Abstract
Plasmodium falciparum lines differ in their ability to infect mosquitoes. The Anopheles gambiae L3-5 refractory (R) line melanizes most Plasmodium species, including the Brazilian P. falciparum 7G8 line, but it is highly susceptible to some African P. falciparum strains such as 3D7, NF54, and GB4. We investigated whether these lines differ in their ability to evade the mosquito immune system. Silencing key components of the mosquito complement-like system [thioester-containing protein 1 (TEP1), leucine-rich repeat protein 1, and Anopheles Plasmodium-responsive leucine-rich repeat protein 1] prevented melanization of 7G8 parasites, reverting the refractory phenotype. In contrast, it had no effect on the intensity of infection with NF54, suggesting that this line is able to evade TEP1-mediated lysis. When R females were coinfected with a line that is melanized (7G8) and a line that survives (3D7), the coinfection resulted in mixed infections with both live and encapsulated parasites on individual midguts. This finding shows that survival of individual parasites is parasite-specific and not systemic in nature, because parasites can evade TEP1-mediated lysis even when other parasites are melanized in the same midgut. When females from an extensive genetic cross between R and susceptible A. gambiae (G3) mosquitoes were infected with P. berghei, encapsulation was strongly correlated with the TEP1-R1 allele. However, P. falciparum 7G8 parasites were no longer encapsulated by females from this cross, indicating that the TEP1-R1 allele is not sufficient to melanize this line. Evasion of the A. gambiae immune system by P. falciparum may be the result of parasite adaptation to sympatric mosquito vectors and may be an important factor driving malaria transmission.
Keywords: immune evasion, mosquito immunity, refractory mosquito, thioester-containing protein 1 activation
Human malaria is a disease caused by Plasmodium parasites that threatens one-half of the world’s population. In 2009, malaria infection affected 225 million people and resulted in 781,000 deaths, mostly of young African children (1). Plasmodium is transmitted by anopheline mosquitoes and undergoes a complex sexual developmental cycle in the insect host (2). Mosquitoes become infected when they ingest vertebrate blood containing gametocytes, and fertilization and zygote formation take place in the lumen of the mosquito midgut. Zygotes mature into motile ookinetes that must traverse the midgut and avoid destruction by the mosquito immune system to complete their development. Plasmodium parasites suffer great loses during these initial stages in the mosquito, and only a few ookinetes succeed. This natural bottleneck in the number of parasites in the vector is an attractive target to block malaria transmission (3).
Anopheles gambiae is the main vector of the human malaria parasite P. falciparum in large regions of Africa. This mosquito species exhibits a wide range of susceptibility to infection with a given P. falciparum line (4, 5), and different Plasmodium isolates also vary in their ability to infect a given mosquito strain (4, 6, 7). This suggests that genetic differences in both the mosquito and the parasite affect the efficiency of mosquito infection and disease transmission.
A stable line of A. gambiae highly refractory to P. cynomolgi infection was obtained by genetic selection of refractory females over a few generations (5). In this refractory strain, ookinetes form and invade the midgut, but they are killed as they come in contact with the mosquito hemolymph and covered with melanine, an insoluble dark pigment (5, 8). This refractory line was also able to eliminate and melanize many different Plasmodium species, including P. vivax, several primate malaria parasites (P. gonderi, P. inui, and P. knowlesi), P. berghei, and P. gallinaceum (5). Interestingly, dramatic differences were observed in the ability of several P. falciparum strains to survive in these refractory mosquitoes, and survival seemed to correlate with their geographical origin. For example, the New World (SL and 7G8) and Asian (Indo3) strains were effectively encapsulated with melanine, whereas some of the African strains (LE5 and NF54) survived well, although encapsulation was occasionally observed (5). This refractory line was lost, but a second line was selected using the same strategy. This A. gambiae (L3-5) refractory strain has been widely used as an experimental model; for example, it was used to map mosquito loci involved in P. cynomolgi B encapsulation (9). The L3-5 refractory (R) strain was used in most of the experiments reported in this manuscript, and for convenience, it will be referred to as the R strain.
The thioester-containing protein 1 (TEP1), a complement 3-like protein, is a final effector of the mosquito immune system that mediates a powerful antiplasmodial response against P. berghei parasites; TEP1 is active even in mosquito strains such as A. gambiae G3 that are highly susceptible to infection (10). TEP1 circulates in the hemolymph as a complex with two other proteins, the leucine-rich repeat protein 1 (LRIM1) and the Anopheles Plasmodium-responsive leucine-rich repeat protein 1 (APL1). These two leucine-rich proteins are required to stabilize TEP1, because silencing either LRIM1 or APL1 triggers premature TEP1 activation, resulting in mosquitoes devoid of a functional complement-like system (10–12). TEP1 also mediates killing and encapsulation of P. berghei in the A. gambiae R strain (13).
TEP1 has been reported to mediate P. falciparum lysis in the A. gambiae Keele laboratory strain (14, 15), and APL1 (isoform C) silencing prevents P. falciparum lysis in the A. gambiae Ngousso laboratory strain (16). In contrast, LRIM1 silencing in the A. gambiae Younde strain from Cameroon had no effect on sympatric P. falciparum isolates (17). More recent studies have shown that the TEP1-rB allele found in natural populations of A. gambiae mosquitoes is associated with higher P. falciparum (ND37 strain, a line derived from NF54) infection than the TEP1-S allele (18). In general, the TEP1 system seems to be more effective in eliminating P. berghei than P. falciparum. The difference in the efficiency of TEP1-mediated immunity against P. falciparum isolates suggests that some P. falciparum lines may be able to evade the mosquito immune system; however, comparisons between studies are inconclusive because different combinations of A. gambiae and P. falciparum strains have been used. In this study, we analyze whether the survival of different P. falciparum lines in the A. gambiae R strain is caused by differential activation of the mosquito TEP1 system and whether these effects on the mosquito immune response are local or systemic. We provide experimental evidence that parasite genetic factors can modulate the ability of a mosquito to activate TEP1-mediated lysis, allowing some parasite strains to survive even in a highly refractory mosquito strain.
Results
Infectivity of Different P. falciparum Lines to the R Strain.
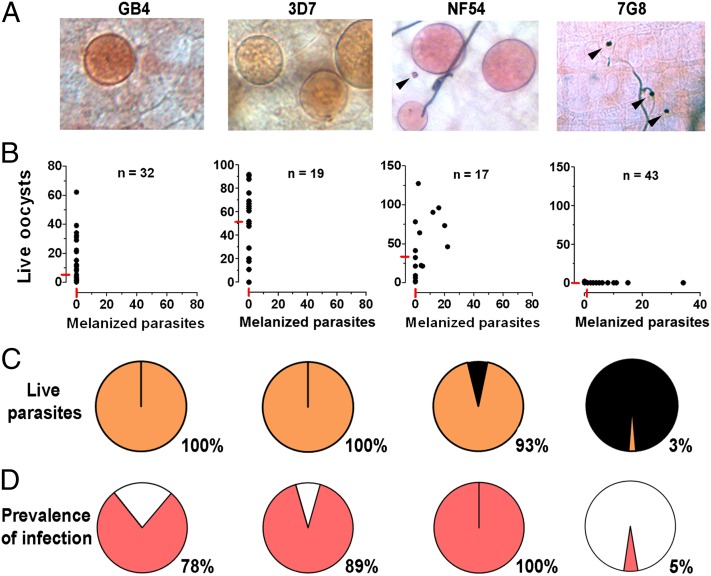
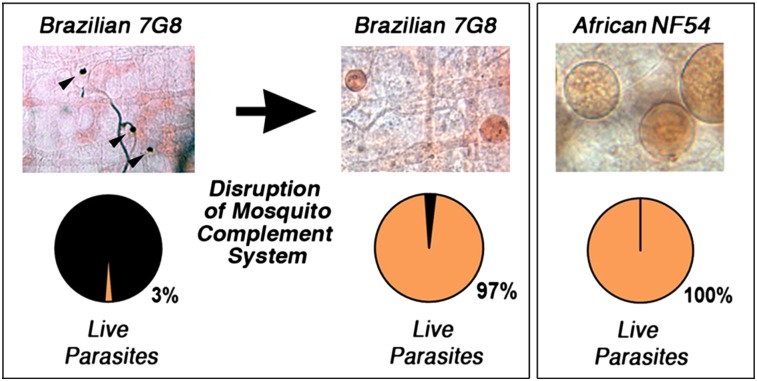
We investigated whether two P. falciparum lines that were known to differ in their survival in the first refractory mosquito strain reported in 1986 [the Brazilian 7G8 that was highly melanized and the NF54 strain that survived very well (5)] had similar phenotypes in the A. gambiae R strain that was reselected in 1997 (9). We confirmed that the NF54 (African origin) and 7G8 (Brazilian) strains differ greatly in their infectivity to the R strain (Fig. 1), with a proportion of melanized parasites of 7% and 97% (Fig. 1 A–C) (P < 0.001, χ2) and a prevalence of mosquito infection of 100% and 5%, respectively (Fig. 1D) (P < 0.001, χ2). Two other strains of African origin, the 3D7 (a parasite line cloned from NF54) and GB4 (Ghana) strains, also survived very well in R females (Fig. 1). No melanized parasites were observed with either strain (Fig. 1 A–C), and the prevalence of mosquito infection was 89% and 78% for the 3D7 and GB4 lines, respectively (Fig. 1D). These findings indicate that P. falciparum strains differ in their ability to survive in the A. gambiae R strain.
Fig. 1.
Survival of different P. falciparum lines (GB4, 3D7, NF54, and 7G8) in the R A. gambiae (L3-5 strain) 7–9 d postfeeding. (A) Mercurochrome staining of infected midguts. (B) Live and melanized parasites on individual mosquito midguts. The medians are indicated with red lines. (C) Proportion of live (orange) and melanized (black) parasites. (D) Prevalence of mosquito infection. All parasite phenotypes were confirmed in two or three independent experiments.
Mosquito TEP1 Activation and P. falciparum Survival.
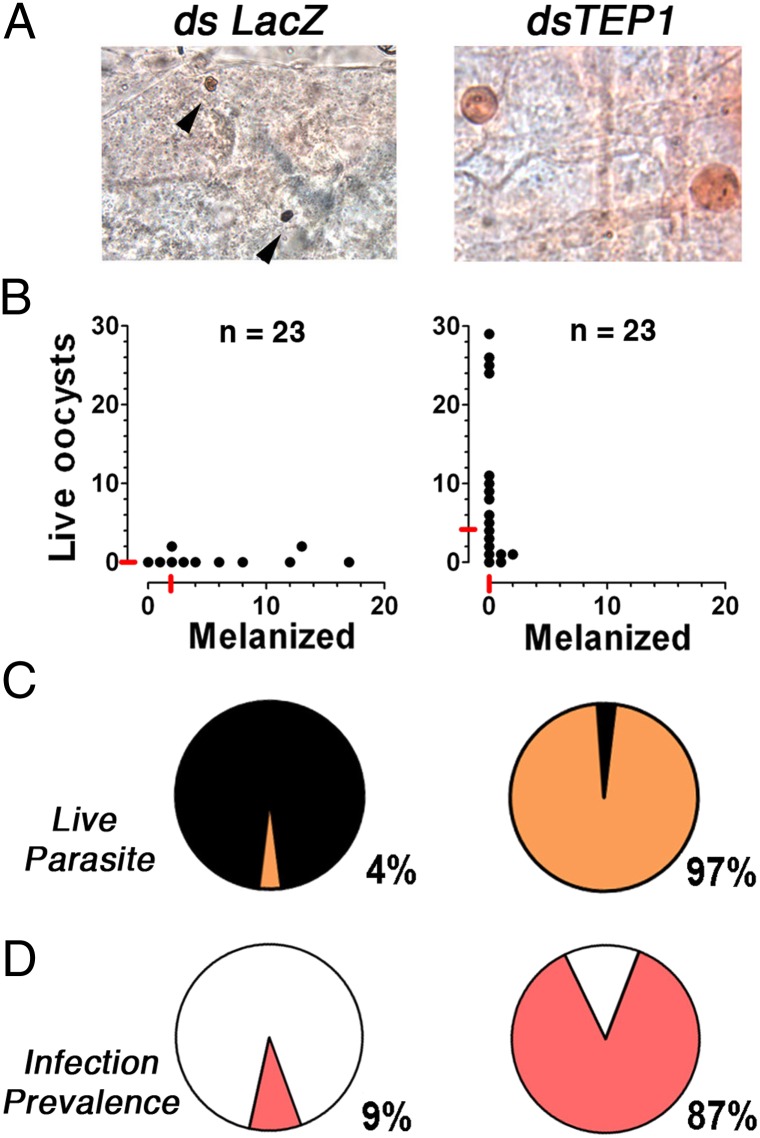
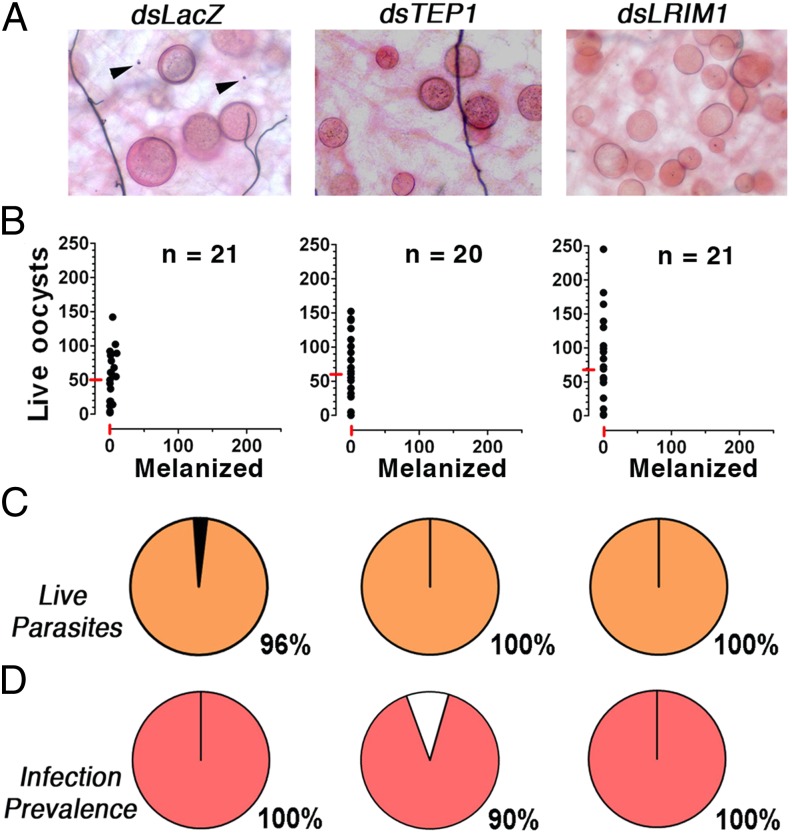
The potential involvement of the mosquito complement-like system in the antiparasitic response to P. falciparum 7G8 parasites was investigated by silencing TEP1 expression in R females. Systemic injection of dsTEP1 reduced endogenous TEP1 mRNA levels by 84% (Fig. S1), reduced the proportion of melanized parasites from 96% to 3% (Fig. 2 A–C) (P < 0.001, χ2), increased the median number of live oocysts from zero to four (Fig. 2B) (P < 0.001, χ2), and increased the prevalence of infection from 9% to 87% (Fig. 2D) (P < 0.001, χ2) relative to the dsLacZ-injected control. The participation of TEP1-mediated lysis in the antiplasmodial response to 7G8 parasites was confirmed by silencing LRIM1 and APL1, two genes required to stabilize the TEP1 complex. Silencing LRIM1 or APL1 alone also had a dramatic effect, because all parasites were melanized in the dsLacZ control group; however, melanization was no longer observed when either one of these two genes were silenced (Fig. S2 A–C) (P < 0.001). The prevalence of mosquito infection increased from 0% in the dsLacZ group to 53% and 68% in the LRIM1- and APL1-silenced groups, respectively (Fig. S2D) (P < 0.001, χ2).
Fig. 2.
Effect of TEP1 silencing in R A. gambiae (L3-5 strain) females on P. falciparum (7G8) infection. R females were injected with dsLacZ control or dsTEP1 3 d before feeding on a P. falciparum (7G8) gametocyte culture, and midgut infection was assessed 8 d postfeeding. (A) Mercurochrome staining of midguts. (B) Live and melanized parasites on individual mosquito midguts. The medians are indicated with red lines. (C) Proportion of live (orange) and melanized (black) parasites. (D) Prevalence of mosquito infection. All gene silencing phenotypes were confirmed in two or three independent experiments.
Although the NF54 line infects the R strain efficiently with minimal encapsulation (Fig. 1), it was possible that TEP1 eliminated a substantial number of NF54 parasites through a lytic mechanism. To explore this possibility, the effect of disrupting the mosquito complement-like system on NF54 infection was established. Silencing TEP1 or LRIM1 had no effect on the intensity of infection with NF54 (Fig. 3). However, it rescued the small percentage (3–4%) of parasites that were encapsulated in the dsLacZ-injected controls (Fig. 3C). Taken together, these findings indicate that, in the R strain, TEP1 is activated in response to infection with 7G8 parasites and mediates a very effective antiparasitic response. In contrast, in the same mosquito strain, TEP1 is not limiting infection with NF54 parasites, indicating that this African strain can infect the mosquito and evade the complement-like system.
Fig. 3.
Effect of TEP1 and LRIM1 silencing in R A. gambiae (L3-5 strain) females on P. falciparum (NF54) infection. R females were injected with dsLacZ control, dsTEP1, or dsLRIM1 3 d before feeding on a P. falciparum (NF54) gametocyte culture, and midgut infection was assessed 8 d postfeeding. (A) Mercurochrome staining of midguts. (B) Live and melanized parasites on individual mosquito midguts. The medians are indicated with red lines. (C) Proportion of live (orange) and melanized (black) parasites. (D) Prevalence of mosquito infection. All gene silencing phenotypes were confirmed in two or three independent experiments.
General Mechanism of P. falciparum Evasion of the Mosquito Complement-Like System.
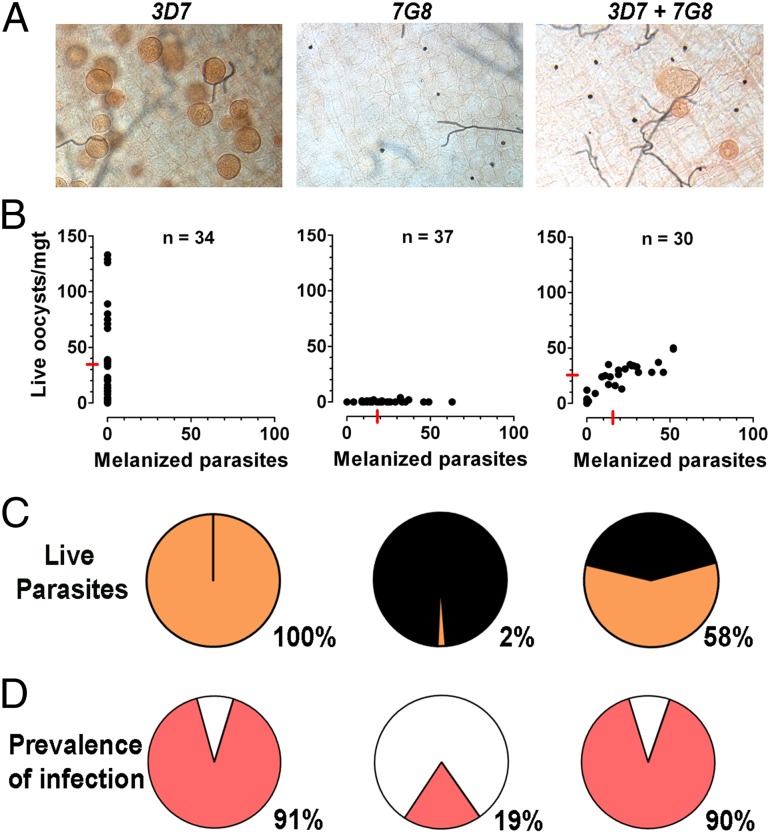
We explored whether P. falciparum strains could have systemic effects on mosquito immune response by either infecting R mosquitoes with one of two parasite lines (3D7 and 7G8) that differ greatly in survival, or coinfecting them with both strains. As expected, no melanizations were observed in mosquitoes infected with 3D7 parasites, and the prevalence of infection was high (91%) (Fig. 4). In contrast, 98% of 7G8 parasites were melanized, and the prevalence of infection was much lower (19%; P < 0.001, χ2) (Fig. 4). Coinfection with 3D7 and 7G8 parasites resulted in mixed phenotype in which each mosquito midgut contained both live and encapsulated parasites (Fig. 4), an intermediate level of melanized parasites (42%), and a high prevalence of infection (90%). There was a strong correlation (r2 = 0.757, P < 0.0001) between the number of live and encapsulated parasites for a given midgut (Fig. 4B), and therefore, midguts with a large number of live parasites also had the largest number of melanized parasites. The intensity and prevalence of infection was not significantly different in the coinfected mosquitoes (3D7 + 7G8) compared with the mosquitoes infected with 3D7 alone (Fig. 4 B and D). In several instances, live oocysts were localized physically close to encapsulated parasites in the midgut (Fig. 4A).
Fig. 4.
Coinfection of P. falciparum 3D7 and 7G8 strains in R A. gambiae (L3-5) females. Gametocyte cultures of P. falciparum 3D7 and 7G8 strains were fed to R females either separately or as a 1:1 mixture of the two strains. Midgut infection was assessed 8 d postfeeding. (A) Mercurochrome staining of midguts. (B) Live and melanized parasites on individual mosquito midguts. The medians are indicated with red lines. (C) Proportion of live (orange) and melanized (black) parasites. (D) Prevalence of mosquito infection. All parasite phenotypes were confirmed in two or three independent experiments.
A. gambiae TEP1 Alleles and P. falciparum 7G8 Melanization.
Previous studies have shown that the A. gambiae G3 strain is fixed for a TEP1 allele (S3) that is associated with susceptibility to infection with P. berghei, whereas the R strain (L3-5) is fixed for a refractory allele (R1) associated with melanization (13). Furthermore, allele-specific silencing of the F1 heterozygous progeny of a genetic cross between these two lines showed that the R1 allele is dominant for the melanization phenotype (13). We confirmed that our G3 and R colonies are also fixed for the S3 and R1 TEP1 alleles, respectively (Table 1). We identified an R strain A. gambiae colony that had been contaminated with G3 females, and this colony was allowed to breed for over 30 generations without any selection to maximize genetic recombination. Genotyping of 250 individuals confirmed that the three possible combinations of TEP1 alleles (S3/S3, S3/R1, and R1/R1) were present in 43%, 41%, and 16% of mosquitoes, respectively (Table 1) and that the frequency of these three TEP1 genotypes was in Hardy–Weinberg equilibrium (P = 0.7, χ2).
Table 1.
Genotype of the TEP1 locus in A. gambiae lines G3, L3-5, and G3 × L3-5 laboratory cross
| Strain | n | S3/S3 (%) | S3/R1 (%) | R1/R1 (%) |
| G3 | 100 | 100 | 0 | 0 |
| L3-5 | 222 | 0 | 0 | 100 |
| L3-5 × G3 colony | 251 | 43 | 41 | 16 |
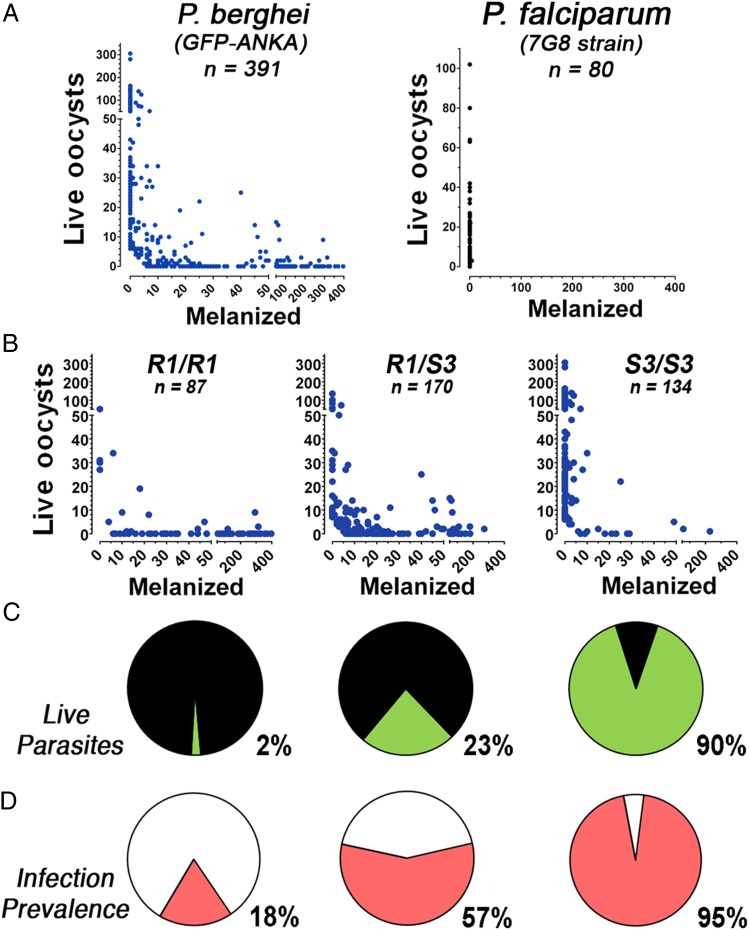
This cross-colony was used to investigate whether the two different TEP1 alleles (S3 and R1) were major determinants of the ability of A. gambiae to melanize P. berghei and P. falciparum 7G8 parasites. As expected, we observed three different phenotypes when mosquitoes were infected with P. berghei ranging from complete susceptibility to complete melanization, and many mosquitoes with both live and melanized parasites were also observed (Fig. 5A). However, when the same cross-colony was infected with P. falciparum 7G8 parasites, which are almost completely melanized by the R strain (Fig. 1B), they were highly susceptible to infection, and only two mosquitoes had very few melanized parasites (Fig. 5B). The mosquitoes infected with P. berghei were genotyped, and a strong association was observed between the TEP1 allele and susceptibility to infection (Fig. 5 C and D), which is in agreement with previous reports (13). The proportion of melanized parasites in R1/R1, S3/R1, and S3/S3 females was 98%, 77%, and 10% (P < 0.001, χ2), respectively, and the prevalence of infection was 18%, 57%, and 95% (P < 0.001, χ2), respectively.
Fig. 5.
Survival of P. berghei or P. falciparum 7G8 (black dots) infections in a laboratory cross line of A. gambiae with different genotypes for the TEP1 locus. Infections were analyzed 6 d postinfection for P. berghei or 8 d postinfection for P. falciparum. (A) Number of live and melanized P. berghei parasites (Left; blue dots) and P. falciparum 7G8 (Right; black dots) on individual mosquito midguts. (B) Number of live and melanized P. berghei parasites on individual mosquitoes with the R1/R1, R1/S3, or S3/S3 TEP1 genotypes. (C) Proportion of live (green) and melanized (black) P. berghei parasites in mosquitoes with the R1/R1, R1/S3, or S3/S3 TEP1 genotypes. (D) Prevalence of infection in mosquitoes with the R1/R1, R1/S3, or S3/S3 TEP1 genotypes.
Discussion
P. falciparum strains differ in their ability to survive in the A. gambiae R (L3-5) strain. As had been previously described for the first refractory strain (5), African strains of P. falciparum that are sympatric with A. gambiae survive well, whereas strains from different geographical origin, such as the 7G8 strain from Brazil, are almost completely melanized. Furthermore, gene knockdown by dsRNA injections showed that melanization of the 7G8 parasites is mediated by the mosquito immune system and involves the TEP1 complex (TEP1, LRIM1, and APL1). In contrast, NF54 parasites survive very well in the R strain, and disruption of the TEP1 complex does not affect the intensity of infection, suggesting that this African strain is able to evade the antiparasitic response mediated by TEP1. These results indicate that the TEP1 pathway is able to recognize and eliminate some strains of P. falciparum but not others.
The phenotypic difference when the G3/R cross-colony was infected with P. berghei or 7G8 P. falciparum parasites indicates that, although both strains have a very similar phenotype mediated by the TEP1 complex, the contribution of TEP1 alleles to refractoriness is different between these two parasites. We confirmed that the TEP1-R1 allele has a dominant effect on P. berghei melanization, which was previously described (13); however, it was not sufficient for 7G8 P. falciparum parasites to be melanized. The almost complete lack of melanization of 7G8 parasites in the colony cross suggests that multiple mosquito genes are involved. The possibility that some of the genes that mediated refractoriness to 7G8 were deleterious to the mosquito and lost in the colony after several generations cannot be ruled out. Genetic crosses between the R strains and the highly susceptible 4Arr A. gambiae strain also revealed differences in the quantitative trait loci mediating melanization of P. cynomolgi B (9) and P. cynomolgi Ceylon parasites (19).
The TEP1-R1 allele present in R mosquitoes has been shown to be a major determinant of P. berghei melanotic encapsulation (13), and it is closely related to the rB allele that is fixed in some M-form A. gambiae populations from Mali and Burkina Faso (18). The TEP1-R1 allele seems to be the result of recombination of the rA and rB TEP1 alleles that are present at high frequency in some African populations of A. gambiae (18). The rB allele is also dominant for P. berghei melanotic encapsulation, but heterozygous S/rB females do not melanize P. falciparum (ND37 strain) parasites (18). This difference in the ability to melanize P. berghei and P. falciparum is similar to what we observed in the L3-5/G3 colony cross, indicating that the R1 or rB TEP1 alleles are enough to melanize P. berghei but not P. falciparum. TEP1–S/rB TEP1 heterozygotes had significantly fewer live oocysts than S/S females when infected with P. falciparum (ND37 strain), suggesting that the rB TEP1 allele may mediate parasite lysis (18). However, we found that silencing TEP1 in the R strain had no effect on the intensity of infection with NF54 parasites, indicating that the TEP1-R1 allele is not mediating lysis of this P. falciparum strain. It is, therefore, possible that some African strains of P. falciparum may have adapted to evade the antiplasmodial responses mediated by A. gambiae TEP1 alleles that can be very effective against other parasite strains or species. As Brazilian 7G8 parasites adapted to mosquito vectors from the New World, they may have lost some of the genetic traits that allow some African strains to survive even in A. gambiae mosquitoes that are highly refractory to most other Plasmodium parasites.
The fact that coinfection of R females with P. falciparum 3D7 and 7G8 parasites resulted in mixed infection, in which all infected midguts had both live and encapsulated parasites, indicates that neither parasite line nor their hybrid progeny is causing a systemic effect in the mosquito immune system. This finding rules out the possibility of systemic activation of TEP1 by 7G8 parasites or a systemic suppression by the 3D7 line. The two parental gametocyte cultures were added in similar amounts, and this finding was confirmed by the similar infection levels between mosquitoes infected with each of these two strains (Fig. 4). In the coinfected group, a similar proportion of live and melanized parasites was obtained in individual mosquitoes, independent of the differences in intensity of infection between mosquitoes. In other words, both parasite strains were effective in invading the midgut of some mosquitoes, whereas both parasites did poorly in others; this finding suggests that how many parasites form and invade a given midgut is determined by factors from the mosquito host, but whether a parasite will survive or be melanized when and if it encounters the mosquito complement-like system is determined by the genotype of the parasite. These results indicate that the surviving phenotype does not dominate over encapsulation or vice versa. In other words, activation of TEP1 by some parasites does not seem to affect the fate of other parasites present in the same mosquito.
The mechanism by which several African strains of P. falciparum, such as NF54, avoid lysis by the TEP1 pathway in A. gambiae R females is unknown, but it may involve avoiding detection by the mosquito immune system, a change on the parasite’s surface that renders ookinetes resistant to TEP1 lysis and/or local deactivation of TEP1. We have recently shown that the nitration responses triggered when a P. berghei ookinete traverses a mosquito midgut cell can modify the parasite, making it visible to the complement-like system (20). The coinfection experiments indicate that the antiplasmodial response mediated by TEP1 is localized and suggest that the fate of each parasite in a given mosquito is genetically determined by the parasite. Parasite survival may depend on the ability of each ookinete to traverse the midgut without being tagged for destruction. The ability of a parasite to evade the mosquito immune system would allow it to be transmitted more effectively to the human host. The genetic differences between P. falciparum strains open the possibility of mapping the parasites loci that allow some P. falciparum strains to evade the mosquito immune system using a classical genetic approach.
Materials and Methods
A. gambiae and Plasmodium Parasites.
The A. gambiae L3-5 refractory strain and a genetic cross between L3-5 and G3 were used (9). Mosquitoes were reared at 27 °C and 80% humidity on a 12-h light to dark cycle under standard laboratory conditions. The four P. falciparum strains used (NF54, 3D7, GB4 and 7G8) were maintained in O+ human erythrocytes using RPMI medium 1640 supplemented with 25 mM Hepes, 50 mg/L hypoxanthine, 25 mM NaHCO3, and 10% (vol/vol) heat-inactivated type O+ human serum at 37 °C and a gas mixture of 5% O2, 5% CO2, and balance N2 (21, 22).
Experimental Infection of Mosquitoes with P. falciparum.
A. gambiae females were infected artificially by membrane feeding with P. falciparum gametocyte cultures. Gametocytogenesis was induced as previously described (23). Mature gametocyte cultures (stages IV and V) that were 14–16 d were used to feed mosquitoes using membrane feeders at 37 °C for 30 min. Midguts were dissected 8 d after feeding, and oocysts were stained with 0.05% (wt/vol) mercurochrome in water and counted by light microscopy. The distribution of parasite numbers in individual mosquitoes between control and experimental groups was compared using the nonparametric Mann–Whitney test. All parasite phenotypes were confirmed in two to three independent experiments.
dsRNA-Mediated Gene Knockdown.
Individual female A. gambiae mosquitoes were injected 1–2 d postemergence as previously described (24). Briefly, mosquitoes were injected with 69 nL 3 μg/μL dsRNA solution 3–4 d before receiving a Plasmodium-infected blood meal. dsRNA was produced using the MEGAscript RNAi Kit (Ambion) using DNA templates obtained by PCR using A. gambiae cDNA and the primers previously described (25) with T7 polymerase promoter sites added in the 5′ end. Gene silencing was assessed in whole-sugar feed mosquitoes by quantitative real-time PCR using primers previously described (25) except for TEP1, which was quantitated using primers TEP1-qF (5′-GTTTCTCACCGCGTTCGT-3′) and TEP1-qR (5′-AACCAATCCAATGCCTTCTC-3′). All gene silencing phenotypes were confirmed in two to three independent experiments.
Genotyping of TEP1 Alleles.
DNA was extracted from legs of individual mosquitoes as previously described (26). Identification of TEP1 allele S3 or R1 in individual mosquitoes was done by PCR with allele-specific reverse primers (TEP1-For: 5′-AAAGCTACGAATTTGTTGCGTCA-3′, TEP1-R1-Rev: 5′-CCTCTGCGTGCTTTGCTT-3′, and TEP1-sR5-Rev: 5′-GTTGATTCACCAACCAATTCAT-3′) as previously described (10).
Supplementary Material
Acknowledgments
We thank Andre Laughinghouse, Kevin Lee, Tovi Lehman, and Robert Gwadz for insectary support and Lois Bangiolo for experimental assistance. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 11078 (volume 109, number 28).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121183109/-/DCSupplemental.
References
- 1.World Health Organization W . World Malaria Report. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Vogel G. The ‘do unto others’ malaria vaccine. Science. 2010;328:847–848. doi: 10.1126/science.328.5980.847. [DOI] [PubMed] [Google Scholar]
- 4.Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar J. 2005;4:3. doi: 10.1186/1475-2875-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins FH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 6.Niaré O, et al. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science. 2002;298:213–216. doi: 10.1126/science.1073420. [DOI] [PubMed] [Google Scholar]
- 7.Riehle MM, et al. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. doi: 10.1126/science.1124153. [DOI] [PubMed] [Google Scholar]
- 8.Paskewitz SM, Brown MR, Lea AO, Collins FH. Ultrastructure of the encapsulation of Plasmodium cynomolgi (B strain) on the midgut of a refractory strain of Anopheles gambiae. J Parasitol. 1988;74:432–439. [PubMed] [Google Scholar]
- 9.Zheng L, et al. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
- 10.Blandin S, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 11.Fraiture M, et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandin SA, et al. Dissecting the genetic basis of resistance to malaria parasites in Anopheles gambiae. Science. 2009;326:147–150. doi: 10.1126/science.1175241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garver LS, Xi Z, Dimopoulos G. Immunoglobulin superfamily members play an important role in the mosquito immune system. Dev Comp Immunol. 2008;32:519–531. doi: 10.1016/j.dci.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitri C, et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e1000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohuet A, et al. Anopheles and Plasmodium: From laboratory models to natural systems in the field. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White BJ, et al. Adaptive divergence between incipient species of Anopheles gambiae increases resistance to Plasmodium. Proc Natl Acad Sci USA. 2011;108:244–249. doi: 10.1073/pnas.1013648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, et al. Quantitative trait loci in Anopheles gambiae controlling the encapsulation response against Plasmodium cynomolgi Ceylon. BMC Genet. 2003;4:16. doi: 10.1186/1471-2156-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira GdeA, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Zolg JW, MacLeod AJ, Dickson IH, Scaife JG. Plasmodium falciparum: Modifications of the in vitro culture conditions improving parasitic yields. J Parasitol. 1982;68:1072–1080. [PubMed] [Google Scholar]
- 23.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 24.Molina-Cruz A, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 25.Jaramillo-Gutierrez G, et al. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154. doi: 10.1186/1471-2180-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truett GE, et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]