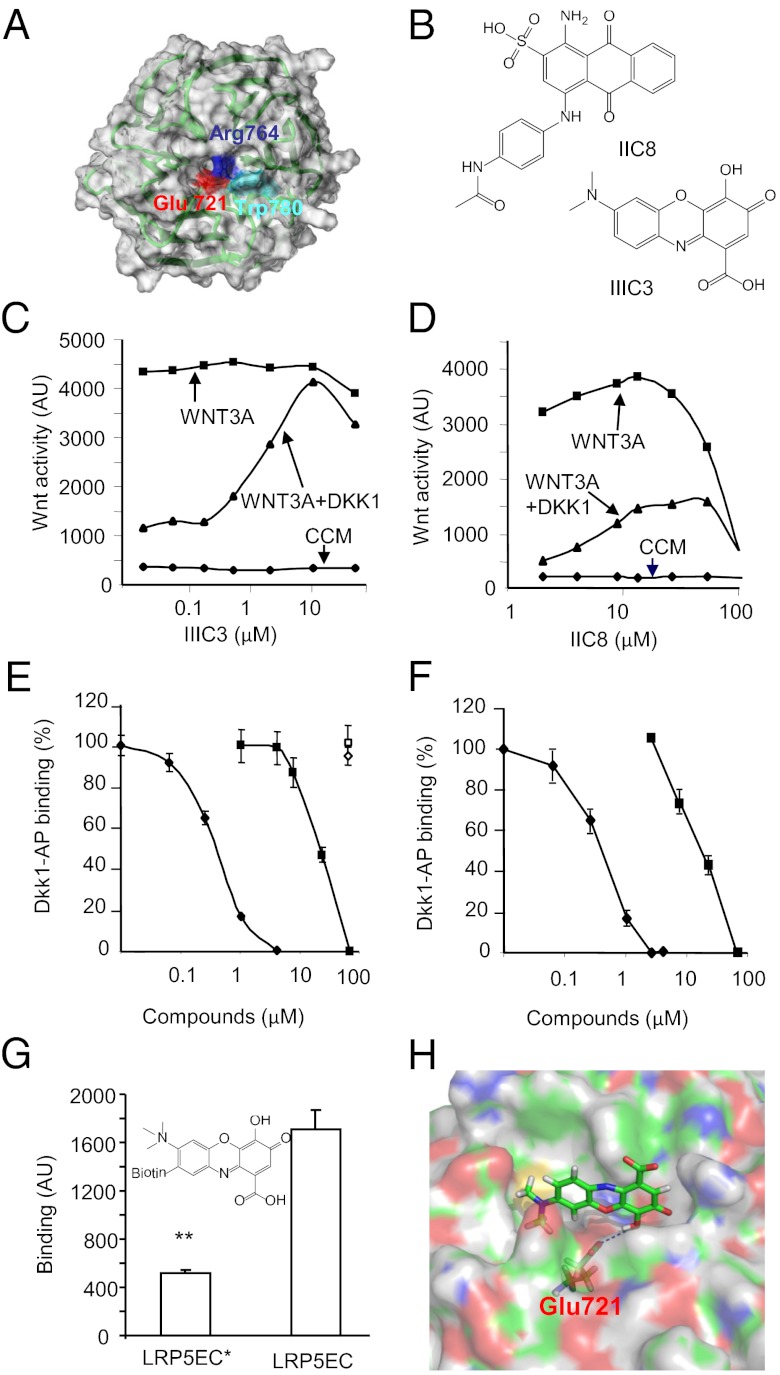
Previously, we had shown that some of the amino acid residues located at the top cavity of the β-propeller structure of the third YWTD repeat domain of human LRP5 are important for DKK binding and DKK-mediated Wnt antagonism (15). Among these residues, Glu721 (Fig. 1A), which is located at the center of the cavity, seemed to be the most critical because its mutation resulted in marked reduction in DKK1 binding to the domain (15). Recent structural studies have confirmed the importance of this residue as well as the cavity in binding of the corresponding LRP6 domain to DKK1 (20–22). Thus, small molecules, which can bind to this cavity, may disrupt the interaction between DKK and LRP5/6. We carried out virtual screens of the National Cancer Institute (NCI) database (http://129.43.27.140/ncidb2) for chemical compounds that could potentially bind to this cavity. This database includes the coordinates of 250,251 small (molecular weight < 1,000 Da) chemical compounds. The initial screen was carried out with the program UNITY (Tripos) based on a structure (Fig. 1A) of the third YWTD repeat domain of human LRP5 modeled on the crystal structure of the YWTD repeat domain of the LDL receptor (17). Of note, the modeled LRP5 structure turned out to be very similar to the crystal structures of LRP6 third YWTD repeat domains reported recently (20–22). The search query consisted of Glu721 as H-bond donor and Arg764 as H-bond acceptor with 0.3 Å tolerance and a hydrophobic center with 1.0 Å tolerance that is 3.2 Å away from Trp780 (15) pointing toward the cavity (Fig. 1A). To take the flexibility of the compounds into consideration, the Directed Tweak algorithm in the program UNITY, which allows a rapid and conformationally flexible 3D search (30), was applied. The candidate compounds from the UNITY screen (∼2,000) were then docked into the DKK-binding surface using the program FlexX (Tripos), which can quickly and flexibly dock ligands to protein-binding sites (31, 32). Human LRP5 residues Glu721, Trp863, Tyr719, Arg764, Asp887, Phe888, Gly781, Trp780, and Met890, which have been shown to be involved in DKK binding (15), were included in defining the receptor region for docking. Following the docking procedures, the compounds were ranked based on their predicted binding affinities for the DKK-binding pocket using the program Cscore. Cscore generates a relative, consensus score from the scores generated by multiple scoring functions (a Gold-like function, a Dock-like function, ChemScore, a PMF function, and FlexX). The results from Cscore were then subjected to a final manual inspection.
Fig. 1.
Identification of small-molecule Dkk antagonists. (A) The homology model of the third YWTD repeat domain of human LRP5 based on the crystal structure of the LDL receptor. The three key residues that were used in our initial receptor-based virtual screen are highlighted. (B) Two-dimensional chemical structures of DKK antagonists IIC8 and IIIC3. (C and D) Effects of IIC8 and IIIC3 on DKK1 CM-mediated antagonism of WNT3A CM. The errors are less than 5%. CCM, control conditioned medium. (E and F) Effects of IIC8 (square) and IIIC3 (diamond) on DKK1-AP binding to LRP5 (closed symbols) or Kremen1 (open symbols) (E) or its mutant lacking the first two YWTD repeat domains (F). (G) Direct binding of biotinylated IIIC3a to the LRP5 extracellular domain (LRP5EC). LRP5EC* contains three Ala substitution mutations at Asp111, Asp419, and Glu721. Data are present as means ± SEM **P < 0.01 (Student t test). (H) Docking of IIIC3 to the homology model of LRP5 third YWTD repeat domain. Glu721 presumably forms a hydrogen bond with IIIC3.
We requested 100 compounds that were ranked highest in the virtual screen and were provided with 54 by the NCI. These 54 compounds were screened for their ability to reverse DKK1-mediated inhibition of WNT3A-stimulated reporter gene activity in NIH 3T3 cells (Table S1). Compounds that could reverse DKK1 conditioned medium (CM)-mediated inhibition by more than 15% were retested, and 2 of these 54 compounds showed reproducible effects. These two compounds, designated as IIC8 and IIIC3 (gallocyanine) (Fig. 1B), were further characterized using the Wnt reporter gene and DKK binding assays. IIIC3 appeared to be more effective and more potent in reversing DKK1 CM-mediated inhibition and had a wider range of effective concentrations than IIC8. IIIC3 had an EC50 of ∼5 μM (Fig. 1C), whereas IIC8 had an EC50 of ∼15 μM (Fig. 1D). Both compounds also inhibited the binding of DKK1-alkaline phosphatase (AP) to HEK293 cells expressing the wild-type LRP5 (Fig. 1E) or a LRP5 mutant (Fig. 1F) that lacks the first two YWTD repeat domains (15) without any effect on the binding to cells expressing Kremen1 (Fig. 1 E and F). IIIC3 with an IC50 of about 3 μM is more effective than IIC8 (an IC50 of 10 μM) in the inhibition of DKK1 binding, which is consistent with its increased effectiveness in its reversal of DKK-mediated antagonism of Wnt signaling. Because of the potency and effectiveness of IIIC3, this compound and its analogs were further characterized and used in following studies.
Characterization of IIIC3 and Its Analogs.
We obtained IIIC3 and several of its analogs from Enzo Life Sciences (Fig. S1 A–D) and tested them using the Wnt reporter gene assay; IIIC3 from Enzo Life Sciences showed similar potency in the reversal of DKK1 CM-mediated Wnt antagonism as the compound obtained from the NCI (Fig. S1A). One of its analogs, IIIC3a, which has an additional methyl group on the amine of IIIC3, showed similar activity in reversing DKK antagonism, but appears to be more stable in solution (Fig. S1B). IIIC3 analogs with modification of the ketone or carboxyl group in the gallic acid part of the molecule diminished the activity (Fig. S1 C and D). IIIC3a was also compared for its effects on all of the three Wnt antagonistic Dkk isoforms; it showed similar abilities to block the inhibition of Wnt signaling by these three Dkk isoforms (Fig. S1E), whereas it showed no effects on sFRP4-mediated inhibition of Wnt signaling (Fig. S1E). In addition, IIIC3a inhibited DKK2-AP binding to cells expressing LRP5 with a similar IC50 value for inhibition of DKK1-AP binding (Fig. S1F).
We also examined the effect of IIIC3 on the binding of DKK2C to a recombinant protein containing LRP6 third and fourth YWTD repeat domains (E3E4) by using biolayer interferometry. We obtained the dissociation constant KD of 16 ± 3 nM for DKK2C and LRP6-E3E4 (Fig. S1G), which is compatible with previous reported KD value for LRP6-E3E4 with DKK1 (21). We measured the apparent KD values of DKK2C with LRP6-E3E4 as a function of IIIC3 concentration, which allowed us to calculate the inhibition constant KI of 14 ± 4 μM for IIIC3 (Fig. S1G).
Next, we wanted to determine whether IIIC3a can directly bind to the extracellular domain of LRP5. IIIC3a was biotinylated (Fig. 1G) and bound to a surface in wells of a multiwell plate via streptoavidin that was covalently linked to the wells. The biotinylated IIIC3a, which show a similar efficacy to reverse DKK2-mediated Wnt antagonism (Fig. S1H), could capture the AP-LRP5 extracellular domain fusion protein from the conditioned medium (Fig. 1G). Importantly, mutation of LRP5 extracellular domain residues Glu721 to Ala led to significant reduction in the binding to biotinylated compound (Fig. 1G). Glu721 was used in our virtual screen calculations, and its carboxyl group presumably forms a hydrogen bond with the hydroxyl group of IIIC3 based on the docking model (Fig. 1H). The docking model also predicted that the Glu721 to Ala mutation markedly reduced the affinity for IIIC3a, which is consistent with the results in Fig. 1G. We also modeled the binding of IIIC3 to LRP6-E3 based on the DKK1 and LRP6-E3E4 complex structure (20). As shown in Fig. S1I, IIIC3 binds to the same surface to which DKK1 also binds. Thus, these results taken together support the conclusion that IIIC3 directly binds to the LRP5/6 YWTD repeat domains to specifically compete with DKK.
Effects of Dkk Inhibitors on Bone Formation.
DKK1 is highly expressed in bones and may be an important negative regulator for bone formation by antagonizing canonical Wnt signaling (33–37). Therefore, we tested if IIIC3 could stimulate bone formation in mice. We first used a local bone formation assay. IIIC3, control vehicle, or positive control (b-FGF, 12.5 μg⋅kg⋅d) were injected into the subcutaneous tissue over the right side of the calvaria of 6-wk-old CD-1 mice three times a day for 5 d. Calvarias were collected 22 d after the first injection and fixed for sectioning. As shown in Fig. S2A, new bones were found in both b-FGF and IIIC3 treated calvarias; IIIC3 appeared to be as effective as FGF in stimulating bone formation (Fig. S2B). In a separate experiment, the dosage effect of the compound on bone formation was assessed. In addition, the effect of WNT3A was tested; IIIC3 dosage-dependent increases as well as WNT3A-stimulated increase in bone formation were observed (Fig. S2C). However, intraperitoneal administration of IIIC3 at 3.5 or 7.0 mg⋅kg⋅d did not meaningfully change whole-body bone mineral density (BMD), even though the group receiving 3.5 mg⋅kg⋅d IIIC3 showed statistically significant increases in BMD by 3% at the end of treatment (Fig. S2D). The bone histomorphometric analysis of these mice receiving vehicle or 3.5 mg⋅kg⋅d did not revealed any significant difference between these two groups of mice. We also tested IIIC3a, and its treatment did not result in significant changes in BMD. However, we noticed that the mice treated with IIIC3 (Fig. S2E) had significant lower blood-glucose concentrations, which was revealed by routine blood tests as part of toxicity monitoring required by the protocol. This fortuitous finding suggests a possible involvement of Dkk in the regulation of glucose metabolism.
Effect of DKK2 Deficiency on Glucose Metabolism.
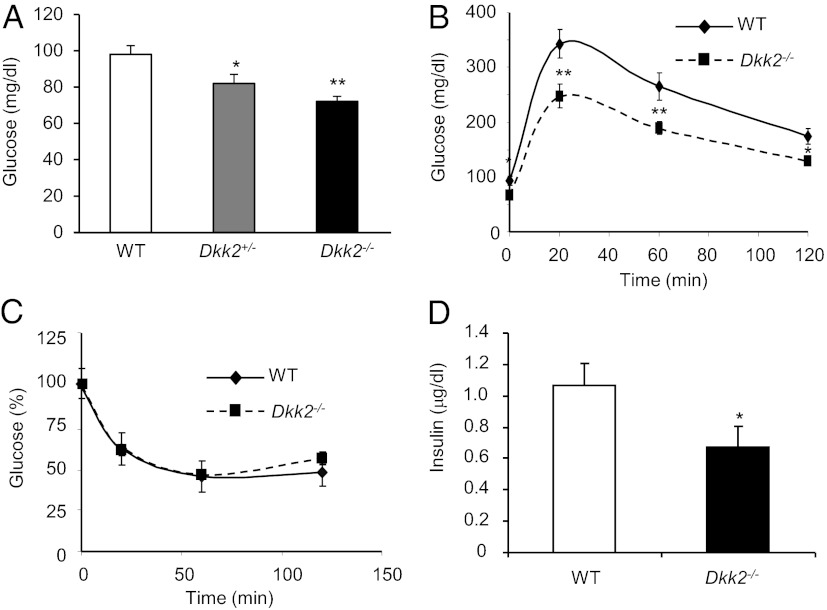
We previously generated a mouse line that lacks DKK2 (33). We measured blood-glucose concentrations of DKK2-deficient mice and their wild-type littermates on chow diet after 16-h fasting. Both male (Fig. 2A) and female (Fig. S2F) Dkk2−/− mice had significantly lower blood-glucose concentrations than their respective wild-type littermate controls. Even Dkk2+/− had significantly lower blood-glucose concentrations than the wild-type controls (Fig. 2A). There were also significant differences between wild-type and Dkk2−/− mice in the blood-glucose concentrations after 2-h fasting (Fig. S2G). We performed the glucose tolerance test (GTT) and insulin tolerance test (ITT). Dkk2−/− mice showed improved glucose tolerance because they had significantly lower blood-glucose concentrations than the wild-type mice at every test point in the GTT (Fig. 2B). There were, however, no significant differences between these mice in the ITT (Fig. 2C). In addition, Dkk2−/− mice had lower plasma insulin concentrations than wild-type mice (Fig. 2D). Taken together, these results suggest that DKK2 deficiency does not have an apparent effect on insulin sensitivity or production.
Fig. 2.
Effects of DKK2 deficiency on glucose metabolism. (A) Basal blood-glucose concentrations. Dkk2−/− mice and wild-type heterozygous littermates on chow diet (10-wk-old, male) were fasted for 16 h before their blood-glucose concentrations were determined by a glucometer. (B and C) GTT and ITT. Dkk2−/− mice and wild-type littermates (4-mo-old, male) were subjected to a GTT or ITT. Blood-glucose concentrations were determined at indicated time points. The percentages of glucose concentrations were calculated based on the glucose concentration at time 0 for ITT. (D) Plasma insulin concentrations. Dkk2−/− mice and wild-type littermates were fasted for 16 h, and their plasma insulin concentrations were determined. Data are presented as means ± SEM *P < 0.05; **P < 0.01 (Student t test, n > 12).
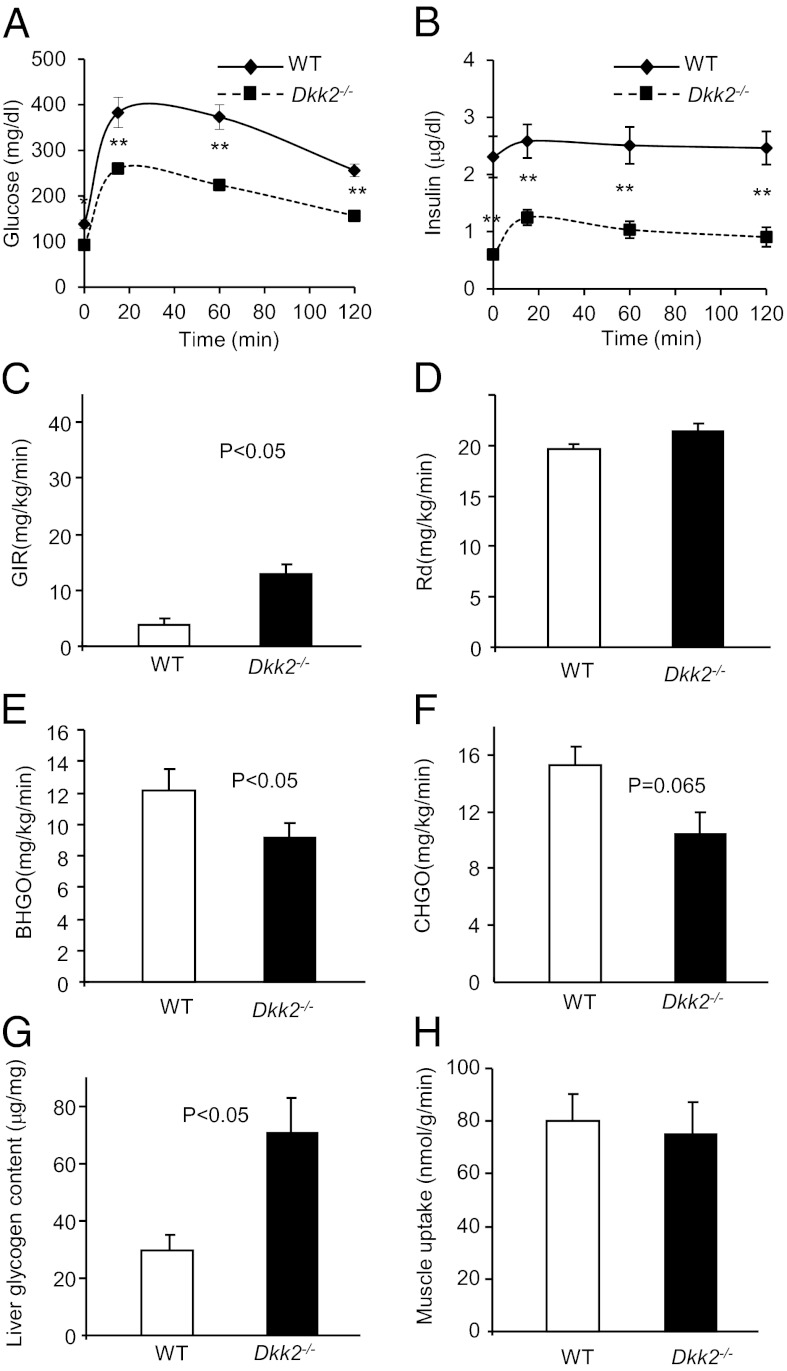
We performed hyperinsulinemic-euglycemic clamp studies using wild-type and Dkk2−/− mice on regular chow, but did not observe significant changes, even though some trends were observed. We postulated that there may be greater differences in glucose metabolism between these mice on high-fat diet (HFD). Although HFD-fed Dkk2−/− mice showed no differences in the body weight, food intake, fat mass, energy expenditure, muscle mass, or water intake from the wild-type mice (Fig. S3 A–F), they had significantly lower fasting blood-glucose concentrations than the wild-type mice (Fig. 3A). These Dkk2−/− mice also exhibited significantly improved glucose tolerance (Fig. 3A). In addition, the plasma insulin concentrations of these mice were also significantly lower than the wild-type mice (Fig. 3B). The hyperinsulinemic-euglycemic clamp studies revealed that the HFD-fed Dkk2−/− mice required a significantly higher glucose infusion rate (Fig. 3C) to maintain euglycemia during the clamp. However, the whole-body glucose uptake was similar to the wild-type mice (Fig. 3D). Instead, the Dkk2−/− mice had significantly lower in the basal, fasted state (Fig. 3E) and a trend for lower (Fig. 3F) endogenous glucose production during the hyperinsulinemic-euglycemic clamp than the wild-type mice. These Dkk2−/− mice also had significantly higher hepatic glycogen concentrations than the wild-type mice (Fig. 3G). Together with the observation that DKK2 deficiency did not significantly affect muscle uptake of glucose (Fig. 3H), we concluded that DKK2 might regulate glucose metabolism primarily through increasing glycogen accumulation in the liver without significantly altering peripheral insulin sensitivity.
Fig. 3.
Glucose metabolism in DKK2-deficient mice on HFD. (A and B) GTT and plasma insulin concentrations. Dkk2−/− and wild-type littermate male mice at 8 wk old were fed with HFD for 6 wk and subjected to GTT. Blood-glucose concentrations (A) and plasma insulin concentrations (B) were determined at indicated timepoints. Data are presented as means ± SEM *P < 0.05; **P < 0.01 (Student t test, n > 12). (C–H) Hyperinsulinemic-euglycemic clamp experiment. Dkk2−/− mice and WT littermates (4-mo-old, male) were fed with high fat diet for 6 wk and subjected to the hyperinsulinemic-euglycemic clamp studies. GIR, Glucose infusion rate; Rd, Glucose disposal rate; BHGO, basal hepatic glucose output; CHGO, clamp hepatic glucose output. Data are presented as means ± SEM n > 6, Student t test.
To further characterize the mechanisms for DKK2 to regulate hepatic glucose output, we examined the expression of phosphoenolpyruvate carboxylase (Pepck), glucose-6-phosphatase (G6pc), and pyruvate carboxylase (Pcx), the genes encoding key enzymes in glucose output regulation, and found no difference in their expression between wild-type and Dkk2−/− livers (Fig. S4 A–C). We also examined the hepatic expression of several other genes related to glycogen metabolism, including PTG (protein target to glycogen, Ppp1r3), liver glycogen phosphorylase (Pygl), glycogen synthase 2 (Gys2), and phosphorylase kinase α2 (Phka2). No significant differences between wild-type and Dkk2−/− samples were observed except for Ppp1r3. The expression of Ppp1r3 was modestly increased in Dkk2−/− liver samples (Fig. S4D).
DKK2 Deficiency Increases Intestinal Wnt Signaling and Plasma GLP-1 Concentration.
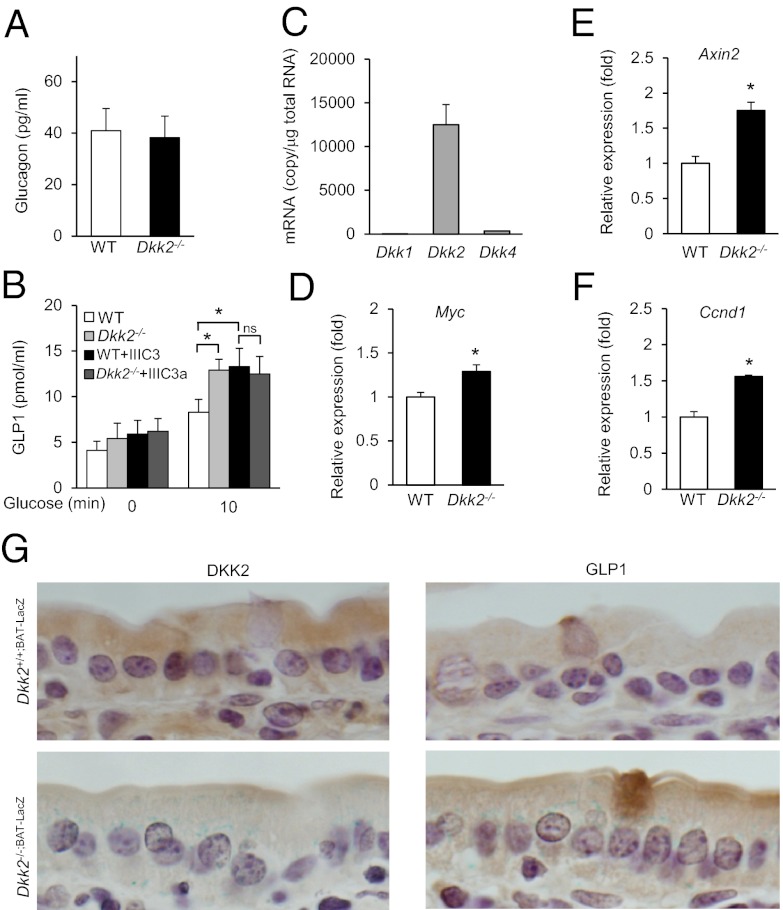
Glucagon and GLP-1 are two hormones that can regulate hepatic glucose output independently of insulin (38, 39). We compared the plasma contents of these two hormones between wild-type and Dkk2−/− mice. Although there were no significant differences in the glucagon contents, there were higher GLP-1 concentrations in Dkk2−/− than wild-type plasma (Fig. 4 A and B). Given that Wnt-β-catenin signaling has been shown to stimulate GLP-1 production in enteroendocrine cells (26), we looked into the possibility that DKK2 deficiency may lead to an increase in Wnt activity in the intestine. We first examined the expression levels of the three Wnt antagonistic Dkk genes in intestines using quantitative RT-PCR analysis. The expression of Dkk2 is markedly higher than Dkk1 or Dkk4 (Fig. 4C), suggesting that DKK2 is a major DKK expressed in mouse intestines. In addition, the expression of several Wnt target genes, including Axin2, Myc, and Ccnd1, was elevated in the Dkk2−/− intestines compared with that in the wild-type intestines (Fig. 4 D–F), suggesting there may be an increase in canonical Wnt signaling activity in the Dkk2−/− intestines. To further characterize the effects of DKK2 deficiency on Wnt-β-catenin signaling and GLP-1 production, we cross Dkk2−/− mice with a Wnt-β-catenin reporter mouse line [B6.Cg-Tg(BAT-lacZ)3Picc/J] to generate Dkk2−/−;BAT-LacZ mice. The intestines were stained for LacZ activity in situ, followed by histological sectioning. LacZ staining was readily visible in the intestinal epithelial cells from Dkk2−/−;BAT-LacZ mice, but not from Dkk2+/+;BAT-LacZ mice (Fig. 4E), suggesting that there was elevated Wnt-β-catenin signaling in Dkk2−/− intestinal epithelial cells. In addition, DKK2 protein was detected in the intestinal epithelial cells of wild-type, but not Dkk2−/−, samples (Fig. 4E). We also detected GLP-1+ enterocytes in both DKK2 wild-type and null intestines (Fig. 4E). In Dkk2−/− intestines, the GLP-1+ enterocytes were also LacZ+ (Fig. 4E). Thus, DKK2 deficiency appeared to be associated with increased Wnt-β-catenin signaling activity in the intestinal enterocytes, including those producing GLP-1, and plasma GLP-1 concentrations.
Fig. 4.
Plasma glucagon and GLP-1 measurement and Wnt signaling in the intestines. (A and B) Plasma glucagon and GLP-1 contents in 4-mo-old Dkk2+/+ and Dkk2−/− mice. Data were presented as mean ± SEM *P < 0.05 (Student t test, n = 7–12). (C–F) Quantitative RT-PCR analysis of gene expression. The mRNA concentrations of Dkk1, Dkk2, and Dkk4 in wild-type mouse intestines (C) and relative expression of Wnt target genes in the intestines of Dkk2−/− mice and WT littermates (D–F) are presented as means ± SEM *P < 0.05 (Student t test, n > 3). (G) Intestinal segments from Dkk2−/−;BAT-LacZ and Dkk2+/+;BAT-LacZ mice were stained for LacZ activity (blue) in situ, followed by sectioning and immunostaining (brown). (Magnification: 100×.)
Dkk Inhibitor’s Effect on Blood Glucose Depends on DKK2.
Because both treatment of the Dkk inhibitor IIIC3 or IIIC3a and DKK2 deficiency caused reduction in blood-glucose concentrations, we tested whether the Dkk inhibitors may regulate glucose metabolism by antagonizing DKK2. To determine the contribution of DKK2 to IIIC3a’s effect on blood glucose, we treated Dkk2−/− and their wild-type littermates with IIIC3a. After 24-d treatment of IIIC3a, the mice were subjected to a GTT. IIIC3a treatment of wild-type mice resulted in significantly improved glucose tolerance (Fig. S5A). However, the compound had little effect on glucose tolerance of Dkk2−/− mice (Fig. S5A), even though metformin, which acts through an inhibition of hepatic gluconeogenesis (40), could (Fig. S6A). The similar effects of IIIC3a on plasma GLP-1 concentrations of wild-type and Dkk2−/− mice were also observed (Fig. 4B). In addition, IIIC3a, like DKK2 deficiency, did not significantly alter insulin production or sensitivity (Fig. S5 B and C). Moreover, there were significantly higher contents of hepatic glycogen (Fig. S6B) in IIIC3a-treated mice, which are consistent with the findings made with Dkk2−/− mice. Therefore, we conclude that the effect of gallocyanine compounds on glucose metabolism may be at least in part mediated through DKK2.
Dkk Inhibitor Improves Glucose Tolerance in db/db Mice.
We next investigated whether IIIC3a was able to improve glucose tolerance in the db/db mice, a type 2 diabetic model. The db/db mice were administered 1.5 mg⋅kg⋅d of IIIC3a or vehicle control daily via intraperitoneal injection, starting at the age of 6 wk. Four weeks later a GTT was performed. IIIC3a treatment not only lowered basal fasting blood-glucose concentrations, but also significantly improved glucose tolerance (Fig. S7A). Although IIIC3a treatment did not significantly alter insulin levels in the blood (Fig. S7B), the treatment appeared to improve insulin sensitivity (Fig. S7C). Because IIIC3 treatment did not alter insulin sensitivity in non-db/db mice, the compound may improve insulin sensitivity indirectly by delaying the development of insulin resistance in the db/db mice. Therefore, these results together suggest that DKK may be a potential target for treating type 2 diabetes.
Discussion
In this article we used chemical biological and genetic approaches to investigate the role of Wnt antagonist Dkk in the regulation of glucose metabolism led by our serendipitous finding that small molecule DKK inhibitors reduce blood-glucose concentrations. Because the DKK inhibitor IIIC3a showed no additional effect on fasting blood-glucose concentrations or glucose tolerance in mice lacking DKK2, the inhibitor may in large part act through the inhibition of DKK2 in its regulation of glucose metabolism. The study of DKK2-deficient mice revealed that DKK2 may regulate glucose metabolism by regulating hepatic glucose output, probably through increasing hepatic glycogen concentrations.
We have examined the expression of a number of glucose metabolism and output-related genes in the liver. There were no significant difference in their expression between wild-type and Dkk2−/− livers except that of Ppp1r3. Ppp1r3 encodes a regulatory subunit of protein phosphatase 1 that plays an important role in glycogen metabolism and binds directly to glycogen synthase, phosphorylase, and phosphorylase kinase (41). Thus, up-regulation of Ppp1r3 can contribute to the elevated accumulation of glycogen in the livers lacking DKK2. However, it is not clear whether Wnt-β-catenin signaling directly regulates Ppp1r3 expression. In mouse livers, like intestines, DKK2 is expressed at significantly higher levels than DKK1 or DKK4. However, we could not detect elevated LacZ activity in the livers of Dkk2−/−;BAT-LacZ mice. In contrast, DKK2 deficiency is associated with elevated Wnt-β-catenin signaling activity in the intestinal epithelial cells, including those producing GLP-1 in Dkk2−/− mice and increased plasma concentrations of GLP-1. GLP-1 is a hormone that reduces hepatic glucose output through mechanisms, including the stimulation of hepatic glycogen synthesis. Thus, it is reasonable to postulate that intestines may be an important site of action of DKK2 in its regulation of glucose metabolism. Although GLP-1 can stimulate insulin and inhibit glucagon production in pancreases, we interpret the lack of an increase in insulin levels or a decrease in glucagon levels in DKK2-null mice to suggest that the effects of increased GLP-1 on the pancreas may be counteracted by the effect of reduced blood-glucose levels on insulin and glucagon production in the pancreas. Our study also does not exclude other possible mechanisms of action, in which DKK2 may even act through other tissues or organs. Zeve et al. recently showed that increases in Wnt activity in fat progenitors improved glucose metabolism through a humoral factor that acts on muscles (42).
The failure for these Dkk inhibitors to increase BMD in mice may be attributed to many possible factors. In addition to pharmacokinetic factors, complicated effects of DKK isoforms on bone biology may contribute to this lack of effect. Although DKK1 inhibition is associated with an increase in BMD in mice (35, 36, 43), DKK2 deficiency resulted in osteopenia because of its role in osteoblast terminal differentiation (37). Thus, specific targeting of DKK1 may be required for increasing BMD.
Protein–protein interactions are generally considered to be difficult to disrupt by small-molecule compounds. In this study, we demonstrated a screening approach that combines molecular modeling, computation-based virtual screening, and biological assays to identify small molecule compounds that can efficiently disrupt Dkk-LRP5 interaction. Although the virtual screening technique has a long history, it has struggled to meet its initial promise because of a number of difficulties. The field has recently had important successes, but mostly in identifying inhibitors for enzymes or template-based searches (44). In this study, we carried out a de novo initial virtual screen for chemicals based only on a predicted structure of a target without any small-molecule template. We were able to identify effective compounds from physical screening of merely 54 compounds. The difficulty in disruption of protein–protein interactions is primarily attributed to the large interaction surfaces. However, the key sites for Dkk interaction in the YWTD repeat domains are located in the cavities formed by the β-propellers. The cavities are also ideal for high-affinity binding of small molecules, which may explain our current success and a previous success (45) in targeting YWTD-repeat domains for disruption of protein–protein interactions. It is of great interest to determine if the approach is applicable to other types of targets. Nevertheless, the approach has allowed us to identify effective Dkk inhibitors that helped us to identify Dkk, more specifically DKK2, as being an important regulator for glucose metabolism in mice. In addition, these compounds may provide useful tools for therapeutic target validation and basic research in Wnt signaling. Given that DKK2 deficiency does not appear to cause spontaneous tumor formation (up to 12 mo of age) or overt increases in β-catenin levels in contrast to APC or β-catenin stabilization mutations, we speculate that a modest increase in Wnt activity by inhibiting Wnt antagonists may not pose a major risk for increasing cancer incidents. Therefore, DKK2 may be a potential therapeutic target for treating type 2 diabetes.
Materials and Methods
The homology models of the third YWTD repeat domains of LRP5 were built with the software ICM (Molsoft) by using the structure of LDL receptor YWTD-EGF domain (PDB ID, 1IJQ) (17) as the template. The UNITY module of the SYBYL software package (Tripos) was then used to screen the NCI small-molecule 3D database for chemical compounds that could fit into the DKK binding groove of the third YWTD domain of LRP5. The candidate compounds obtained from the UNITY search were docked into the binding groove by using the FlexX module of SYBYL (Tripos). An SGI Octane computer was used to carry out all of the computational studies.
IIIC3 was docked into the third YWTD repeat domain by ICM for further optimization. Its binding energy, re-evaluated with an implicit solvent model, is −23.46 kcal/mol. However, when Glu721 is mutated to Ala, the binding energy increases to −19.90 kcal/mol, which may result from the loss of a hydrogen bond between the carboxyl group of Glu721 and the hydroxyl group of IIIC3.
Methods for DKK binding and evaluation of bone formation and glucose metabolism are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Michelle Orsulak for technical help and Dr. Taosheng Chen for kindly providing the FortéBio Octet RED instrument for Bio-Layer Interferometer experiments. This work is supported by National Institutes of Health Grants CA132317 and CA139395 (to D.W.), R01 DK-40936 (to G.I.S.), U24 DK059635 (to V.T.S. and G.I.S.), and GM081492 and CA21765 to (J.J.Z.), the American Lebanese Syrian Associated Charities (to J.J.Z), and a Veterans Affairs Merit (to V.T.S.).
Footnotes
Conflict of interest statement: X.L. and D.L. are affiliated with Enzo Therapeutics, a subsidiary of Enzo Biochem. Enzo Life Sciences, another subsidiary of Enzo Therapeutics, has provided products used in this study.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205015109/-/DCSupplemental.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monaghan AP, et al. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 6.Krupnik VE, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 7.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 8.Semënov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 9.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Mao J, Sun L, Liu W, Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem. 2002;277:5977–5981. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- 12.Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, et al. Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J Biol Chem. 2008;283:23364–23370. doi: 10.1074/jbc.M802375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, et al. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem. 2008;283:23371–23375. doi: 10.1074/jbc.M802376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: A common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 17.Jeon H, et al. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001;8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- 18.Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 19.Takagi J, Yang Y, Liu JH, Wang JH, Springer TA. Complex between nidogen and laminin fragments reveals a paradigmatic beta-propeller interface. Nature. 2003;424:969–974. doi: 10.1038/nature01873. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, et al. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, et al. Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev Cell. 2011;21:848–861. doi: 10.1016/j.devcel.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn VE, et al. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev Cell. 2011;21:862–873. doi: 10.1016/j.devcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin T. The WNT signalling pathway and diabetes mellitus. Diabetologia. 2008;51:1771–1780. doi: 10.1007/s00125-008-1084-y. [DOI] [PubMed] [Google Scholar]
- 24.Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 25.Scott LJ, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 27.Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujino T, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rulifson IC, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst T. Flexible 3D searching—The directed tweak technique. J Chem Inf Comput Sci. 1994;34:190–196. [Google Scholar]
- 31.Hoffmann D, et al. Two-stage method for protein-ligand docking. J Med Chem. 1999;42:4422–4433. doi: 10.1021/jm991090p. [DOI] [PubMed] [Google Scholar]
- 32.Kramer B, Rarey M, Lengauer T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins. 1999;37:228–241. doi: 10.1002/(sici)1097-0134(19991101)37:2<228::aid-prot8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Li X, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 34.Williams BO, Insogna KL. Where Wnts went: The exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald BT, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glantschnig H, et al. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem. 2010;285:40135–40147. doi: 10.1074/jbc.M110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Hamdah R, et al. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;13(Suppl 1):118–125. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest. 2010;120:2267–2270. doi: 10.1172/JCI43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- 42.Zeve D, et al. Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab. 2012;15:492–504. doi: 10.1016/j.cmet.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 44.Shoichet BK. Virtual screening of chemical libraries. Nature. 2004;432:862–865. doi: 10.1038/nature03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonacci TM, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.