Abstract
The NLRP3 (nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3) inflammasome mediates production of inflammatory mediators, such as IL-1β and IL-18, and as such is implicated in a variety of inflammatory processes, including infection, sepsis, autoinflammatory diseases, and metabolic diseases. The proximal steps in NLRP3 inflammasome activation are not well understood. Here we elucidate a critical role for Ca2+ mobilization in activation of the NLRP3 inflammasome by multiple stimuli. We demonstrate that blocking Ca2+ mobilization inhibits assembly and activation of the NLRP3 inflammasome complex, and that during ATP stimulation Ca2+ signaling is pivotal in promoting mitochondrial damage. C/EPB homologous protein, a transcription factor that can modulate Ca2+ release from the endoplasmic reticulum, amplifies NLRP3 inflammasome activation, thus linking endoplasmic reticulum stress to activation of the NLRP3 inflammasome. Our findings support a model for NLRP3 inflammasome activation by Ca2+-mediated mitochondrial damage.
Keywords: innate immunity, mitochondria
Inflammation must be tightly regulated to meet the demands of host defense and stress adaptation while limiting immunopathology and the detrimental effects of chronic inflammation. Sensors of the innate immune system, such as pattern recognition receptors, couple detection of proinflammatory triggers to induction of inflammation in both adaptive and maladaptive settings, so the regulation of this interaction is absolutely critical in control of inflammation (1). A particularly intriguing “sensor” is the NLRP3 (nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3) inflammasome complex, which detects cellular stress in a variety of infectious and “sterile” settings and links this recognition to the induction of a critical inflammatory pathway (2–5).
The NLRP3 inflammasome is a cytoplasmic complex consisting of the regulatory subunit NLRP3, the adaptor ASC, and the effector subunit caspase-1. Stimulus dependent assembly of the complex activates the proteolytic activity of caspase-1, which is required for the processing and secretion of the inflammatory cytokines IL-1β and IL-18 (2–5). In recent years, many different molecules have been shown to activate the NLRP3 inflammasome complex. These molecules include: extracellular ATP released from dying cells (6); alum, the adjuvant activity in human vaccines (7); nigericin, a bacterial toxin with potassium ionophore activity (6); and cholesterol crystals (8), which contribute to the development of atherosclerotic plaques. Such findings underscore the critical role of the NLRP3 inflammasome in inducing inflammation in a variety of diseases.
How these diverse stimuli activate the NLRP3 inflammasome is not clear, but the prevailing view is that they generate some signal of cellular stress that is often referred to as signal 2. Although signal 1 is important for transcriptional up-regulation of the NLRP3 subunit and is typically provided by signaling through Toll-like receptors (9), three cellular processes have been suggested to provide signal 2 (2, 3, 10). The first is K+ efflux, which has been linked to most/all NLRP3 inflammasome activators, but just how K+ efflux can activate the inflammasome in a specific manner is not obvious; moreover, modulating the ionic milieu in other ways also affects NLRP3 inflammasome activation (11, 12). Second, crystals trigger phagolysosomal membrane damage/rupture (13); but how this is linked to the proximal steps in NLRP3 inflammasome activation is not clear, nor is its relevance for stimuli that are not phagocytosed. Finally, other studies implicate mitochondrial damage, including increased mitochondrial reactive oxygen species (mROS) production, loss of membrane potential (ΔΨ), and release of mtDNA into the cytosol (14–16). Importantly, how NLRP3 inflammasome activators trigger mitochondrial damage has not been characterized.
Ca2+ mobilization controls diverse cellular processes, including proliferation and differentiation, transcription, cellular metabolism, and cell death (17). Such Ca2+ mobilization is made possible by maintaining low levels of cytoplasmic Ca2+ at basal state, so that signal-dependent influx of Ca2+ from the extracellular space and intracellular Ca2+ stores (e.g., endoplasmic reticulum, ER) leads to a rapid increase in cytoplasmic Ca2+ levels, activation of Ca2+ binding proteins, and induction of various cellular responses. A key player in Ca2+ signaling is the mitochondria, which take up Ca2+ from the ER or the extracellular space to regulate spatiotemporal patterns of Ca2+ signaling (18–20). However, excessive or sustained mitochondrial Ca2+ uptake can lead to mitochondrial damage and cell death (21–23).
In this article, we show that activation of the NLRP3 inflammasome requires Ca2+ signaling. Several NLRP3 inflammasome activators mobilize Ca2+, disruption of which inhibits NLRP3 inflammasome activation. During ATP stimulation, the crucial role of Ca2+ mobilization is in inducing mitochondrial damage. C/EPB homologous protein (CHOP), a protein known to regulate Ca2+ release from the ER during ER stress, amplifies NLRP3 inflammasome activation.
Results
Ca2+ Signaling Is Critical for NLRP3 Inflammasome Activation by Extracellular ATP.
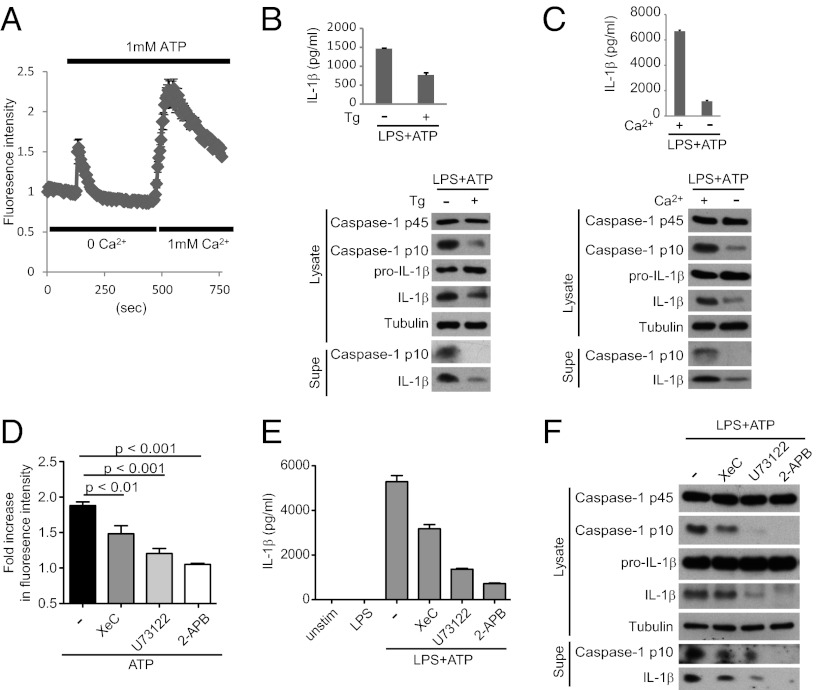
Extracellular ATP activates the NLRP3 inflammasome through P2X7R (24), a ligand-gated ion channel of the purinergic receptor family. Using Ca2+-sensitive fluorometric probes and analysis by time-lapse microscopy to measure cytosolic Ca2+ levels, we confirmed previous findings (25) that Ca2+ is mobilized from both extracellular and intracellular pools during ATP stimulation (Fig. 1A). To determine which pool contributes to NLRP3 inflammasome activation, we used experimental manipulations common in Ca2+ signaling research: treatment with thapsigargin (Tg), an inhibitor of the SERCA (sarcoplasmic/ER Ca2+-ATPase) pump to deplete ER Ca2+ stores, or incubation in Ca2+ free media, to block extracellular Ca2+ entry. We found that brief Tg pretreatment (Fig. 1B) and incubation in Ca2+-free media (Fig. 1C) both attenuate NLRP3 inflammasome activation by ATP stimulation (as measured by caspase-1 processing to the active p10 subunit), IL-1β processing, and release of processed caspase-1 and IL-1β into culture supernatants. This finding suggests that ER Ca2+ release and influx of extracellular Ca2+ are both required to activate the NLRP3 inflammasome.
Fig. 1.
Extracellular ATP mobilizes Ca2+ to activate the NLRP3 inflammasome. (A) LPS-primed BMDMs were loaded with Fura-2 followed by stimulation with 1 mM ATP and analysis of Ca2+ flux by time-lapse microscopy. Ca2+ is released from intracellular stores during the initial stimulation in Ca2+-free buffer, and exchange to 1 mM Ca2+-containing buffer permits extracellular Ca2+ influx. (B and C) LPS-primed BMDMs were treated or not with Tg for 30 min to deplete ER Ca2+ (B) or switched to Ca2+ free (−) or Ca2+-containing (+) media immediately before ATP stimulation (C). NLRP3 inflammasome activation was assessed by Western blotting of lysates and supernatants (supes) as well as IL-1β ELISA. (D–F) BMDMs were pretreated with XeC, U73122, or 2-APB, followed by ATP stimulation and analysis of Ca2+ flux (D) and NLRP3 inflammasome activation (E and F).
We sought to further characterize the mechanisms that regulate Ca2+ mobilization during ATP stimulation. Phospholipase C (PLC) proteins are key players in Ca2+ signaling through their production of the second messengers inositol triphosphate (IP3) and diacylglycerol (17). Using macrophages expressing a PLC reporter (consisting of the PLC pleckstrin homology domain fused to GFP) (26), we showed that the protein translocates from the plasma membrane to the cytosol during ATP stimulation, indicative of PLC activation (Fig. S1A). Importantly ATP-induced Ca2+ flux could be blocked by the PLC inhibitor U73122 (Fig. 1D). PLC activation can be coupled to ER Ca2+ release through the IP3 receptor (IP3R), and indeed treatment with the IP3R inhibitor XeC attenuates ATP-mediated Ca2+ signaling (Fig. 1D). Finally, ER Ca2+ release can trigger extracellular Ca2+ influx through store-operated Ca2+ entry (SOCE) (27). The chemical 2-aminoethoxydiphenyl borate (2-APB), which blocks SOCE at multiple steps (including oligomerization and translocation of the STIM proteins that sense ER Ca2+ depletion, as well as the activity of the Ca2+ channels ORAI), also blocks Ca2+ mobilization (Fig. 1D). Inhibition of Ca2+ flux was strongest with 2-APB and weaker with XeC and U73122, suggesting that other Ca2+-releasing second messengers or ER Ca2+ release mechanisms may be triggered by P2X7R.
These findings indicate that during ATP stimulation, Ca2+ mobilization may be regulated by PLC proteins, IP3R-mediated Ca2+ release, and SOCE. Next, we addressed the role of such Ca2+ mobilization in activation of the NLRP3 inflammasome, using multiple read-outs of NLRP3 inflammasome activity. We found that XeC, U73122, and 2-APB blocked ATP-induced caspase-1 and IL-1β processing and release of processed caspase-1 and IL-1β into culture supernatants (Fig. 1 E and F). As with Ca2+ flux (Fig. 1D), inhibition was strongest with 2-APB and weakest with XeC. Finally, Ca2+ signaling inhibitors blocked assembly of the NLRP3 inflammasome complex as visualized by immunofluorescence (Fig. S2). Although many ATP-stimulated cells (>25%) accumulate foci corresponding to activated NLRP3 inflammasome complexes, cotreatment with Ca2+ signaling inhibitors restricts formation of such foci (<5%). This finding demonstrates that Ca2+ signaling regulates NLRP3 inflammasome activation at an upstream step, proximal to complex assembly.
Of note, XeC, U73122, and 2-APB had no effect on the transcriptional induction of NLRP3 (signal 1) or pro–IL-1β by LPS (Fig. S1B), indicating that Ca2+ mobilization does not regulate expression of the NLRP3 inflammasome or LPS signaling. We also rule out a role for the inhibitors in modulating cell viability (Fig. S1C). In conclusion, Ca2+ signaling—probably regulated by PLC, ER Ca2+ release through the IP3R, and SOCE—is important for NLRP3 inflammasome activation by extracellular ATP.
Ca2+ Signaling May Have a Central Role in NLRP3 Inflammasome Activation.
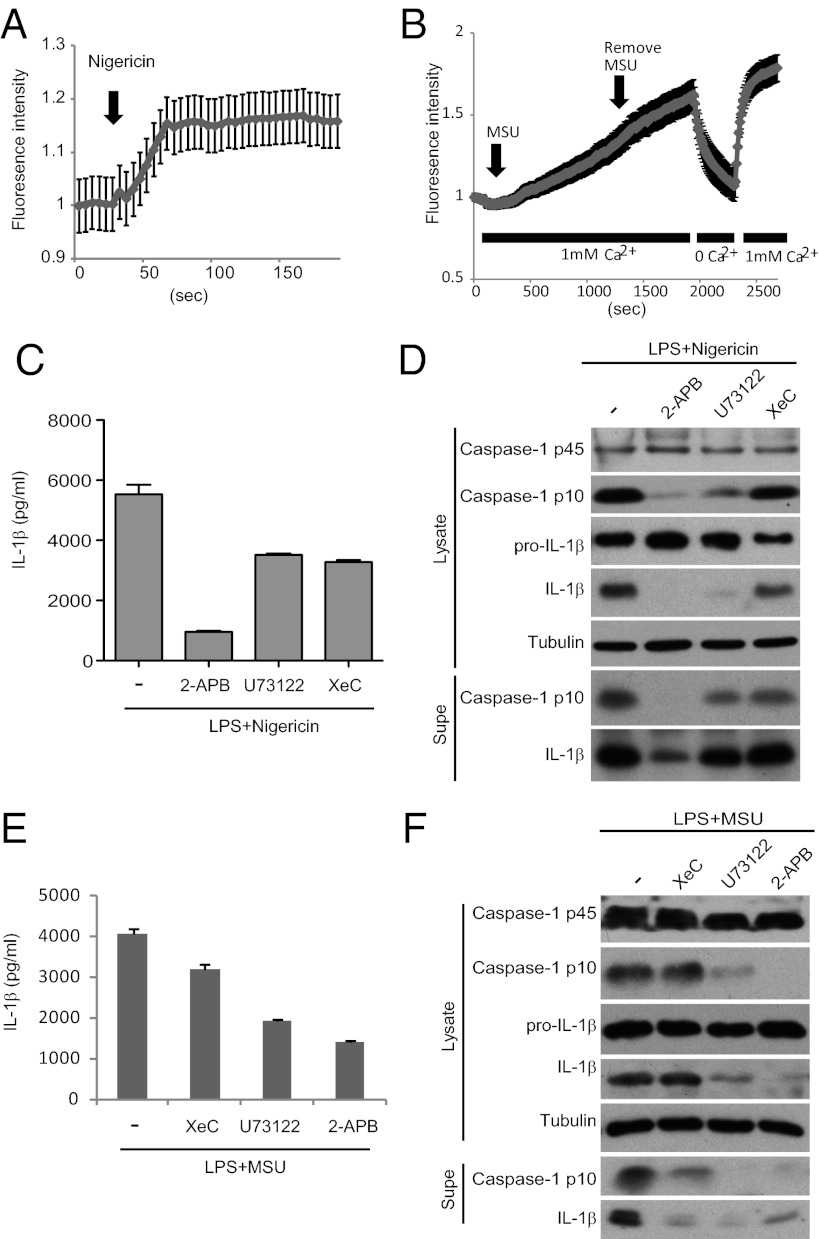
Next, we asked if Ca2+ signaling plays a role in NLRP3 inflammasome activation by other stimuli. We found that nigericin stimulation led to Ca2+ mobilization (Fig. 2A) (28), as did monosodium uric acid crystals (MSU) (Fig. 2B). We were initially not successful in detecting alum-induced Ca2+ flux, but because this effort could be confounded by high rates of Ca2+ efflux from the cytosol, we took an alternative experimental strategy (29) and asked if alum pretreatment could block well-established mechanisms of ER Ca2+ release. Indeed, alum pretreatment inhibited Ca2+ mobilization during P2Y receptor activation, strongly suggesting that it triggered ER Ca2+ release and depletion of ER Ca2+ stores (Fig. S3A). Thus, multiple stimuli that activate the NLRP3 inflammasome induce Ca2+ signaling.
Fig. 2.
Ca2+ signaling may be a common mechanism for NLRP3 inflammasome activation. (A and B) LPS-primed BMDMs stimulated with nigericin (A) or MSU (B) were analyzed by Ca2+ imaging. In B, buffer exchange to remove (0 Ca2+) and replace Ca2+ (1 mM Ca2+) indicates contribution of store-operated Ca2+ entry to Ca2+ flux. (C–F) BMDMs stimulated as indicated were examined for NLRP3 inflammasome activation.
Next, we asked if such Ca2+ mobilization plays a role in NLRP3 inflammasome activation. Indeed XeC, U73122, and 2-APB blocked caspase-1 processing and IL-1β production during stimulation with nigericin, MSU, and alum (Fig. 2 C–F and Fig. S3 B and C). Incubation in Ca2+-free media also attenuated NLRP3 inflammasome activation by MSU and alum stimulation (Fig. S4). In contrast, activation of the NLRC4 inflammasome by FliC was attenuated by the PLC inhibitor U73122 but not by the IP3R or SOCE inhibitors (Fig. S5). This result may reflect “noncanonical” functions of PLC (e.g., nuclear PLC) in settings where the roles of IP3R and SOCE are not well-defined (30, 31). U73122 is unlikely to have off-target effects during ATP stimulation because it blocked Ca2+ mobilization (Fig. 1D) concomitantly with NLRP3 inflammasome activity (Fig. 1E); moreover, the inactive analog U73343 did not have such effects (Fig. S1D). Collectively, these findings indicate that Ca2+ signaling may be a common step in NLRP3 inflammasome activation.
Ca2+ Signaling Promotes Mitochondrial Damage.
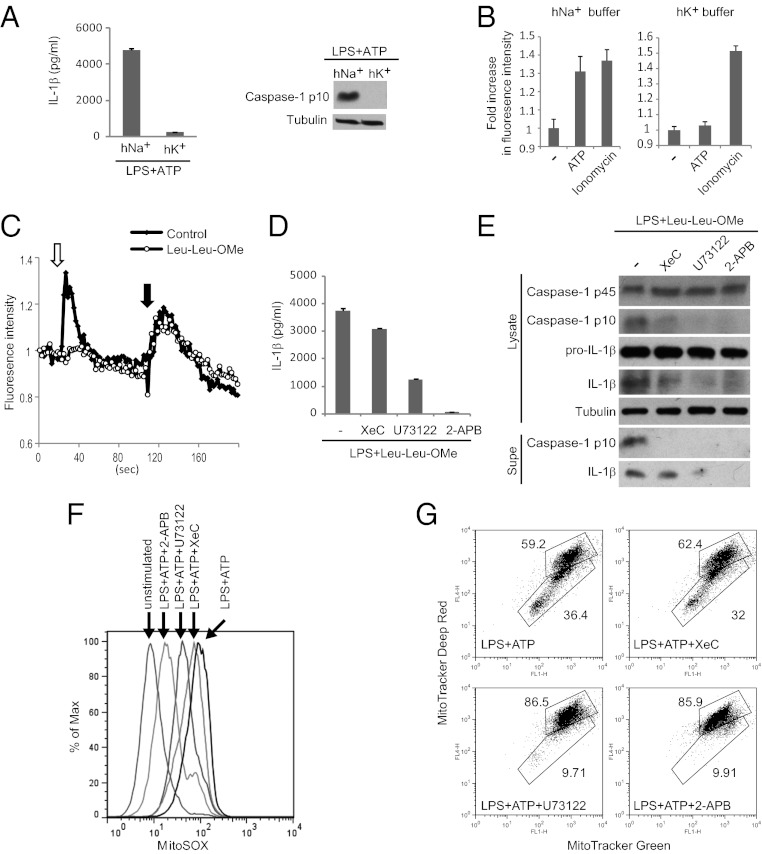
Next, we asked how the requirement for Ca2+ signaling could be integrated with current models of NLRP3 inflammasome activation. K+ efflux has been implicated by studies showing that high extracellular K+ blocks NLRP3 inflammasome activation by multiple stimuli (Fig. 3A) (10). K+ efflux is not directly linked to activation of specific signaling pathways but can regulate Ca2+ signaling through its effects on plasma membrane polarization, and during extracellular Ca2+ entry K+ efflux counteracts membrane depolarization, thus promoting further Ca2+ influx. Indeed, we found that ATP-mediated Ca2+ flux is strongly reduced in the presence of high extracellular K+ (Fig. 3B), suggesting that K+ efflux may promote Ca2+ influx to activate the NLRP3 inflammasome.
Fig. 3.
Ca2+ signaling promotes mitochondrial damage during ATP stimulation. (A) BMDMs were stimulated with ATP in the presence of 40 mM K+ (hK+) or Na+ (hNa+), followed by analysis of NLRP3 inflammasome activation. (B) Ca2+ imaging of ATP-stimulated BMDMs in the presence of hK+ or hNa+ buffers. Fold-increase represents the ratio of maximal fluorescence to baseline fluorescence. Ionomycin stimulation was included as a control for Fura-2 loading. (C) BMDMs were pretreated with Leu-Leu-OMe or not (control), followed 1 h later by stimulation with 100 μM ATP in Ca2+-free HBSS (white arrow) to induce ER Ca2+ release through the P2Y receptor. Stimulation with the Ca2+ ionophore Ionomycin (black arrow) mobilized Ca2+ from distinct Ca2+ stores resistant to Leu-Leu-OMe and P2YR activation and served as a positive control for Fura-2 loading. (D and E) BMDMs, stimulated as indicated, were examined for NLRP3 inflammasome activation. (F and G) BMDMs stimulated as indicated were stained with MitoSOX (F) or Mitotracker Green and Deep Red (G) followed by flow cytometry analysis.
Phagolysosomal rupture has been posited to be crucial in NLRP3 inflammasome activation by crystals. In support of this, a lysosomotropic peptide (Leu-Leu-OMe) that induces phagolysosomal rupture through a completely distinct mechanism—osmotic stress of the lysosome—can activate the NLRP3 inflammasome (13). Importantly, the lysosomal compartment constitutes an intracellular Ca2+ store that can be released in a signal-dependent way to trigger ER Ca2+ release, indicative of functional coupling between these Ca2+ stores (32). Thus, we hypothesized that phagolysosomal rupture may induce Ca2+ mobilization to activate the NLRP3 inflammasome. Indeed, we demonstrated that Leu-Leu-OMe stimulation induces ER Ca2+ release, because it blocks subsequent Ca2+ mobilization by P2YR activation (Fig. 3C). Furthermore, Ca2+ signaling inhibitors block NLRP3 inflammasome activation by Leu-Leu-OMe, indicating that such Ca2+ mobilization is critical (Fig. 3 D and E). Thus, phagolysosomal rupture may induce Ca2+ mobilization to activate the NLRP3 inflammasome.
Finally, mitochondrial damage has been linked to NLRP3 inflammasome activation. ATP stimulation leads to increased mROS production, loss of membrane potential (ΔΨ), and release of mtDNA into the cytosol (14–16), and manipulations that block any of these processes reduce inflammasome activation. Importantly, mitochondria play a key role in shaping the spatiotemporal dynamics of Ca2+ signaling by uptake and release of cytosolic Ca2+ (18–20). However, excessive or sustained Ca2+ uptake can lead to mitochondrial damage characterized by increased production of mROS, mitochondrial permeability transition, and eventually rupture of the mitochondria (21–23). Thus, we hypothesized that the requirement for Ca2+ mobilization in activation of the NLRP3 inflammasome may reflect the ability of Ca2+ signaling to trigger mitochondrial damage. Consistent with this idea, Ca2+ signaling inhibitors block mROS production during ATP stimulation as measured by MitoSOX, a specific probe of mROS (Fig. 3F). Ca2+ signaling inhibitors also rescue ATP-mediated loss of membrane potential as measured by staining with Mitotracker Deep Red, a dye that accumulates in a ΔΨ-dependent manner (Fig. 3G). Moreover, Ca2+ signaling inhibitors block release of mtDNA into the cytosol during ATP stimulation (Fig. S6). Thus, we propose that Ca2+ mobilization is required for inducing mitochondrial damage. Together with recent studies, our results support the model whereby Ca2+ signaling critically regulates NLRP3 inflammasome activation by triggering mitochondrial damage.
CHOP Amplifies Activation of the NLRP3 Inflammasome.
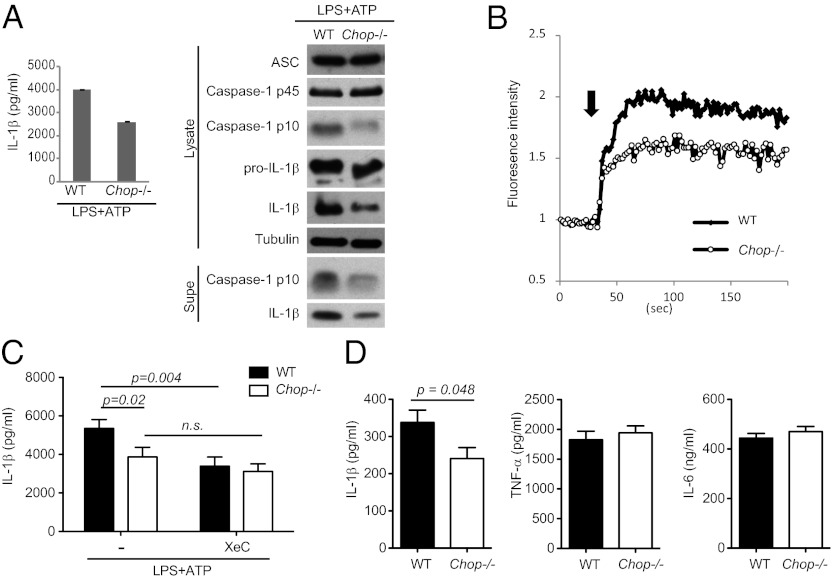
Our results implicate a critical role for Ca2+ mobilization in NLRP3 inflammasome activation, thus we hypothesized that regulators of this process may modulate NLRP3 inflammasome activity. CHOP is a transcription factor that can regulate ER Ca2+ release through the IP3R, and during ER stress CHOP deficiency leads to attenuated ER Ca2+ release concomitant with reduced oxidative stress and improved cellular survival (33–35). To test if CHOP plays any role in modulating NLRP3 inflammasome activity, we examined NLRP3 inflammasome activation in WT and Chop−/− bone marrow-derived macrophages (BMDMs). We observed a consistent decrease in NLRP3 inflammasome activation in Chop−/− BMDMs in response to ATP stimulation (Fig. 4A). In contrast, LPS-mediated IL-6 and TNF-α production (Fig. S7B); NLRP3 and pro–IL-1β up-regulation (Fig. S7C); and NLRC4 inflammaction activation were normal (Fig. S7D), suggesting no general defect in inflammatory responses. Expression of Asc, P2X7R, and cathepsin B was also normal (Fig. 4 and Fig. S7A).
Fig. 4.
CHOP amplifies NLRP3 inflammasome activation. (A) WT and Chop−/− BMDMs were examined for inflammasome activation. (B) Ca2+ flux in ATP-stimulated WT and Chop−/− BMDMs was examined by Ca2+ imaging. (C) WT and Chop−/− BMDMs were pretreated with XeC or not followed by ATP stimulation. (D) WT and Chop−/− mice were injected with LPS to induce sepsis, followed by analysis of serum cytokine levels. ns, not significant.
These findings suggested a specific involvement of CHOP in modifying NLRP3 inflammasome activity, and we asked whether such a role could be related to control of ER Ca2+ release via the IP3R. First, we showed that ATP-mediated Ca2+ mobilization is reduced in Chop−/− BMDMs (Fig. 4B). Second, we reasoned that if CHOP and IP3R function in the same pathway, XeC should block NLRP3 inflammasome activity in WT but not Chop−/− BMDMs. Indeed, although XeC treatment and CHOP deficiency both inhibited ATP-induced IL-1β production by ∼30–40% in WT BMDMs, no further block of IL-1β production was observed upon XeC addition to Chop−/− BMDMs (Fig. 4C). Thus, CHOP may amplify NLRP3 inflammasome activity by regulating IP3R-mediated Ca2+ release.
To demonstrate physiological relevance, we turned to a mouse model of sepsis in which IL-1β production is dependent on the NLRP3 inflammasome (6, 36). WT and Chop−/− mice were injected with high dose LPS (40 mg/kg), followed by analysis of serum IL-1β levels. Significantly reduced IL-1β secretion was found in Chop−/− mice compared with WT mice, but levels of TNF-α and IL-6 were similar (Fig. 4D). Thus, CHOP has a specific role in regulation of the NLRP3 inflammasome in vivo.
Discussion
In this study, we elucidate a critical role for Ca2+ signaling in activation of the NLRP3 inflammasome. Previous studies relying mainly on 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA)-AM treatment or incubation in Ca2+-free media have made somewhat inconsistent conclusions regarding the role of Ca2+ in NLRP3 inflammasome activation (28, 37–39). Given the pleitropic effects of BAPTA-AM on many cellular processes including LPS signaling (40), we wished to more carefully examine this issue by defining specific regulators of Ca2+ mobilization. Using XeC, U73122, and 2-APB, we implicate PLC proteins, IP3R-mediated Ca2+ release, and SOCE as critical regulators during ATP stimulation (Fig. 1D). Although not well-characterized, induction of such a pathway by stimuli that induce Ca2+ influx across the plasma membrane is not without precedence (41, 42). Definitive demonstration of the role of this pathway requires genetic models, but pharmacological inhibitors are commonly used in the field of Ca2+ signaling because of redundancy in regulation (i.e., multiple PLCs) or, conversely, lethality of cells and mice lacking critical regulators (i.e., IP3R1). Most importantly, we correlate block of Ca2+ mobilization (Fig. 1D) with NLRP3 inflammasome activity (Fig. 1 E and F), and use independent methods to implicate ER Ca2+ release and extracellular Ca2+ entry (Fig. 1 B and C). Ca2+ signaling inhibitors do not regulate signal 1 (Fig. S1B) or affect cell viability (Fig. S1C), but block NLRP3 inflammasome activation at every step examined, including complex assembly (Fig. S2), caspase-1 processing, IL-1β processing, and IL-1β release to culture supernatants (Fig. 1 E and F). Collectively, these data indicate a role for Ca2+ mobilization in regulating the upstream, proximal steps in NLRP3 inflammasome activation (although not ruling out additional effects more distally).
How can our findings be integrated into existing models for activation of the NLRP3 inflammasome? K+ efflux and phagolysomal rupture have been implicated in NLRP3 inflammasome activation but the critical intermediate events have not been defined. Our results are consistent with the idea that high K+ buffers perturb Ca2+ mobilization and that K+ efflux may regulate Ca2+ signaling (Fig. 3B), while rupture of phagolysosomes leads to ER Ca2+ release (Fig. 3 C–E), likely as a result of Ca2+ mobilization from the lysosomal compartment. Thus, we propose that K+ efflux and phagolysosomal rupture promote Ca2+ mobilization to activate the NLRP3 inflammasome. Conversely, we report that Ca2+ signaling inhibitors block ATP-mediated mitochondrial Ca2+ damage, as measured by mROS production (Fig. 3F), loss of ΔΨ (Fig. 3G), and release of mtDNA into the cytosol (Fig. S6). Because these processes are required for NLRP3 inflammasome activation, this finding suggests that the critical role of Ca2+ signaling, at least during ATP stimulation, is to mediate mitochondrial damage. Consistent with this theory, activation of the NLRP3 inflammasome by transfection of oxidized mitochondrial DNA (14) is not blocked by Ca2+ signaling inhibitors (Fig. S8).
Additionally, we find that Ca2+ is mobilized during stimulation with alum, MSU, and nigericin, and that such Ca2+ signaling is required for NLRP3 inflammasome activation (Fig. 2, and Figs. S3 and S4). This finding suggests that Ca2+ mobilization is a common, proximal step in activation of the NLRP3 inflammasome. We propose that the multitude of stimuli that activate the NLRP3 inflammasome can be rationalized in light of the many mechanisms for mobilizing Ca2+, including G protein-coupled receptors, receptor tyrosine kinases, immunoreceptor tyrosine-based activation motif-coupled receptors, and Ca2+ entry channels (17). However, we note that Ca2+ signaling cannot be sufficient for NLRP3 inflammasome activation, as indicated by the inability of the Ca2+ ionophore ionomycin to induce IL-1β production (Fig. S9), despite triggering Ca2+ mobilization and mitochondrial Ca2+ uptake (43). Presumably, many other stimuli that induce “physiological” Ca2+ signaling would also not activate the NLRP3 inflammasome. Importantly, Ca2+ signaling is well-established to be necessary but not sufficient for mitochondrial damage (21–23), and we propose that only stimuli that mobilize Ca2+ in a manner leading to mitochondrial damage would activate the NLRP3 inflammasome (Fig. 5).
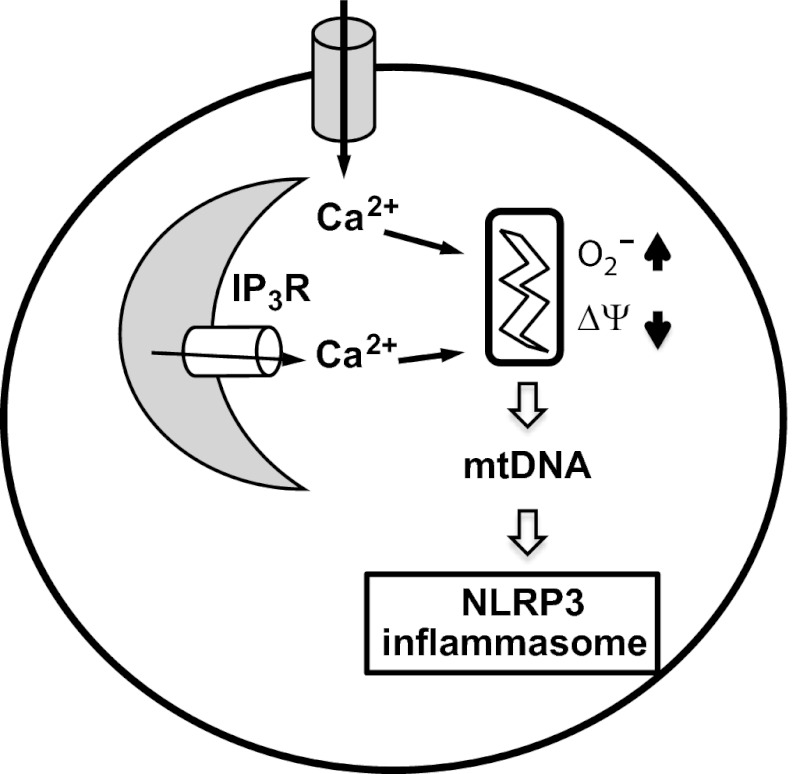
Fig. 5.
Model. Our results support a model in which Ca2+ signaling is critical for NLRP3 inflammasome activation. In response to ATP and perhaps other stimuli, Ca2+ mobilization from ER stores and the extracellular space triggers mitochondrial damage, including increased mROS production, loss of membrane potential, and release of mtDNA into the cytosol.
Finally, we wondered about a potential role for the NLRP3 inflammasome in “sensing” dysregulated Ca2+ homeostasis and inducing inflammation in chronic diseases, such as neurodegeneration, atherosclerosis, and obesity-associated metabolic diseases (44–46). In considering this possibility, we focused on CHOP, an effector of the ER-stress pathway (35). ER stress is closely linked to dysregulated Ca2+ homeostasis and CHOP can regulate ER Ca+ levels (33); moreover, a recent report links ER stress to NLRP3 inflammasome activation (47). Indeed, CHOP deficiency attenuates ATP-mediated NLRP3 inflammasome activation (Fig. 4A) and IL-1β production during sepsis (Fig. 4D), but other inflammatory responses were not affected (Fig. 4D and Fig. S7). The underlying mechanism is likely to be attenuated Ca2+ release from the IP3R, as supported by Ca2+ imaging studies (Fig. 4B) and the inability of IP3R inhibition to further block inflammasome activation (Fig. 4C). Somewhat surprisingly, alum stimulation of Chop−/− BMDMs leads to normal NLRP3 inflammasome activation (Fig. S10 A and B), but it also depletes ER Ca2+ stores comparably in WT and Chop−/− BMDMs (Fig. S10C). Less ROS is generated by alum stimulation compared with ATP (Fig. S10 D and E), and CHOP has been proposed to regulate ER Ca2+ release in a manner dependent on ER redox status, so one possible interpretation of these results is that CHOP amplifies NLRP3 inflammasome activation in a manner dependent on ROS levels. Our results reveal CHOP as a modulator of the NLRP3 inflammasome pathway and support the idea that in many chronic diseases, CHOP and ER stress may amplify NLRP3 inflammasome activity to augment inflammation.
Materials and Methods
Mice.
B6 and Chop−/− mice were from the Jackson Laboratories and Nlrp3−/−, Asc−/−, and Caspase-1−/− mice were gifts of Visha Dixit (Genentech, San Francisco, CA) and Ruslan Medzhitov (Yale University, New Haven, CT). All mice are on the B6 background. Animals were bred and maintained at Harvard Medical School and all animal experiments were done with approval by and in accordance with regulatory guidelines and standards set by the Institutional Animal Care and Use committee of Harvard Medical School.
Cells and Stimulations.
To activate the NLRP3 inflammasome, BMDMs (48) were primed with LPS before stimulation with ATP (1–5 mM, 30 min), alum (200 μg/mL, 4 h), nigericin (20 μM, 1 h), MSU (100 μg/mL, 4 h), and Leu-Leu-OMe (1 mM, 5 h). To activate the NLRC4 inflammasome, LPS-primed BMDMs were transfected with FliC (80 ng/0.7 × 106 cells) using the Lipofectamine 2000 reagent (8 μL) 5 h before harvest. Transfection of oxidized mtDNA was done as previously described (14). U73122 and U73343 (10 μM), 2-APB (100 μM), XeC (5 μM), and YVAD (40 μM) were added following LPS priming, 30 min before addition of the NLRP3 inflammasome activators. Ca2+ free media (Invitrogen) was added 10 min before addition of NLRP3 inflammasome activators, as was KCl or NaCl (40 mM final concentration). To transiently express PLC-PH-GFP, BMDMs were transfected with DNA using Nucleofector technology (Lonza) and rested for 4 h before stimulation; 100 μM ATP was used to activate the P2YR.
Antibodies.
Antibodies were obtained from Santa Cruz (caspase-1), Alexis (NLRP3), Sigma (Tubulin), R&D Systems (IL-1β), and National Cancer Institute-Frederick Biological Resources Branch Repository (IL-1β).
Western Blotting and ELISA.
In some Western blotting experiments, supernatants were concentrated by addition of TCA (20% final volume, 10 min at 4 °C), followed by centrifugation (17,000 × g, 5 min at 4 °C) and two washes of the protein pellet with ice-cold acetone. IL-1β ELISA kit was from eBioscience, and IL-6 and TNF-α ELISA kits were from Biolegend.
Ca2+ Imaging.
BMDMs were loaded with the Ca2+-sensitive indicator dye Fura-2-AM (1.5 μM, 30 min, 37 °C). Ca2+ flux was measured by time-lapse microscopy, and fluorescence images were collected using either a Yokogawa Spinning Disk microscope equipped with a Sutter DG-4 illuminator and a 40× objective or a Nikon TE200 inverted microscope with Nomarski optics. The 340/380 ratio was calculated using ImageJ (National Institutes of Health).
Flow Cytometry.
MitoSOX, MitoTracker Green and Red, and DCFDA staining were done according to manufacturer's instructions (Invitrogen). Data were acquired with a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo analytical software (TreeStar).
Measuring Cytosolic mtDNA.
Measurements of cytosolic mtDNA were done following an established protocol (15).
Sepsis.
Mice age 6–10 wk were injected intraperitoneally with 40 mg/kg LPS. After 3 h, mice were killed and blood was obtained by cardiac puncture. Serum levels of IL-1β, TNF-α, and IL-6 were analyzed by ELISA.
Statistical Analysis.
Statistics were calculated using GraphPad Prism 5. Comparisons of two groups were analyzed using two-tailed t test, and comparisons of multiple groups were analyzed using one-way or two-way ANOVA as indicated. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank K. Fitzgerald, V. Dixit, and R. Medzhitov for sharing Nlrp3−/−, Asc−/−, Caspase-1−/− mice, and femurs. This work was supported in part by the Uehara Memorial Foundation, the Kanae Foundation for the Promotion of Medical Sciences, and a Japan Society for the Promotion of Science Fellowship (T.M.); a Postdoctoral Fellowship from the Swedish Research Council (to J.O.); and a Harvard School of Public Health Career Incubator Fund and a Pilot and Feasibility Grant from the Harvard Digestive Diseases Center (to T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.P.Y.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117765109/-/DCSupplemental.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 11.Perregaux DG, Gabel CA. Human monocyte stimulus-coupled IL-1beta posttranslational processing: Modulation via monovalent cations. Am J Physiol. 1998;275:C1538–C1547. doi: 10.1152/ajpcell.1998.275.6.C1538. [DOI] [PubMed] [Google Scholar]
- 12.Perregaux DG, Laliberte RE, Gabel CA. Human monocyte interleukin-1beta posttranslational processing. Evidence of a volume-regulated response. J Biol Chem. 1996;271:29830–29838. doi: 10.1074/jbc.271.47.29830. [DOI] [PubMed] [Google Scholar]
- 13.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada K, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 17.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001;20:2672–2679. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44:6–13. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchen MR. Mitochondria and calcium: From cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 24.Solle M, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 25.Stober CB, et al. ATP-mediated killing of Mycobacterium Bovis bacille Calmette-Guérin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol. 2001;166:6276–6286. doi: 10.4049/jimmunol.166.10.6276. [DOI] [PubMed] [Google Scholar]
- 26.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 27.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 28.Brough D, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 29.Roy J, Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. The omega-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a “store-operated” mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G715–G722. doi: 10.1152/ajpgi.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitale M, et al. Interleukin 2 activates nuclear phospholipase Cbeta by mitogen-activated protein kinase-dependent phosphorylation in human natural killer cells. FASEB J. 2001;15:1789–1791. doi: 10.1096/fj.01-0008fje. [DOI] [PubMed] [Google Scholar]
- 31.York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 32.Haller T, Dietl P, Deetjen P, Völkl H. The lysosomal compartment as intracellular calcium store in MDCK cells: A possible involvement in InsP3-mediated Ca2+ release. Cell Calcium. 1996;19:157–165. doi: 10.1016/s0143-4160(96)90084-6. [DOI] [PubMed] [Google Scholar]
- 33.Li G, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 35.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 38.Lee HM, et al. Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu J, et al. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86:1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai D, et al. Induction of TLR4-target genes entails calcium/calmodulin-dependent regulation of chromatin remodeling. Proc Natl Acad Sci USA. 2009;106:1169–1174. doi: 10.1073/pnas.0811274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau BW, et al. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology. 2005;128:695–707. doi: 10.1053/j.gastro.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 42.Okubo Y, Kakizawa S, Hirose K, Iino M. Visualization of IP(3) dynamics reveals a novel AMPA receptor-triggered IP(3) production pathway mediated by voltage-dependent Ca(2+) influx in Purkinje cells. Neuron. 2001;32:113–122. doi: 10.1016/s0896-6273(01)00464-0. [DOI] [PubMed] [Google Scholar]
- 43.Monteith GR, Blaustein MP. Heterogeneity of mitochondrial matrix free ca2+: Resolution of Ca2+ dynamics in individual mitochondria in situ. Am J Physiol. 1999;276:C1193–C1204. doi: 10.1152/ajpcell.1999.276.5.C1193. [DOI] [PubMed] [Google Scholar]
- 44.Celsi F, et al. Mitochondria, calcium and cell death: A deadly triad in neurodegeneration. Biochim Biophys Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2792–2797. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menu P, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signaling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.