Abstract
Prenatal life encompasses a critical phase of human brain development, but neurodevelopmental consequences of normative differences in prenatal growth among full-term pregnancies remain largely uncharted. Here, we combine the power of a within-monozygotic twin study design with longitudinal neuroimaging methods that parse dissociable components of structural brain development between ages 3 and 30 y, to show that subtle variations of the in utero environment, as indexed by mild birth weight (BW) variation within monozygotic pairs, are accompanied by statistically significant (i) differences in postnatal intelligence quotient (IQ) and (ii) alterations of brain anatomy that persist at least into late adolescence. Greater BW within the normal range confers a sustained and generalized increase in brain volume, which in the cortical sheet, is specifically driven by altered surface area rather than cortical thickness. Surface area is maximally sensitive to BW variation within cortical regions implicated in the biology of several mental disorders, the risk for which is modified by normative BW variation. We complement this near-experimental test of prenatal environmental influences on human brain development by replicating anatomical findings in dizygotic twins and unrelated singletons. Thus, using over 1,000 brain scans, across three independent samples, we link subtle differences in prenatal growth, within ranges seen among the majority of human pregnancies, to protracted surface area alterations, that preferentially impact later-maturing associative cortices important for higher cognition. By mapping the sensitivity of postnatal human brain development to prenatal influences, our findings underline the potency of in utero life in shaping postnatal outcomes of neuroscientific and public health importance.
Prenatal life encompasses a foundational and universally critical phase of human brain development. Consequently, research aimed at understanding how prenatal influences play out in later life cannot only illuminate mechanisms of fundamental importance to basic developmental neuroscience, but may also have public health implications.
Exquisite sensitivity of the developing brain to prenatal environmental insults has been well established through experimental animal models (1). In humans, where experimental approaches are not feasible, numerous observational studies have linked frank insults during prenatal life [as indexed by premature delivery (2); abnormally low birth weight for gestational age (3); and maternal exposure to radiation (4) or starvation (5)], to altered postnatal brain development. However, by virtue of their relative rarity, such frank prenatal insults may be less impactful at the population level than more subtle variations of the in utero environment occurring among the uncomplicated pregnancies from which most people are born (6). Evidence that subtle alterations of the prenatal environment may have meaningful consequences for postnatal development in humans can be found in large-scale epidemiological studies that use birth weight (BW) as a global proxy measure of uterine “optimality.” These studies find that lowered BW, even among people born full term and at weights considered appropriate for gestational age, is associated with an elevated risk for multiple common forms of mental illness (including schizophrenia and affective and anxiety disorders) (7) and reduced cognitive abilities (8). Such data raise the possibility that normative BW variation may be associated with detectable differences in postnatal human brain development that persist beyond childhood. This hypothesis is yet to be tested, despite its potential to inform both developmental neuroscience and public health policy.
Our current understanding of the relationship between BW variation and later brain development in humans largely stems from cross-sectional neuroimaging studies that compare healthy controls born at full-term with weights appropriate for gestational age to individuals born small for gestational age (BW below the 10th centile for gestational age) (3, 9, 10) or at a very low BWs (less than 1,500 g regardless of gestation length) (9, 11, 12). This approach focuses on extreme rather than normative BW variation, while rarely disambiguating low BW from prematurity. The few existing neuroimaging studies of normative BW variation among full-term pregnancies (13, 14) are cross-sectional in design and relate BW to brain anatomy in childhood without controlling for the large number of often interrelated factors that could influence both BW (15) and brain anatomy (16, 17) [e.g., gestational age, birth order, maternal age, ethnicity, socioeconomic status (SES), and unmeasured genetic and environmental variation].
In the current study, we sought to simultaneously address these multiple challenges by relating BW variation at full-term to postnatal brain development and cognition in 139 complete twin-pairs [85 monozygotic (MZ), 54 same-sex dizygotic (DZ)] and 170 unrelated singletons, on whom a total of 1,022 structural magnetic resonance imaging (sMRI) brain scans had been acquired between ages 3 and 30 y at ∼2-y intervals. Studying BW differences within MZ pairs provides a near-experimental model, within which prenatal environmental variation can be linked to brain outcomes in isolation from genetic, gestational, and maternal differences. To optimize generalizability of MZ-based analyses, we: (i) only considered MZ pairs with mild levels of BW discordance [(heavy twin – light twin)/(heavy twin) < 0.2] that are thought to largely exclude pathological sources of BW variation within MZ twin-pairs that are qualitatively different from the sources of normative BW variation among singletons (18); and (ii) assessed replicability of MZ-based finding in an independent sample of DZ twins and unrelated singletons. In addition to considering cognitive ability [full scale, verbal, and performance intelligence quotient (FSIQ, VIQ, and PIQ, respectively)] and global brain volumes [total brain volume (TBV), total gray matter volume (GMV), and total white matter volume (WMV)] as postnatal outcomes of interest, we focused on the relationship between BW and postnatal anatomy of the cerebral cortex.
The cerebral cortex undergoes especially rapid growth during in utero life (19) and represents a key biological substrate for many of the postnatal outcomes that are sensitive to normative BW variation. Prospective ultrasound studies of fetal growth indicate that prenatal influences on eventual BW are already operative by the second trimester of pregnancy (20, 21), when a number of foundational aspects of cortical development are underway, including: (i) a growing role for the subventricular zone in generation of new cortically bound progenitor neurons (22); and (ii) subsequent migration of these neurons into the cortical plate via the expanding subplate (23), which plays a pivotal role in formation of early neuronal circuits (24). Environmentally determined disturbances of such early processes could well have behaviorally relevant consequences for cortical development throughout the lifespan (25), which may be accompanied by neuroanatomical alterations detectable by in vivo neuroimaging. Prefrontal cortices are particularly salient in this regard given their protracted maturational course (26–28) and involvement in multiple domains of higher cognition. To provide a detailed picture of the relationship between BW variation and postnatal cortical development, we used “surface-based morphometry,” to measure three distinct morphometric properties of the cortical sheet from sMRI: cortical volume (CV) and its two sole determinants [mean cortical thickness (CT) and total cortical surface area (SA)]. These two CV subcomponents are evolutionarily (29), genetically (30), and developmentally (31) distinct and may, therefore, be differentially sensitive to normative BW variation. We related BW to global differences in CT and SA, as well as CT and SA measures taken at 80,000 points across the cortical sheet. This spatially fine-grained analysis allowed us to map regional variation in neurodevelopmental vulnerability to prenatal influences, in recognition of the fact that different cortical regions undergo different rates of prenatal (32) and postnatal (26, 27) maturation.
We show that subtle variations of the prenatal environment, as indexed by small BW differences within healthy MZ twin-pairs born at full-term, are associated with statistically significant differences in postnatal cognition and robust alterations of brain anatomy that persist into early adulthood. Environmentally determined increases in BW, within the normative BW range, confer increases in postnatal cognitive ability and developmentally stable increases in TBV, WMV, GMV, and CV. BW influences on CV are largely driven by alterations of SA rather than CT, and vulnerability of SA to differences in the prenatal environment shows marked regional heterogeneity. By comparing BW correlates between MZ and DZ twins, we show that genetic differences between individuals do not obscure the postnatal consequences of those prenatal environmental differences indexed by within MZ BW variation. We further establish generalizability of our twin-based findings to an independent sample of unrelated singletons. Our findings both help to specify aspects of early human brain development that are especially vulnerable to subtle differences in the prenatal environment, and carry potential public health relevance because they point toward mechanisms that operate in healthy full-term pregnancies and hold across different genetic and familial contexts.
Results
Sample Characteristics.
Participant characteristics are summarized in Table 1. BW was highly correlated between twins (P < 0.0005) and although this did not differ in a statistically significant manner between MZ and DZ groups, the estimated Pearson correlation coefficient for BW (controlling for gestation length) was marginally higher in MZ (r = 0.7) than DZ (r = 0.6) twins. The total BW of both twins combined, and singleton BW, were positively correlated with gestational age (twins: r = 0.5, P < 0.0005; singletons: r = 0.2, P = 0.002), but the magnitude of absolute or proportional BW discrepancy between twins was not. Neither total twin-pair BW nor measures of within-pair BW discrepancy were related to sex or SES. Singleton BW was greater in males than females (P = 0.04) and did not vary as a function of SES. In both twin and singletons groups, BW was positively associated with height and weight throughout the age range examined. Within twin-pairs, differences in BW were associated with significant differences in the trajectory of postnatal increases in height (interaction between BW and cubic age, P = 0.004), and weight (interaction between BW and cubic age, P = 0.007). Across singletons, after accounting for sex and gestation, greater BW was associated with developmentally stable increases in both postnatal height (t = 2.4; P = 0.02) and weight (t = 1.9; P = 0.06). The apparent lack of a developmentally dynamic relationship between BW and later height and weight in singletons compared with twins may reflect a smaller sample size or the use of residualized rather than raw BW.
Table 1.
Participant characteristics
| Characteristic | MZ | DZ | Singletons |
| No. of individuals | 170 | 108 | 172 |
| No. of complete twin-pairs | 85 | 54 | — |
| Male | 98 | 48 | 81 |
| Age, y | |||
| Mean (SD) | 13.7 (3.9) | 12.7 (4.4) | 14.3 (5.4) |
| Range | 5.5–25.6 | 5.3–25.9 | 3.7–32.8 |
| SES | |||
| Mean (SD) | 44 (18.5) | 43 (15.4) | 41 (18.8) |
| Range | 20–89 | 20–77 | 20–95 |
| GEST, wk | |||
| Mean (SD) | 38 (1.4) | 38 (1.5) | 40 (1.3) |
| Range | 36–41 | 36–41 | 37–43 |
| BW, g | |||
| Mean (SD) | 2,779 (458.2) | 2,871 (352.2) | 3,566 (471.5) |
| Range | 1,729–4252 | 2,182–3770 | 2,126–4,791 |
| BW discordance, g | |||
| Mean (SD) | 237 (175.8) | 221 (163.1) | — |
| Range | 14–737 | 14–595 | — |
| BW discordance, % of heavy twin | |||
| Mean | 8 | 7 | — |
| Range | 0.5–20 | 0.4–20 | — |
| IQ, mean (SD) | 109 (12.4) | 112 (12.2) | 116 (13.7) |
| VIQ, mean (SD) | 109 (13.9) | 110 (12.9) | 115 (14.5) |
| PIQ, mean (SD) | 108 (12.0) | 111 (12.7) | 113 (13.2) |
| No. of scans | |||
| >1 | 126 | 70 | 111 |
| >2 | 68 | 38 | 59 |
| >3 | 22 | 18 | 29 |
| Total no. of scans | 390 | 236 | 396 |
MZ, DZ, and singletons are shown separately. BW, SES (as measured by the Hollingshead scale), IQ, VIQ, PIQ (Full Scale, Verbal and Performance Intelligence Quotient respectively). —, not applicable.
BW Variation and Postnatal Outcome Within MZ Twins.
As shown in Table 2, greater BW within MZ twin-pairs was associated with greater FSIQ and PIQ, whereas there was no statistically significant relationship between BW and VIQ. Relationships between BW and measures of cognitive ability were robust to inclusion of sex, gestation length, height, weight, and SES as covariates.
Table 2.
BW and postnatal outcomes in MZ twins
| Effect of BW |
Estimated change with 500-g BW increase |
|||
| Measure | t | P | Absolute | Relative |
| IQ | 2.3 | 0.02 | 2.0 points | 0.13 SD |
| VIQ | 0.9 | 0.4 | 0.9 points | 0.06 SD |
| PIQ | 2.5 | 0.02 | 2.3 points | 0.15 SD |
| Total brain volume | 9.2 | <0.0005 | 27 cm3 | 2%, 0.25 SD |
| GMV | 5.7 | <0.0005 | 19 cm3 | 2.1%, 0.27 SD |
| WMV | 3.7 | 0.0002 | 10 cm3 | 1.9%, 0.17 SD |
| Total cortical volume | 5.8 | <0.0005 | 16 cm3 | 2.3%, 0.27 SD |
| Mean cortical thickness | 2.3 | 0.02 | 0.025 mm | 0.6%, 0.15 SD |
| Total surface area | 8.3 | <0.0005 | 35.7 cm2 | 1.8%, 0.3 SD |
Main effect of BW is shown for cognitive [IQ, VIQ, PIQ (Full Scale, Verbal and Performance Intelligence Quotient respectively)] and global anatomical outcomes.
With regard to global measures of postnatal brain development (see Table 2), BW variation within MZ twin-pairs was significantly and positively associated with TBV, GMV, and WMV. These associations did not vary in a statistically significant manner with age. BW influences on TBV appeared to be driven more by GMV than WMV. The strong relationship between BW and total GMV held for both cortical (i.e., CV) and noncortical (t = 3.2; P = 0.0019) components of gray matter. The influence of BW on CV was driven more by SA than CT: while greater BW was associated with statistically significant and developmentally static increases in both SA and CT, the effect-size estimate for BW influences on SA was almost twice that for CT.
In keeping with the muted effect of BW on mean CT relative to total SA, BW influences on regional CT were much less pronounced than those for regional SA. Specifically, we were unable to detect any statistically significant relationships between BW variation and regional CT after applying a false-discovery rate (FDR) correction for multiple comparisons. At an uncorrected nominal P value threshold of 0.01, however, a positive, and developmentally static, main effect of BW on CT became apparent in middle frontal sulcus, bilateral posterior superior temporal sulcus, left superior parietal lobule, and left middle temporal gyrus (Fig. S1).
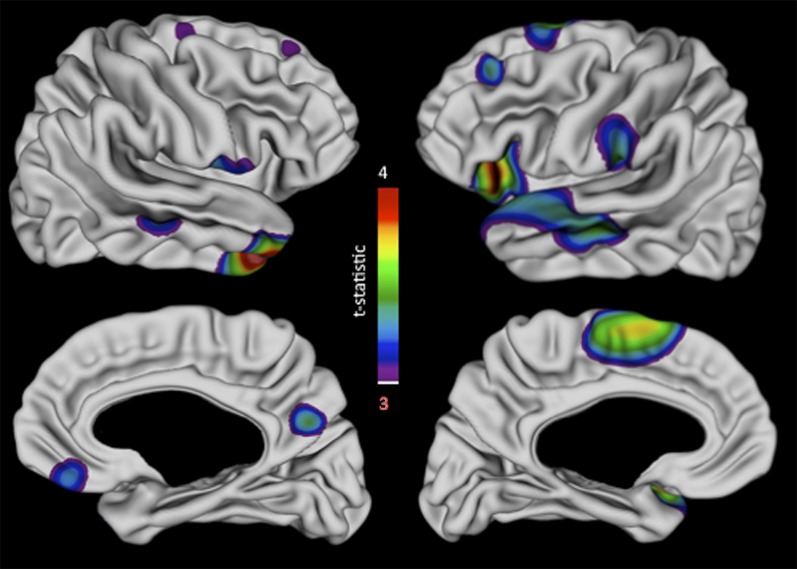
In contrast to out vertex-based CT findings, BW had a robust influence on regional SA that survived FDR correction for multiple comparisons (Fig. 1). Greater BW was associated with developmentally fixed SA increases within bilateral superior frontal gyrus, bilateral perisylvian cortices (including inferior frontal gyrus, superior temporal gyrus, and left angular gyrus), and bilateral middle temporal gyrus.
Fig. 1.
Vertex maps showing the relationship between BW variation within MZ twin-pairs and postnatal differences in cortical surface area. Associations between BW and vertex-level measures of surface area (SA) are shown as t-statistic maps after application of a FDR correction for multiple comparisons with q (the expected proportion of falsely rejected null hypotheses) set at 5%. As shown in the color bar, “warmer” colors indicate a more statistically significant positive association between BW and SA.
All significant relationships between BW and anatomical outcomes were robust to inclusion of sex, gestation length, height, weight, and SES as covariates.
Influence of Zygosity on Postnatal Correlates of BW Variation.
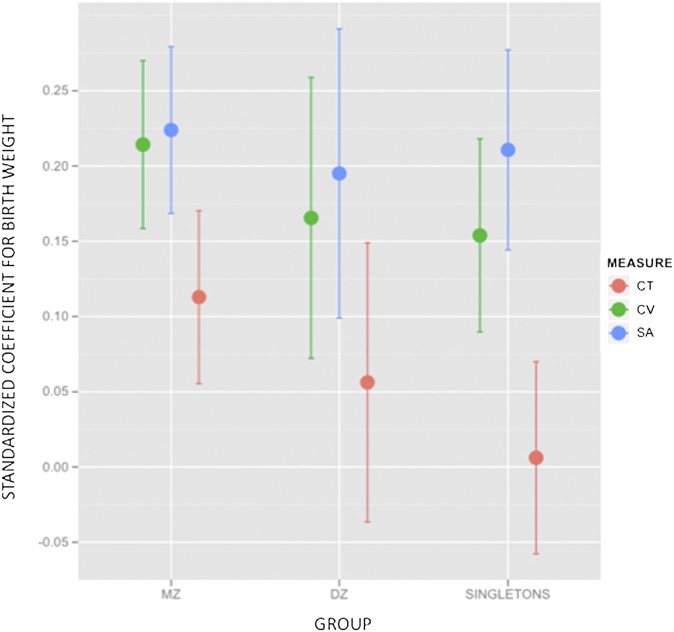
None of the statistically significant relationships between BW and postnatal outcomes within MZ twins showed a statistically significant interaction with zygosity in analyses that compared within DZ BW effects to those observed within MZ twins (Eq. S3). Fig. 2 illustrates the comparability of within-MZ and within-DZ analyses with respect to the importance of SA (relative to CT) in driving BW associations with CV.
Fig. 2.
Whisker plot showing standardized coefficients (and 95% coefficient confidence intervals) for the age-invariant main effect of BW variation on CV, CT, and cortical SA within each of the three groups examined in this study. Note that changes in CV and SA for changes in BW are both similar to each other within each group as well as across groups.
Variation in BW and Postnatal Outcome Across Unrelated Singletons.
Differences in BW (residualized for gestational age and sex) across unrelated singletons were not related to IQ measures in a statistically significant manner but were related to postnatal brain development in a manner that largely replicated MZ twin-based findings (Table S1). Thus, among singletons, greater BW was associated with statistically significant increases in TBV (t = 2.6; P = 0.01), GMV (t = 2.2; P = 0.03), WMV (t = 2.2; P = 0.03), and CV (t = 2.3; P = 0.02). As was found within MZ analyses, BW influences on CV in singletons were largely mediated through SA (t = 3; P = 0.003) rather than CT (t = 0.04; P = 0.9). Also, as for MZ-twin based findings, BW variation across singletons showed statistically significant positive associations with regional SA but not regional CT (see SI Methods). All significant findings in singletons were robust to inclusion of sex, gestation length, height, weight, and SES as covariates.
Discussion
Our study related normative BW variation in full-term pregnancies to postnatal measures of global cognitive functioning and longitudinal brain development between early childhood and late adolescence/early adulthood. Focusing on these relationships within MZ twins allowed us to use BW as a relatively (33) “pure” marker for variation in the prenatal environment and to thus map the consequences such variation has for postnatal human cortical development. Testing for stability of MZ findings within DZs and across unrelated singletons allowed us to determine whether the postnatal neurodevelopmental signatures of environmentally driven BW variation held under more common conditions, when BW variation indexes a host of factors beyond the in utero environment including genetic, gestational, familial, and societal influences. Thus, while MZ-only analyses provide a rare opportunity to test basic developmental neuroscience hypotheses in humans using a near-experimental design, DZ and singleton analyses provide information about the generalizability and related potential for public health relevance of findings in MZs. We now address these two main outputs of our study in turn.
Modeling Prenatal Environmental Influences on Brain Development Using MZ Twins.
Our study adds to previous reports that BW differences within MZ twin-pairs are accompanied by postnatal differences in cognition (34) and extends upon this knowledge by charting associations between BW variation within MZ twins and patterns of postnatal brain development from ages 3 through 30 y. By simultaneously controlling for multiple potential confounds, MZ twins allow more specific inferences to be made regarding the causal processes that might underlie statistical associations between BW and postnatal brain maturation.
Differences between MZ twins are classically thought to reflect environmental influences [although potential genetic sources of MZ discordance are recognized (33)]. In keeping with this notion, most studies of the mechanisms underlying BW differences between MZ twins have focused on the placenta as the chief determinant of the in utero environment, linking within-twin differences in prenatal growth to inequitable access to placental tissue (35). The capacity of placental mechanisms for BW variation to also impact brain development has been established through experimental modification of effective placental transfer in animal models of placental insufficiency (1) and maternal undernutrition (36, 37). In humans, ultrasonographic markers of placental insufficiency have been shown to predict neurobehavioral outcomes during fetal and early postnatal life (38, 39). These data suggest that the differences in postnatal brain maturation we find to be associated with within MZ BW variation are, at least in part, reflective of within-MZ twin-pair differences in placental support impacting both somatic and brain growth in utero.*
Of the neuroanatomical measures examined in this study, SA appeared to be most vulnerable to the prenatal environmental differences indexed by within-MZ twin-pair BW differences, and the effect size for BW association with SA was twice that of BW influences on cognition as estimated in our own study and in prior work (34). Although the in vivo neuroimaging measures examined in our study do not provide information about the cellular mechanisms that might link postnatal SA variation in humans to differences in the prenatal environment, a number of plausible candidate mechanisms are suggested by postmortem studies of typical mammalian cortical development in utero, and its sensitivity to environmental factors. For example, amplified progenitor cell division within the subventricular zone (SVZ) is thought to be an important prenatal determinant of final SA in primates, and in humans, the SVZ may continue to provide neurons for the tangentially expanding cortical sheet well into the second trimester of pregnancy (22, 40), when prenatal determinants of BW variation appear to be active (20, 21). Similarly, the proper incorporation of new neurons and incoming afferents into the developing cortical sheet is thought to rely heavily on the subplate, a transient structure that becomes most prominent during the second trimester of primate pregnancy, and especially so under nascent associative cortices (23). Although speculative, the notion that SVZ and subplate processes might be particularly vulnerable to alterations of the prenatal environment capable of modifying BW has been lent support by a recently published study that provides experimental data on in utero adversity and brain development in primates (37). In this landmark study, modest maternal calorie restriction not only altered the balance between rates of cell birth and apoptosis in midgestation baboon SVZ but also reduced subplate neuronal network density. The subplate has also been proposed as a locus of vulnerability to pre- and perinatally incurred insults based on neuroimaging studies of prematurely born humans and experimental animal models of prenatal brain injury (41), although the generalizability of these theories to more subtle alterations of the prenatal environment remains unclear.
Our data suggest that the sustained alterations of postnatal SA that accompany variation in the prenatal environment, as captured by within MZ BW differences, may preferentially alter late-maturing prefrontal and temporal cortices. These findings provide empirical support to neurodevelopmental theories that emphasize the potential for prenatally instilled differences to have temporally delayed postnatal “echoes,” of relevance for the emergence of psychopathology (25). From a mechanistic perspective, regional specificity of our findings could potentially reflect regional differences in the timing of prenatal cortical development (23, 32) or the fact that frontal and temporal cortices show a protracted postnatal developmental course relative to other regions (26–28). Because many of the frontotemporal regions where SA appears to be most sensitive to BW variation within MZ twins have been separately implicated in multiple common psychiatric disorders (42, 43), our data also propose a potential neurodevelopmental mechanism for the observed association between normative BW variation in full-term pregnancies and later risk for psychopathology (7).
Generalizability of Findings Beyond MZ Twins.
BW correlates within DZ twins were not significantly different from those observed within MZ twins. Therefore, genetic differences between DZ twins do not obscure the capacity for subtle variation in the prenatal environment to manifest as coordinated differences in both BW and neurodevelopment. This conclusion is in keeping with quantitative genetic reports that BW at full term (44), and associations between BW and later cognitive outcome (44, 45), are largely determined by non-genetic sources of variation. The fact that environmental influences on neurocognitive development are not “drowned out” by genetic variation is highly relevant from a public health perspective because (i) there is greater potential to optimize neurodevelopment outcomes through modification of the prenatal environment than by modification of genes; and (ii) it is a favorable (but not sufficient) condition for the success of any potential population-level attempt to modify neurodevelopmental outcomes through environmental interventions targeted at BW optimization (46).
We were able to replicate twin-based associations between BW variation and later brain anatomy in an independent sample of unrelated singletons. This demonstrates empirically that our findings in twins are generalizable to singleton pregnancies from which most people are born. Thus, subtle interindividual differences in BW, within the normal range, and among individuals born at full term, are associated with replicable differences in postnatal brain development that can still be detected in late adolescence/early adulthood. The lack of an association between BW and cognitive ability in singletons may reflect the fact that, unlike BW variation within twins, BW variation across unrelated singletons also indexes differences in gestational age and maternal age, parity, and smoking (15). Such factors may operate to obscure the subtle relationship between BW variation and postnatal cognitive ability that is detectable within twins.
In general, the relationships we find between BW variation and postnatal outcome are small in magnitude and may, therefore, play a far smaller role in predicting morbidity at the individual level than gross prenatal insults. However, small BW-related shifts in outcome among the vast number of healthy full-term pregnancies that give rise to offspring with weights appropriate for their gestational age could have significant population-level consequences (6).
Limitations and Caveats.
Our findings should be interpreted in light of certain limitations and caveats. First, although BW difference within MZ twins constitutes a good summary measure for environmentally driven variation of in utero growth, it is likely to encompass diverse patterns of divergent growth between twins that vary in their timing and underlying physiological mechanism. Second, the capacity of BW variation to predict differences in later brain anatomy may be preceded by BW-related differences in head size at birth. Clarifying the extent of this mediation will help quantify the relative importance of in utero vs. postnatal mechanisms in forging the links we observe between prenatal growth and brain anatomy into late adolescence. Third, our analyses did not identify the curvilinear “inverted-U” relationship that has been documented between BW variation and some measures of postnatal outcome (47), which may reflect our focus on healthy children who had been born at weights appropriate for gestational age. Fourth, our sample has a relatively higher IQ and SES than the general population, and any genetic and environmental differences that this might index could potentially modify the relationship between normative BW variation and postnatal neurodevelopment. Finally, in vivo structural neuroimaging does not provide any information about cellular or molecular phenotypes that might explain variation in SA for example. Addressing these issues will require continued work within animal models.
Despite these limitations and caveats, our study identifies replicable relationships between normative BW variation in healthy full-term pregnancies and postnatal neurodevelopment. Our combined use of twin samples, longitudinal neuroimaging, and sophisticated methods for analysis of sMRI data allows us to establish that relationships between purely environmental sources of BW variation and postnatal brain development persist into late adolescence and, in the cortex, seem to specifically target SA rather than CT. These findings delineate neuroanatomically dissociable consequences of in utero environmental variation in humans and, thus, help direct subsequent biological research into the molecular and cellular pathways that mediate such consequences. Also, our focus on normative BW variation, and replication of MZ-twin-based findings within DZ twins and across singletons, argues for generalizability of results to that large portion of the population that is born at full term with weights appropriate for gestational age. Consequently, our findings provide a neuroscientific account that may be of relevance to public health policy planners concerned with optimization of fetal development (48).
Methods
Sample.
Participant characteristics are summarized in Table 1. Overall, our study examined a total of 450 individuals on whom 1022 sMRI brain scans had been gathered longitudinally. We included 139 complete same-sex twin-pairs, of which 85 pairs were MZ. Zygosity was determined by buccal cheek swabs using 9–12 unlinked short tandem repeat loci for a minimum certainly of 99% (BRT Laboratories). Twin-pairs were scanned longitudinally and in tandem, such that a total of 626 scans were gathered from the participants in our twin sample between ages 5 and 25 y. Our singleton sample consisted of 172 unrelated individuals of European descent on whom a total of 396 scans had been gathered between ages 5 and 32 y. Recruitment and screening procedures for all participants have been described previously (49) and are detailed in SI Methods.
All participants had an FSIQ of greater than 80. [FSIQ, VIQ, and PIQ were estimated using age-appropriate Wechsler intelligence scales (50).] All twins had been born between 36 and 42 wk gestation and had not been exposed to psychotropic drugs prenatally or experienced significant perinatal complications (as indexed by the need for admission to a special or intensive care baby unit). Details regarding gestational length and BW were drawn from medical records and parental report [which have been established to show good agreement with each other for these variables (51)]. The institutional review board of the National Institutes of Health approved the research protocol used in this study, and written informed consent and assent to participate in the study were obtained from parents and children, respectively.
Neuroimaging.
All sMRI brain scans were T-1 weighted images with contiguous 1.5-mm axial slices, obtained on the same 1.5-T General Electric Signa scanner using a 3D spoiled gradient recalled echo sequence (see SI Methods for scan acquisition parameters). Native sMRI scans were submitted to the CIVET pipeline† for automated morphometric analysis, as described previously (31). This well-validated set of image-processing algorithms generates estimates total GMV, WMV, and CSF, as well as creating a 3D reconstruction of the convoluted cortical sheet for each scan, from which four metrics are derived: total CV and SA, mean CT, and estimates of both CT and SA at 800,000 points (vertices) across the cortical sheet. (See SI Methods for image-processing details.)
Statistical Analysis.
For MZ twins, within twin-pair BW variation was related to within twin-pair variation in cross-sectional cognitive and longitudinal anatomical outcomes using mixed models, with family, and person nested within family as random effects, respectively. This approach allows the relationship between BW and outcomes to vary across pairs and uses this variation to derive an estimate for the fixed effect of BW on cognition and neuroanatomy across all twin-pairs. Application of mixed models to longitudinal neuroimaging data allows the inclusion of multiple measurements per person at different ages and irregular intervals between measurements, thereby increasing statistical power (52). The potential for MZ-based findings to be modified by zygosity was assessed by running models that had included significant BW terms within MZ-twin based analyses, in a combined sample of MZ and DZ twins, and testing for the presence of a statistically significant interaction between BW terms and zygosity. For cognitive measures, we tested whether BW influences were altered by interactive or main effects of sex, gestational length, and SES. For longitudinally measured neuroanatomical outcomes, we first determined the best-fitting age model for each outcome using a previously published (31) approach, which stepped down through cubic → quadratic → linear age-models, and replicated our earlier findings (31, 49) of predominantly curvilinear trajectories for the anatomical indices considered in this current report. BW effects were then examined within these curvilinear age models, by first assessing whether interactions between BW and age terms improved upon an interaction-free model (using F tests) and only presenting the simpler main-effects model in the absence of statistical evidence for BW-related alterations of neurodevelopmental trajectory “shape.” We tested for robustness of BW findings to inclusion of covariates as described for cognitive measures and further tested for stability of BW findings to covarying for height and weight at time of scan. Illustrative statistical models for each step of this process are provided in SI Methods. In singletons, general linear models were used to relate BW and cognition. Mixed models were used to relate BW variation and longitudinal measures of anatomy, as already described for twins, except that person identifier was used as a sole random effect, rather than person identifier nested within family identifier. Rather than using raw BW measures in singletons, we residualized BW for sex and gestational age, to make analyses more comparable to those applied in MZ and same-sex DZ twins.
Supplementary Material
Acknowledgments
We thank the participants who took part in this study and their families. We thank Prof. Daniel Geschwind for helpful comments during our early formulations of the analyses presented in this paper.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11062.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203350109/-/DCSupplemental.
*This hypothesis is (i) consistent with BW variation having a direct causal impact on brain development or environmental variation exerting simultaneous, but causally dissociable, influences on BW and brain development; and (ii) compatible with the presence of additional postnatal contributions to the observed relationship between BW and brain maturation (e.g., differences in BW could index later difference in the timing of pubertal influences on brain development or differences in the evoked environment).
†Ad-Dab’bagh Y, et al., The CIVET image-processing environment: A fully automated comprehensive pipeline for anatomical neuroimaging research. Sixth Annual Meeting of the Organization for Human Brain Mapping (OHBM), June 2006, Florence, Italy.
References
- 1.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 3.De Bie HM, et al. Global and regional differences in brain anatomy of young children born small for gestational age. PLoS ONE. 2011;6:e24116. doi: 10.1371/journal.pone.0024116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schull WJ, Norton S, Jensh RP. Ionizing radiation and the developing brain. Neurotoxicol Teratol. 1990;12:249–260. doi: 10.1016/0892-0362(90)90096-u. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Abel KM, et al. Birth weight, schizophrenia, and adult mental disorder: Is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923–930. doi: 10.1001/archgenpsychiatry.2010.100. [DOI] [PubMed] [Google Scholar]
- 8.Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: A systematic review. Psychol Bull. 2004;130:989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- 9.Martinussen M, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–2596. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 10.Dubois J, et al. Primary cortical folding in the human newborn: An early marker of later functional development. Brain. 2008;131:2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ment LR, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy Z, Lagercrantz H, Hutton C. Effects of preterm birth on cortical thickness measured in adolescence. Cereb Cortex. 2011;21:300–306. doi: 10.1093/cercor/bhq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Soelen IL, et al. Effects of gestational age and birth weight on brain volumes in healthy 9 year-old children. J Pediatr. 2010;156:896–901. doi: 10.1016/j.jpeds.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Qiu A, et al. Birth weight and gestation influence striatal morphology and motor response in normal six-year-old boys. Neuroimage. 2012;59:1065–1070. doi: 10.1016/j.neuroimage.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Breeze AC, Lees CC. Prediction and perinatal outcomes of fetal growth restriction. Semin Fetal Neonatal Med. 2007;12:383–397. doi: 10.1016/j.siny.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Shaw P, et al. Parental age effects on cortical morphology in offspring. Cereb Cortex. 2011;22:1256–1262. doi: 10.1093/cercor/bhr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blickstein I, Goldman RD, Mazkereth R. Adaptive growth restriction as a pattern of birth weight discordance in twin gestations. Obstet Gynecol. 2000;96:986–990. doi: 10.1016/s0029-7844(00)01079-6. [DOI] [PubMed] [Google Scholar]
- 19.Scott JA, et al. Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci. 2011;29:529–536. doi: 10.1016/j.ijdevneu.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox NS, et al. Second-trimester estimated fetal weight and discordance in twin pregnancies: Association with fetal growth restriction. J Ultrasound Med. 2011;30:1095–1101. doi: 10.7863/jum.2011.30.8.1095. [DOI] [PubMed] [Google Scholar]
- 21.Salomon LJ, Hourrier S, Fanchin R, Ville Y, Rozenberg P. Is first-trimester crown-rump length associated with birthweight? BJOG. 2011;118:1223–1228. doi: 10.1111/j.1471-0528.2011.03009.x. [DOI] [PubMed] [Google Scholar]
- 22.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 24.Kostović I, Judaš M, Sedmak G. Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: Relevance for schizophrenia. Int J Dev Neurosci. 2011;29:193–205. doi: 10.1016/j.ijdevneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Ari Y. Neuro-archaeology: Pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008;31:626–636. doi: 10.1016/j.tins.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somel M, et al. Transcriptional neoteny in the human brain. Proc Natl Acad Sci USA. 2009;106:5743–5748. doi: 10.1073/pnas.0900544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 30.Panizzon MS, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raznahan A, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajagopalan V, et al. Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci. 2011;31:2878–2887. doi: 10.1523/JNEUROSCI.5458-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gringras P, Chen W. Mechanisms for differences in monozygous twins. Early Hum Dev. 2001;64:105–117. doi: 10.1016/s0378-3782(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 34.Newcombe R, Milne BJ, Caspi A, Poulton R, Moffitt TE. Birthweight predicts IQ: Fact or artefact? Twin Res Hum Genet. 2007;10:581–586. doi: 10.1375/twin.10.4.581. [DOI] [PubMed] [Google Scholar]
- 35.Fick AL, et al. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am J Obstet Gynecol. 2006;195:178–183. doi: 10.1016/j.ajog.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 37.Antonow-Schlorke I, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baschat AA. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol. 2011;37:501–514. doi: 10.1002/uog.9008. [DOI] [PubMed] [Google Scholar]
- 39.Roza SJ, et al. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol. 2008;168:1145–1152. doi: 10.1093/aje/kwn233. [DOI] [PubMed] [Google Scholar]
- 40.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 41.Miller SP, Ferriero DM. From selective vulnerability to connectivity: Insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gogtay N, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 43.Peterson BS, et al. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gielen M, et al. Modeling genetic and environmental factors to increase heritability and ease the identification of candidate genes for birth weight: A twin study. Behav Genet. 2008;38:44–54. doi: 10.1007/s10519-007-9170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torche F, Echevarría G. The effect of birthweight on childhood cognitive development in a middle-income country. Int J Epidemiol. 2011;40:1008–1018. doi: 10.1093/ije/dyr030. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32:5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization . Promoting Optimal Fetal Health Development. Geneva: WHO; 2003. [Google Scholar]
- 49.Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 51.Adegboye AR, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG. 2008;115:886–893. doi: 10.1111/j.1471-0528.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinheiro J, Bates DM. Mixed-Effects Models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.