Abstract
In the Drosophila embryo, formation of a bone morphogenetic protein (BMP) morphogen gradient requires transport of a heterodimer of the BMPs Decapentaplegic (Dpp) and Screw (Scw) in a protein shuttling complex. Although the core components of the shuttling complex—Short Gastrulation (Sog) and Twisted Gastrulation (Tsg)—have been identified, key aspects of this shuttling system remain mechanistically unresolved. Recently, we discovered that the extracellular matrix protein collagen IV is important for BMP gradient formation. Here, we formulate a molecular mechanism of BMP shuttling that is catalyzed by collagen IV. We show that Dpp is the only BMP ligand in Drosophila that binds collagen IV. A collagen IV binding–deficient Dpp mutant signals at longer range in vivo, indicating that collagen IV functions to immobilize free Dpp in the embryo. We also provide in vivo evidence that collagen IV functions as a scaffold to promote shuttling complex assembly in a multistep process. After binding of Dpp/Scw and Sog to collagen IV, protein interactions are remodeled, generating an intermediate complex in which Dpp/Scw-Sog is poised for release by Tsg through specific disruption of a collagen IV–Sog interaction. Because all components are evolutionarily conserved, we propose that regulation of BMP shuttling and immobilization through extracellular matrix interactions is widely used, both during development and in tissue homeostasis, to achieve a precise extracellular BMP distribution.
Bone morphogenetic proteins (BMPs) form a conserved family of signaling proteins with important functions during development and disease. BMP activity is regulated by a large number of extracellular proteins that modulate BMP receptor binding, stability, or distribution (1, 2). The early Drosophila embryo serves as an excellent model system to study extracellular mechanisms of BMP regulation, because a gradient of BMP activity specifies cell fates along the dorsoventral axis (3). Although the two BMP ligands, Dpp and Scw, are broadly expressed, a conserved set of extracellular regulators mediate the redistribution of the Dpp/Scw heterodimer to the dorsal midline, where peak signaling occurs (4). In dorsal regions, Dpp and Scw are bound by Sog and Tsg into an inhibitory shuttling complex that is unable to bind to receptors but is capable of moving (5–9). Dpp/Scw is released from the shuttling complex by the protease Tolloid, which cleaves and inactivates Sog (10). If Tolloid cleavage of Sog within the complex occurs in dorsolateral regions near the Sog source, Dpp/Scw is re-bound by Sog/Tsg. Multiple cycles of complex cleavage and reformation can occur, until Tolloid cleaves the complex in dorsalmost regions where the concentration of Sog is low, allowing Dpp/Scw to bind its receptors and activate high levels of signaling (5).
We have shown that the extracellular matrix protein collagen IV is important for BMP gradient formation (11). In embryos mutant for the collagen IV genes viking or Dcg1, peak signaling is lost and Dpp accumulation at the dorsal midline is reduced. In vitro experiments revealed that the C-terminal NC1 domain of collagen IV can bind to both Dpp and Sog. Based on these and additional findings, we proposed that collagen IV enhances BMP gradient formation by facilitating the assembly of the Dpp/Scw-Sog-Tsg shuttling complex, thereby promoting the long-range movement of BMPs (11). Although this model is supported by recent quantitative modeling of the BMP gradient in the Drosophila embryo (12), the molecular mechanism of shuttling complex assembly on collagen IV, and whether it occurs in vivo to regulate BMP distribution, is unknown.
Here, we have mapped the collagen IV binding sites on both Dpp and Sog. Based on these and previous interaction studies, we formulate a multistep, molecular model for the assembly of the Dpp/Scw-Sog-Tsg shuttling complex on collagen IV, which we test in vivo by using a collagen IV binding deficient mutant of Dpp. These experiments also provide in vivo evidence that collagen IV restricts movement of free Dpp ligands. We propose that collagen IV may regulate short- and long-range signaling of BMPs in diverse contexts.
Results
Mapping the Collagen IV binding Site in Dpp.
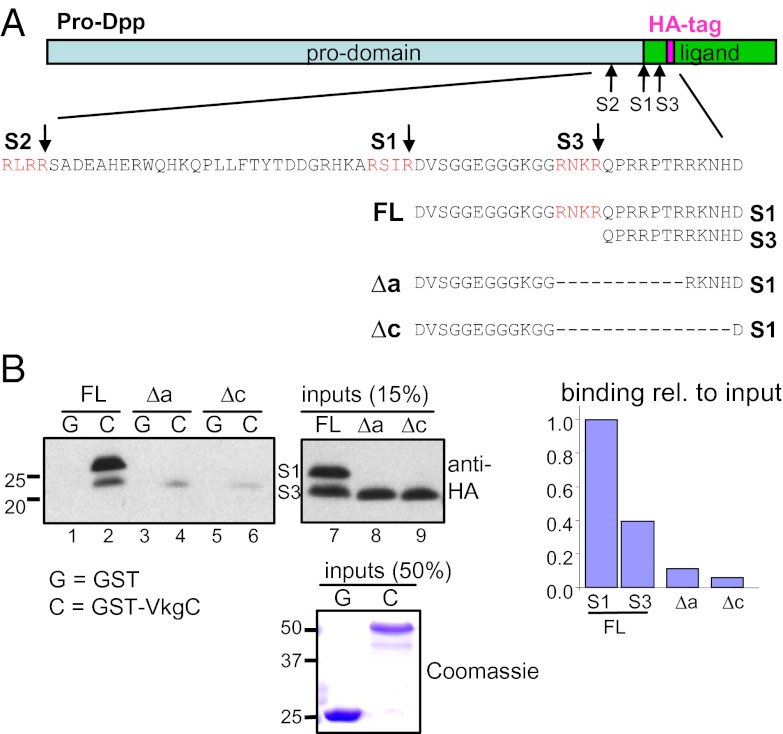
We previously showed that the NC1 domain of the Drosophila collagen IV proteins Viking and Dcg1 (named VkgC and Dcg1C, respectively) can directly interact with an HA-tagged form of Dpp (11). Mature Dpp is secreted from Drosophila S2 cells in two forms resulting from alternative proprotein cleavage at the S1 or S3 site (13). In GST-pulldown (GST-PD) experiments with bacterially purified GST-VkgC, we found that the larger S1 form binds better to collagen IV than the N-terminally truncated S3 form (Fig. 1 A and B; compare ratio of S1 to S3 forms in bound vs. input FL fractions). This result suggested that the N-terminus of mature Dpp is important for binding to collagen IV. The S3 site and downstream residues form a highly basic stretch (Fig. 1A). Partial or complete deletion of this basic region in Dpp-Δa and Dpp-Δc, respectively, strongly reduced binding of Dpp to collagen IV (Fig. 1B), demonstrating that the basic motif is important for its binding to collagen IV. These data also suggest that the observed binding of the S3 form to VkgC (Fig. 1B, lane 2) is likely to be due to its dimerization with the S1 form.
Fig. 1.
A basic region at the start of Dpp mediates binding to collagen IV. (A) Schematic of the Dpp proprotein with the amino acid sequence of Dpp in the region of the furin processing sites (S1, S2, S3) directly upstream of the HA-tagged ligand domain. The sequences of S1/S3 forms of mature wild-type Dpp and the deletion mutants used in B are also shown. Note that our S1/S3 site nomenclature, which is consistent with ref. 31, is opposite to that of ref. 13, with our S1 site corresponding to their S3 site. (B) GST-PD between GST-VkgC and wild-type or deletion mutants of Dpp-HA. Deletion of the basic region strongly reduces binding to GST-VkgC. FL, full-length.
Collagen IV Binding Site Is Not Conserved in Other Drosophila BMPs.
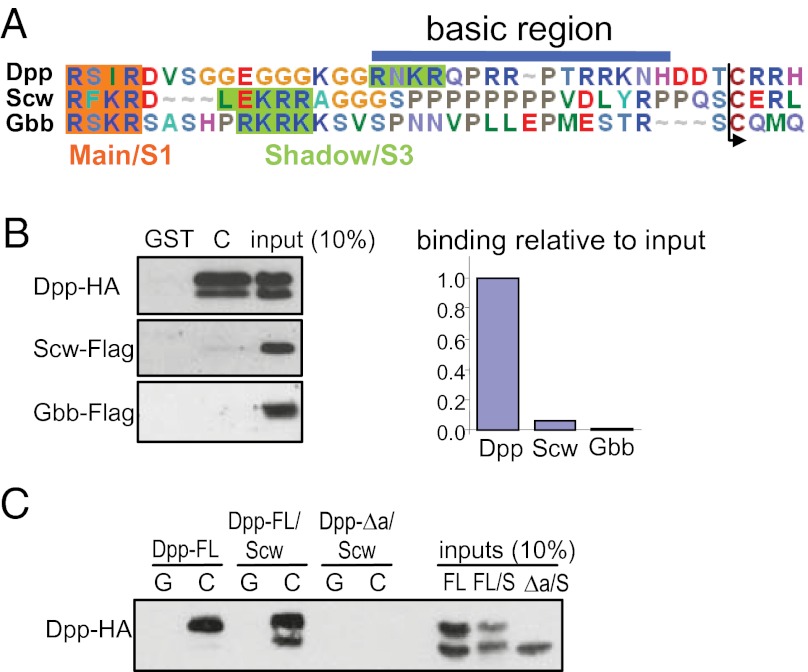
We recently determined the N-termini of the other two Drosophila BMP ligands, Scw and Glass bottom boat (Gbb) (14). Sequence alignment reveals that the Scw and Gbb ligand domains lack the basic stretch found in the S1 form of Dpp (Fig. 2A). Consistent with the absence of a basic motif, Scw and Gbb show little or no binding to VkgC (Fig. 2B). These results further validate the Dpp basic motif as a collagen IV binding site and show that the only Drosophila BMP protein that can interact with the collagen IV NC1 domain is Dpp.
Fig. 2.
Dpp is the only BMP ligand in Drosophila to bind collagen IV. (A) Alignment of Dpp, Gbb, and Scw proteins at the N-terminus of the mature ligand domain. Main (S1 for Dpp) and Shadow (S3 for Dpp) processing sites are highlighted. The basic sequence in Dpp required for binding to collagen IV is not conserved in Gbb or Scw. (B) GST-PD between GST-VkgC and Dpp-HA, Scw-FLAG, or Gbb-FLAG (Left). Scw-FLAG and Gbb-FLAG show very weak binding to GST-VkgC (Right). (C) GST-PD between GST-VkgC and either Dpp-FL monomer, or Dpp-FL/Scw or Dpp-Δa/Scw heterodimer. Dpp-FL/Scw shows strong binding to VkgC, which depends on the collagen IV binding site in Dpp, because Dpp-Δa/Scw cannot bind to VkgC.
In the early embryo, the Dpp/Scw heterodimer is proposed to be both the most potent signaling species and the best substrate for Sog/Tsg-mediated shuttling to the dorsal midline (9). As shown in Fig. 2C, the Dpp/Scw heterodimer displays similar, if not better, binding to VkgC as the Dpp homodimer, again showing relatively more binding of the Dpp S1 form. Consistent with Scw being unable to bind the collagen IV NC1 domain, the collagen IV binding activity of the Dpp/Scw heterodimer is mediated by the basic motif in Dpp, because the DppΔa/Scw heterodimer is unable to interact with VkgC (Fig. 2C).
Mapping the Collagen IV Binding Site in Sog.
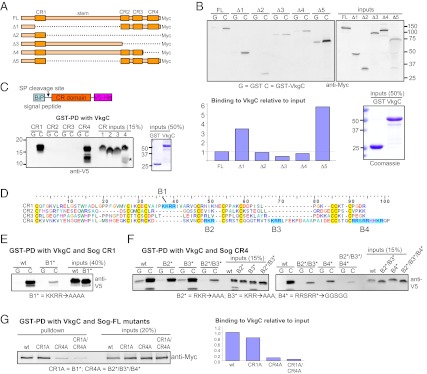
We next mapped the collagen IV binding sites on Sog. Sog is a large glycoprotein consisting of four cysteine rich (CR) domains separated by a long “stem” region between CR1 and CR2 (10) (Fig. 3A). To identify the regions of Sog important for interaction with the collagen IV NC1 domain, we tested binding of a panel of deletion constructs (Fig. 3 A and B). Overall, these experiments suggest that (i) the CR domains mediate binding to collagen IV in a partially redundant fashion (e.g., compare FL vs. Δ3 vs. Δ4), and (ii) the stem region has an inhibitory effect on binding of Sog to collagen IV (compare FL vs. Δ5). We next tested the binding of each CR domain individually. As shown in Fig. 3C, CR1 and CR4 bound efficiently to VkgC, whereas no binding was detected for CR2 and CR3. An alignment of the Sog CR domains revealed that both CR1 and CR4 contain basic motifs (named B1, B2, B3, and B4) not found in CR2 or CR3 (Fig. 3D). For Sog CR1, mutation of B1 greatly reduced binding to VkgC (Fig. 3E). For Sog CR4, B2, B3, or B4 single or double mutants caused a partial loss of binding to VkgC, and the B2/B3/B4 triple mutant further attenuated the interaction (Fig. 3F), suggesting that all three basic motifs in CR4 function with partial redundancy in binding collagen IV. Mutation of either B1 or B2/B3/B4 in the context of full-length Sog also partially blocked binding to VkgC, and binding was very weak when basic motifs were mutated in both CR1 and CR4 (Fig. 3G).
Fig. 3.
Basic sequences in the CR1 and CR4 domains of Sog mediate binding to collagen IV. (A) Domain structure of full-length (FL) Sog and deletion mutants used in B. (B) GST-PD between GST-VkgC and Myc-tagged Sog deletion mutants. Binding of Sog was quantified as bound protein relative to input levels after subtraction of background binding to GST and normalization to Sog-FL. (C) GST-PD between GST-VkgC and individual CR domains of Sog. Only Sog CR1 and CR4 interact with VkgC. CR4 expression results in a degradation product (asterisk). (D) Alignment of Sog CR domains reveals basic motifs in CR1 (B1) and CR4 (B2, B3, B4). (E–G) Mutation of basic motifs in Sog CR1 (E), CR4 (F), or full-length Sog (G) attenuates binding to GST-VkgC. In panel (G) binding was quantified as bound protein relative to input levels, with values of Sog-FL mutants normalized to that of wild-type Sog-FL.
Molecular Model for Shuttling Complex Assembly on Collagen IV.
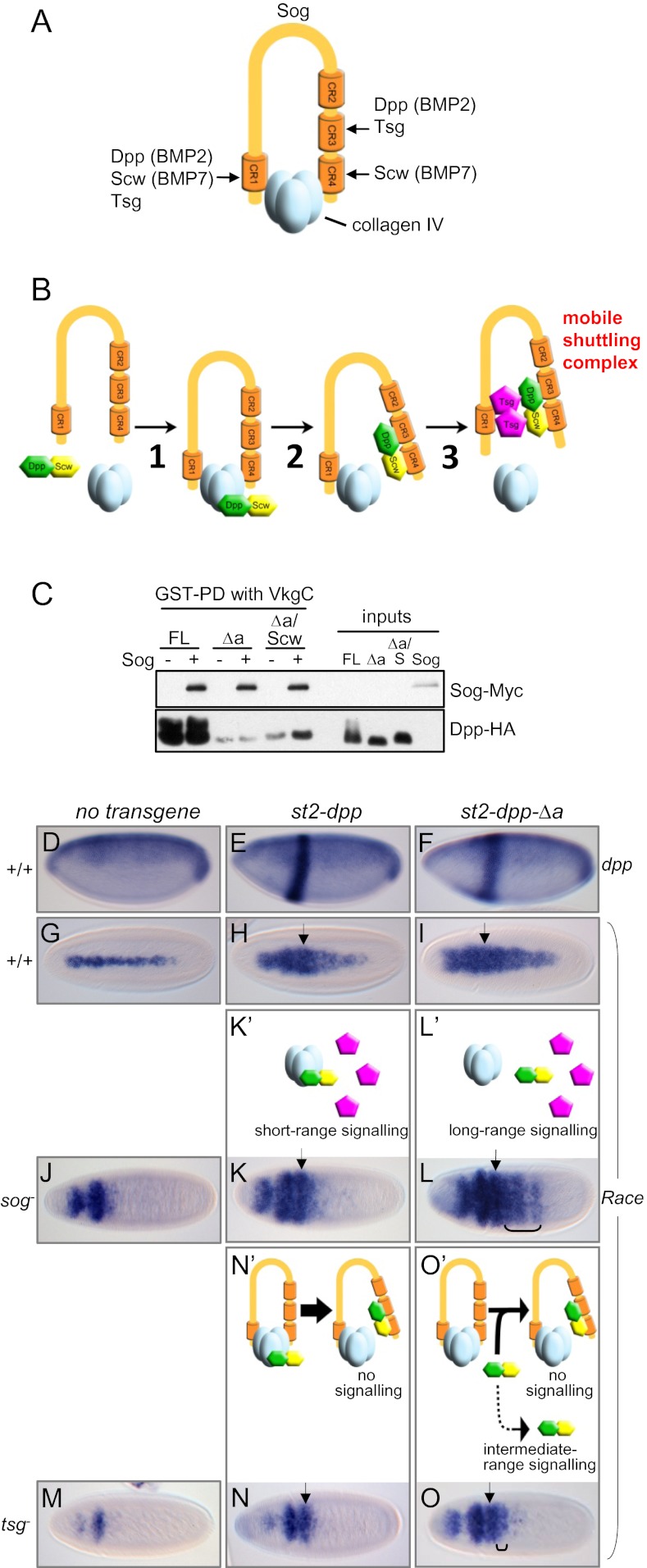
We previously presented evidence that collagen IV enhances BMP transport dorsally and proposed that it acts as a scaffold to enhance shuttling complex formation (11). The identification of the collagen IV binding sites on Sog and Dpp, combined with preexisting data, allows us to formulate a molecular model for the assembly of the Dpp/Scw-Sog-Tsg shuttling complex on collagen IV. Our previous data showed that the Dpp/Scw heterodimer and Sog together can bind VkgC, but the addition of Tsg releases Dpp/Scw and Sog from collagen IV into the Dpp/Scw-Sog-Tsg shuttling complex (11). Here, we show that the Dpp/Scw heterodimer has only one binding site for collagen IV and that Sog has two binding domains, CR1 and CR4. These findings suggest a probable mechanism of release of Dpp/Scw and Sog from collagen IV into the shuttling complex. The Dpp/Scw heterodimer and Tsg together, but neither of them alone, can release Sog from VkgC (11), suggesting that each—Dpp/Scw and Tsg—competes with one of the two collagen IV binding sites on Sog. In vertebrates, the Dpp ortholog BMP2 and Tsg both bind strongly to CR1 and CR3 (15, 16), whereas the Scw-related protein BMP7 interacts preferentially with CR1 and CR4 (15) (Fig. 4A). Because only Scw binds to CR4 with high affinity, we propose that Dpp/Scw interferes with the Sog-CR4/VkgC interaction (via Scw binding to CR4), whereas Tsg competes for binding of collagen IV to Sog-CR1. Furthermore, because neither Sog nor Tsg alone can release Dpp from VkgC (11), we suggest a multistep model for shuttling complex assembly by collagen IV, in which release of Dpp/Scw is coupled to its transfer onto Sog. In the first step, Sog and Dpp/Scw both bind to the NC1 domain of collagen IV within the same NC1 trimer (Fig. 4B, step 1). This binding restricts Dpp and Sog diffusion, but it also promotes the transfer of Dpp/Scw onto Sog, through interactions of Dpp with Sog-CR3 and of Scw with Sog-CR4, which leads to disruption of both Dpp/collagen IV and Sog-CR4/collagen IV interactions (Fig. 4B, step 2). The resulting Dpp/Scw-Sog complex is bound to collagen IV by the Sog–CR1 interaction only and, therefore, poised for release by Tsg, which outcompetes collagen IV for Sog-CR1 binding (Fig. 4B, step 3). Consistent with the formation of a Dpp/Scw-Sog “poised” intermediate on collagen IV, we show that Dpp-Δa/Scw, which lacks the collagen IV binding site, can associate with GST-VkgC in the presence Sog (Fig. 4C).
Fig. 4.
Molecular model for Dpp/Scw-Sog-Tsg shuttling complex assembly on collagen IV. (A) Binding domains on Sog for collagen IV (VkgC), Dpp (BMP2), Scw (BMP7), and Tsg. (B) Molecular model for BMP shuttling complex assembly on collagen IV. (C) GST-PD between GST-VkgC or VkgC-Sog complex and Dpp, Dpp-Δa, or Dpp-Δa/Scw. (D–O) RNA in situ hybridizations for dpp (D–F; lateral views, dorsal up) or the BMP target gene Race (G-O; dorsal views) in control embryos and those expressing st2-dpp or st2-dpp-Δa transgenes. Embryos are wild-type (D–I), sog− (J–L) or tsg− (M–O). Brackets mark ectopic Race induction posterior of the st2 expression domain (arrows). Anterior is left in all images. (K’–O’) Cartoons depicting Dpp/Scw interactions as predicted by our molecular model. Intermediate-range signaling is attributed to a small number of Dpp/Scw molecules undergoing long-range signaling. Signaling in the st2 domain is at least in part attributed to Dpp homodimers (omitted from cartoons for simplicity).
We used the collagen IV binding site mutant of Dpp (Dpp-Δa) to test our model in the context of the early embryo. First, we made use of a system for monitoring Dpp-Δa movement and signaling, based on ectopic expression from a stripe that runs perpendicular to the normal dpp expression domain (Fig. 4 D–F) (17). Activity of the peak threshold BMP target gene Race was visualized, which is normally expressed in a narrow stripe along the dorsal midline (presumptive amnioserosa) (Fig. 4G). As shown (7, 17), misexpression of wild-type Dpp under the even-skipped stripe 2(st2)-enhancer activates BMP signaling in cells within and outside the stripe, leading to expanded Race expression (Fig. 4H), consistent with the ability of the Dpp/Scw heterodimer to move long-range in the early embryo. In st2-dpp-Δa embryos, Race expression is also broadened and extends more posteriorly than in st2-dpp embryos (Fig. 4I), suggesting that Dpp-Δa can signal normally and may be more mobile than wild-type Dpp. Additionally, evidence is presented that the Δa mutation does not affect Dpp protein levels (Fig. S1).
We next used this expression system to test key aspects of our model of shuttling complex assembly by examining the behavior of Dpp-Δa in sog− and tsg− backgrounds. In both mutants, Race expression is lost in the presumptive amnioserosa but expanded in the anterior head region (Fig. 4 J and M), reflecting the loss of peak signaling and uniform intermediate signaling, due to the loss of dorsal BMP shuttling (6). In our model, collagen IV restricts movement of free Dpp (i.e., when not bound to Sog); thus, one prediction is that Dpp-Δa, unlike wild-type Dpp, can move and signal long range in a sog− background (Fig. 4 K′ and L′). When st2-dpp-Δa is expressed in sog− embryos, it induces ectopic Race expression outside of its expression domain (Fig. 4L), suggesting that it is capable of long-range movement. This property is in stark contrast to that of ectopically expressed wild-type Dpp, whose effect is restricted to the st2 expression domain (7) (Fig. 4K). A second feature of our model is that Tsg acts to release the Dpp/Scw-Sog complex from collagen IV by disrupting the Sog-collagen IV interaction. Therefore, we predict Dpp-Δa would, in general, be unable to move and signal long range in a tsg− background, where most Dpp-Δa/Scw-Sog should remain trapped on collagen IV (Fig. 4O′). Indeed, in the absence of Tsg, the effect of Dpp-Δa is largely restricted to its expression stripe (Fig. 4O). Finally, our model proposes that collagen IV enhances the association of Dpp/Scw and Sog, implying that formation of the Dpp/Scw-Sog complex should be less efficient for Dpp-Δa. In the tsg mutant, where assembly of the Dpp/Scw-Sog complex immobilizes BMPs on collagen IV, we therefore predict that some Dpp-Δa/Scw can move out of its expression stripe, whereas all wild-type Dpp/Scw is trapped by collagen IV-Sog (Fig. 4 N′ and O′). Indeed, compared with wild-type Dpp, which is entirely restricted to its expression domain in tsg− embryos (Fig. 4N), some Dpp-Δa can activate signaling in cells away from its source. This intermediate-range signaling is observed as Race induction in a limited number of cells posterior to the st2 domain in the majority (∼70%) of embryos (Fig. 4O). In summary, our in vivo data support three key predictions from our model of shuttling complex assembly.
Discussion
There is ample experimental and theoretical support for the notion that BMP gradient formation in the early embryo involves the concentration of the most potent signaling species, the Dpp/Scw heterodimer, at the dorsal midline in a process involving Sog and Tsg (4). Here, we present in vivo evidence for a role of collagen IV in two key aspects of this shuttling model, which have remained mechanistically unresolved. First, collagen IV functions to immobilize free Dpp, explaining why Sog and Tsg are needed for Dpp movement. Second, collagen IV acts as a scaffold for assembly of the Dpp/Scw-Sog-Tsg shuttling complex. The advantage to BMP gradient formation of assembling the shuttling complex on collagen IV has been suggested by analysis of organism-scale mathematical models (12). These models reveal that the in vitro binding affinity between BMPs and Sog is too low to account for the rate of shuttling complex formation required in vivo. However, by acting as a scaffold, collagen IV would increase complex formation by locally concentrating Dpp/Scw and Sog. Models with a 10–20% reduction in diffusion rates for Dpp/Scw and Sog and an increased apparent affinity of Dpp/Scw for Sog, show the best fit to in vivo data (12).
Our molecular model of shuttling complex assembly occurs in three steps. The first step involves independent binding of Dpp/Scw and Sog to collagen IV. The ability of Dpp-Δa to signal long range in sog− embryos, where wild-type Dpp is trapped in its expression stripe, provides in vivo evidence that the Dpp-collagen IV interaction restricts movement of free Dpp ligands. The result also demonstrates that Sog and Tsg promote long-range movement of Dpp because they release Dpp from collagen IV, and not simply because they prevent Dpp–receptor interactions (8, 18). Restriction of Dpp diffusion by collagen IV may stabilize the gradient by preventing ventral movement of Dpp/Scw after release from Sog/Tsg and promoting Dpp/Scw–receptor interactions at the dorsal midline. It will be interesting, ultimately, to directly visualize Dpp and Dpp-Δa directly in sog and tsg mutant embryos. Although current methods allow detection of high levels of receptor-bound Dpp (8, 9), there are technical limitations associated with specifically detecting the pools of Dpp that would be informative here, i.e., Dpp/Scw heterodimer within the shuttling complex or Dpp-Δa/Scw diffusing between cells. Our data show that Scw is unable to bind the NC1 domain of collagen IV. This lack of collagen IV-dependent immobilization can explain why Scw, unlike Dpp, is capable of long-range signaling in the absence of Sog (8).
Step 2 of shuttling complex assembly involves remodeling of the protein interactions to generate a poised intermediate. Specifically, step 2 is driven by Scw-mediated disruption of the Sog CR4–collagen IV interaction, so that Dpp/Scw is transferred from collagen IV to the Sog CR3-CR4 domains. Scw displacement of the Sog CR4 domain from collagen IV provides molecular insight as to why Scw is needed for Dpp transport (9). In addition to the binding preference of Sog and Tsg for the Dpp/Scw heterodimer (9), only Scw has a high affinity for the Sog CR4 domain. Therefore, Dpp/Scw can be released from collagen IV into the shuttling complex, whereas the Dpp homodimer remains trapped on collagen IV.
In the final step of our model, Tsg mobilizes the shuttling complex by disrupting the Sog CR1–collagen IV interaction. It has been noted that tsg mutants display a more severe reduction in BMP signaling than sog and sog tsg double mutants (8) (also see Race expression in Fig. 4 J and M). This observation has been attributed to a potential Sog-independent pro-BMP activity of Tsg at the level of receptor binding (8). A second contributing factor is suggested by our model, where Sog and Tsg act at distinct steps to allow formation of the shuttling complex. In tsg mutants, Dpp/Scw is loaded onto Sog by collagen IV, but remains locked in this inhibitory poised complex, so that the only BMPs capable of signaling are Dpp and Scw homodimers, which are less potent than the Dpp/Scw heterodimer (9). By contrast, in sog or sog tsg mutants, Dpp/Scw is not shuttled dorsally but is still capable of signaling locally, adding to signaling by Dpp and Scw homodimers. The weaker level of Dpp/Scw signaling in tsg mutants also provides support for our proposed order of steps 2 and 3 in the assembly process, because this order gives rise to the inhibitory intermediate of Dpp/Scw-Sog. Previously it was shown that an N-terminal fragment of Sog, called Supersog, which contains the CR1 domain and a portion of the stem, can partially rescue the loss of peak Dpp/Scw signaling in tsg− embryos (19). Our model suggests that this property of Supersog comes from the ability of its CR1 domain to compete with full-length Sog for binding to collagen IV, thereby releasing Sog-Dpp/Scw, similar to the role of Tsg in shuttling complex assembly. We note that the CR1–collagen IV interaction appears weaker than that of CR4–collagen IV (Fig. 3G), which may facilitate release of Dpp/Scw by Tsg or Supersog-like fragments. After Tsg-mediated release from collagen IV, the mobile shuttling complex can diffuse randomly. Upon Tolloid cleavage of Sog, the liberated Dpp/Scw heterodimer rebinds collagen IV, which either promotes receptor binding (11) or a further round of shuttling complex assembly, depending on the local concentration of Sog.
In addition to collagen IV, the basic region in Dpp/BMP2/4 also binds to heparan sulfate proteoglycans (HSPGs), which can either restrict or enhance BMP long-range movement (20, 21). Indeed, we find that an HSPG-binding mutant, Dpp-ΔN, also binds only weakly to collagen IV (Fig. S2), suggesting that the collagen IV- and HSPG-binding sites on Dpp overlap. It will be interesting to test how HSPGs and collagen IV interact to regulate BMP activity in tissues where they are coexpressed, such as the early vertebrate embryo (20). In the early Drosophila embryo, the absence of glycosaminoglycan chains (22), which largely mediate binding of HSPG to Dpp (23), make it possible to specifically focus on the Dpp–collagen IV interaction.
A shuttling-based mechanism of BMP transport is also used in a number of other developmental contexts, including the early vertebrate embryo (24), specification of the vertebral field in mice (25), and establishment of the posterior cross-vein territory in the Drosophila wing disk (26, 27). Restriction of BMP movement may also be important in other contexts, including several where collagen IV was already shown to regulate a short-range Dpp signal, such as the ovarian stem cell niche and the tip of malpighian tubules (8, 28). The basic collagen IV binding motif is highly conserved among the Dpp/BMP2/4 subfamily (20) and is also found in some other BMPs, including BMP3, consistent with reports that BMP3 and BMP4 can bind collagen IV (11, 29). Overall, these findings support the idea that the collagen IV–BMP interaction is a conserved aspect of extracellular BMP regulation and suggest that the function of collagen IV in both long-range BMP shuttling and local restriction of BMP movement will impact on a number of other contexts in both flies and vertebrates.
Materials and Methods
DNA Constructs.
Cu-inducible Scw and Gbb constructs (pMT-Scw-1xFLAG and pMT-Gbb-3xFLAG) (14), DppΔN-HA (21), and pGEX4T1-VkgC (11) have been described. The coding sequences of Dpp-HA (10) and Sog-Myc (19) were inserted into pMT-V5/His-A (Life Technologies) and then modified by PCR to introduce deletions or mutations (details available on request). To express individual Sog CR domains, regions encoding amino acids 95–186 (CR1), 737–814 (CR2), 825–909 (CR3), or 935–1031 (CR4) were cloned into pMT-Bip-V5/His-A (Life Technologies) in frame with the C-terminal V5-His tag. For transgenesis, the Dpp-Δa deletion was introduced into SK-Asc2-Dpp (17) by PCR and transferred as an AscI fragment into the 22FPE vector (30).
Protein Expression and Purification.
Scw-FLAG, Gbb-FLAG, Dpp-HA, and Sog-Myc proteins were produced in Drosophila S2R+ cells by effectene-mediated transient transfection followed by Cu-induction as described (14). Sog-CR4 was expressed in S2 cells, because secretion from S2R+ cells was inefficient. For Dpp/Scw heterodimers, we cotransfected 3.5 μg of pMT-Dpp-HA and 1.5 μg of pMT-Scw-1xFLAG. Heterodimers were purified from 2 mL of medium by incubation with 50 μL of anti-FLAG M2 matrix (Sigma) according to manufacturer’s instructions. GST and GST-VkgC were expressed in Escherichia coli BL21 cells and purified on GSH-Sepharose beads (GE Healthcare) (details of purification available on request).
GST-Pulldown Experiments and Western Blotting.
Equal amounts of GST-fusion proteins bound to GSH beads were incubated with 20–200 μL of Dpp-HA, Scw-FLAG, Sog-Myc, or Sog-CR-domain-V5-His-transfected medium or 100 μL of affinity-purified Scw-FLAG/Dpp-HA complexes in pulldown buffer (20 mM at Tris pH 8.0, 100 mM NaCl, 1 mM EDTA) at 4 °C for 1–2 h. Beads were washed three times in 1 mL of pulldown buffer + 0.1% Nonidet P-40, and eluted in Laemmli buffer. Samples were separated by reducing SDS/PAGE, followed by Western blotting or Coomassie blue staining. Antibodies used were as follows: mouse anti-HA 1:2,000 (Roche), anti-FLAG M2 1:500 (Sigma), anti-Myc 9E10 1:2,000 (Santa Cruz) or anti-Myc 4A6 1:500 (Millipore), and anti-V5 1:2,000 (Abcam).
Fly Strains.
Flystocks used were y67c23w118, sogS6/FM7c, tsg2/FM7c (both from Bloomington Drosophila Stock Center), st2-dpp (17). st2-dpp-Δa lines were generated at Bestgene by injecting 22FPE-dppΔa into y1w1118 embryos. Transgene expression was activated as described (6).
Embryo Collection and RNA in Situ Hybridization.
Embryos were collected from crosses of yw, sogS6/FM7c or tsg2/FM7c females to homozygous st2-dpp or st2-dppΔa males. Embryos were fixed and processed for RNA in situ hybridization with digU-labeled RNA probes by using standard protocols.
Supplementary Material
Acknowledgments
We thank Abbie Saunders and Mark Ashe for critical reading of the manuscript. This work was supported by Wellcome Trust Studentship 083271/Z/07/Z and Project Grant WT083211AIA, and by the Drosophila Core Research Facility at the Faculty of Life Sciences, University of Manchester, established through funds from the University and the Wellcome Trust (087742/Z/08/Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202781109/-/DCSupplemental.
References
- 1.Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- 2.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holley SA, et al. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 6.Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- 7.Eldar A, et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- 8.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 9.Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marqués G, et al. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang XM, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 12.Umulis DM, Shimmi O, O’Connor MB, Othmer HG. Organism-scale modeling of early Drosophila patterning via bone morphogenetic proteins. Dev Cell. 2010;18:260–274. doi: 10.1016/j.devcel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Künnapuu J, Björkgren I, Shimmi O. The Drosophila DPP signal is produced by cleavage of its proprotein at evolutionary diversified furin-recognition sites. Proc Natl Acad Sci USA. 2009;106:8501–8506. doi: 10.1073/pnas.0809885106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsch C, et al. Different requirements for proteolytic processing of bone morphogenetic protein 5/6/7/8 ligands in Drosophila melanogaster. J Biol Chem. 2012;287:5942–5953. doi: 10.1074/jbc.M111.316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem. 2007;282:20002–20014. doi: 10.1074/jbc.M700456200. [DOI] [PubMed] [Google Scholar]
- 16.Fujisawa T, Huang Y, Sebald W, Zhang J-L. The binding of von Willebrand factor type C domains of Chordin family proteins to BMP-2 and Tsg is mediated by their SD1 subdomain. Biochem Biophys Res Commun. 2009;385:215–219. doi: 10.1016/j.bbrc.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Ashe HL, Mannervik M, Levine M. Dpp signaling thresholds in the dorsal ectoderm of the Drosophila embryo. Development. 2000;127:3305–3312. doi: 10.1242/dev.127.15.3305. [DOI] [PubMed] [Google Scholar]
- 18.Mizutani CM, et al. Formation of the BMP activity gradient in the Drosophila embryo. Dev Cell. 2005;8:915–924. doi: 10.1016/j.devcel.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu K, et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205–209. doi: 10.1016/s0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama T, et al. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol. 2008;313:408–419. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornemann DJ, Park S, Phin S, Warrior R. A translational block to HSPG synthesis permits BMP signaling in the early Drosophila embryo. Development. 2008;135:1039–1047. doi: 10.1242/dev.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkpatrick CA, et al. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev Biol. 2006;300:570–582. doi: 10.1016/j.ydbio.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 25.Zakin L, Chang EY, Plouhinec J-L, De Robertis EM. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev Biol. 2010;347:204–215. doi: 10.1016/j.ydbio.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimmi O, Ralston A, Blair SS, O’Connor MB. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol. 2005;282:70–83. doi: 10.1016/j.ydbio.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda S, Shimmi O. Directional transport and active retention of Dpp/BMP create wing vein patterns in Drosophila. Dev Biol. 2012;366:153–162. doi: 10.1016/j.ydbio.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Bunt S, et al. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paralkar VM, et al. Interaction of osteogenin, a heparin binding bone morphogenetic protein, with type-IV collagen. J Biol Chem. 1990;265:17281–17284. [PubMed] [Google Scholar]
- 30.Kosman D, Small S. Concentration-dependent patterning by an ectopic expression domain of the Drosophila gap gene knirps. Development. 1997;124:1343–1354. doi: 10.1242/dev.124.7.1343. [DOI] [PubMed] [Google Scholar]
- 31.Sopory S, Kwon S, Wehrli M, Christian JL. Regulation of Dpp activity by tissue-specific cleavage of an upstream site within the prodomain. Dev Biol. 2010;346:102–112. doi: 10.1016/j.ydbio.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.