Twenty-five years ago, the neurodevelopmental hypothesis of schizophrenia opened the way for analyzing potential prenatal influences on late postnatal pathogenetic events (1). Since that time, it has been well-established that hypoxia, infection, and other noxious factors can cause fetal brain injury and intrauterine growth restriction and that prenatal brain lesions can cause alterations in the postnatal brain development (2). A study in PNAS (3) suggests that even the subtle normal variations of intrauterine environment may lead to recognizable differences in postnatal brain structure and cognitive functions.
Raznahan et al. (3) perform a longitudinal (early childhood to early adulthood) follow-up study of structural brain development and general cognitive functioning in monozygotic twins. They select monozygotic twins as Nature’s experiment, allowing the detection of in utero environmental influences, which are mainly brought about by placental transfer of nutrients and other materials to the fetuses, on brain development (3). Previous epidemiological studies have already established birth weight as a global proxy of optimal nutritive supply and associated decreased birth weight with an increased risk for mental illness (4) and reduced cognitive abilities (5).
It is not surprising to find positive correlation with postnatal neurocognitive outcome in cases of extreme birth weight variation (below the 10th percentile), because placental transfer in such cases is shifted to a pathological level (2). However, it is not expected that neurocognitive maturation during late adolescence would depend on relatively small differences in the supply of nutrients that fall at the low end of the normal range (near-optimal environment). The work by Raznahan et al. (3) approaches this issue by including in their study only individuals without neurological and psychiatric illness who experience an uncomplicated full-term pregnancy, are healthy at birth, have a birth weight within the normal range for twins, and display within-twin weight differences below 20%. The work by Raznahan et al. (3) estimates that a 500-g increase in birth weight is correlated with an increase in size of cerebral cortex surface of about 35 cm2 as well as a 2-point increase in full-scale and performance intelligence quotient (IQ).
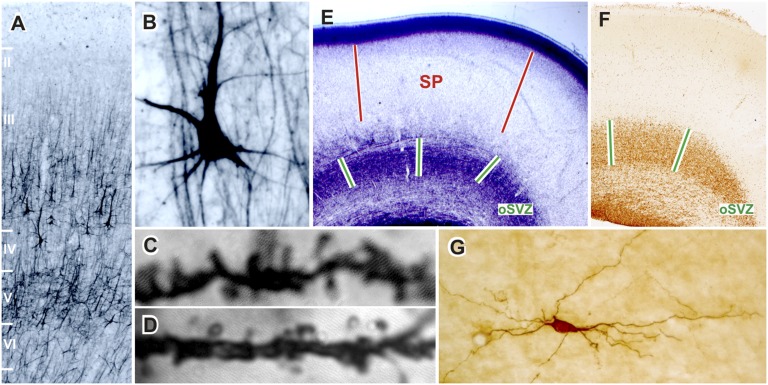
Biological determinants of prenatal circuitry development in the cerebral cortex are thought to be predominantly inherited (6). How is it possible then that a near-optimal fetal environment can still have an impact on structural cortical development when the babies are genetically identical? The answer likely relates to evolutionary changes that have operated on the mechanisms regulating brain development (7, 8) and that have arisen to fabricate the most complex part of the human brain, namely the cerebral cortex (9, 10), with complex cellular and laminar organization (Fig. 1 A and B) and protracted development that is not complete until final maturation of the cortical circuitry in the third decade of life (Fig. 1 C and D) (11, 12). This extended period of genesis also greatly lengthens the time window of neural vulnerability, because increases in the complexity and intensity of molecular and cellular interactions amplify the number of potentially vulnerable targets for disruption. As a consequence, the human fetal cerebral cortex is at risk of environmental insults over an extended period of development (13), and it may also be susceptible to subtle, long-lasting differences in access to important nutrients, such as those nutrients that might be encountered either during childhood or during gestation where twin fetuses compete with each others for maternal resources. The development of the human fetal cerebrum requires immense amounts of cellular proliferation among progenitors of several classes of local circuit and projecting neurons and an accompanying matching with an expanding population of corticocortical connections (13, 14), which are particularly abundant in slowly maturing, associative areas (Fig. 1 C and D). These neurons are produced maximally between weeks 14 and 24 of gestation (9, 15) in the outer subventricular zone (Fig. 1 E and F). Proper incorporation of an increased number of incoming afferent axons projecting into the developing cortical sheet is thought to rely heavily on the subplate (Fig. 1E). Concomitantly with the enhanced number and diversity of corticocortical fibers, the subplate enlarges and increases its complexity (Fig. 1G) and becomes a prominent structure during the second trimester, and it remains well-developed until birth (13).
Fig. 1.
Cellular and laminar composition of postadolescent (A–C, 22 y; D, 44 y) and fetal prefrontal cortex (E and F, 16 gestational wk; G, 24 gestational wk). Human associative cortices are characterized by (A) the dominance of outer cortical layers (II and III) constituted from neurons projecting to other cortical regions as well as (B) the presence of a special class of associative pyramidal layer IIIC neurons (A and B; SMI32 staining) (10, 12). The massive developmental elimination of synapses continues on spines of layer IIIC pyramidal neurons even beyond the adolescence (C and D) (11), although the number of neurons and basic pathways in corticocortical network is determined prenatally by specific interactions in the transient fetal zones that are particularly developed in associative cortices: (E) outer subventricular zone (oSVZ) is the main proliferative compartment (F; Ki67 staining) (13, 15), and (E) subplate (SP) regulates fiber ingrowth and contains a heterogeneous population of neurons (G; neuropeptide staining) (13).
Mechanisms regulating fetal growth and potentially causing birth weight variations are already operative during the second trimester of gestation, because the placenta is well-developed by this stage (2, 5). According to the work by Raznahan et al. (3), the most likely areas of the brain that are affected are high-order associative regions, especially subventricular proliferating precursor cells of neurons destined to project to other cortical regions and the emerging corticocortical pathways through subplate. Recent experimental data correlating in utero adversity with brain development in primates support the above-mentioned conclusions (16). In this primate model, modest energy restriction in mothers during their pregnancies alters the balance between rates of cell birth and cell death by apoptosis in the subventricular zone during the midgestational period and reduces the density of the subplate neuronal network. It is not clear just how the near-optimal placental supply of nutrients, as inferred in the present human twin study (3), affects cortical development at the molecular level, but it is assumed that the differences between the twins are not a consequence of cellular damage, which is the case in severe hypoxia and other pathological conditions (2). Most likely, the mechanisms include epigenetic changes, such as alterations in methylation
Even the subtle normal variations of intrauterine environment may lead to recognizable differences in postnatal brain structure and cognitive functions.
of genes involved in regulating cortical development (17).
According to their IQ levels, which averaged between 109 and 116, the study by Raznahan et al. (3) uses intelligent individuals raised in stimulating environments. During development, education and training have a significant impact on mental capacities (18), probably by influencing structural rearrangement of late-maturing networks involved in the execution of the highest cognitive tasks (11). Moreover, genetic endowment of superior intelligence seems to be linked to increased cortical plasticity (19) and neurocognitive outcomes distinctly influenced by postnatal environmental stimulation. Suboptimal fetal conditions might influence the fine structure of neuronal networks (e.g., dendritic growth) (12), thereby decreasing sensitivity of associative circuitries to postnatal stimulation and limiting final cognitive performance to a level below genetic potential. However, in cases where there are accompanying genetic disturbances, suboptimal fetal conditions might be a tipping factor that promotes direct formation of abnormal circuitry and accounts for late-expressed neuropsychiatric disorders or borderline cognitive abilities (20).
The interactions between genetic programming and postnatal environmental factors are well-recognized as important biomedical and social concepts (6, 11, 18). The work by Raznahan et al. (3) emphasizes that unremarkable but nevertheless significant differences in birth weight and adolescent neurocognitive development are most likely initiated during the second and third trimesters of pregnancy. The data stress the importance of in utero environment on development of brain circuitries that process the most complex, later-maturing mental abilities.
Acknowledgments
The work on prenatal and postnatal development of the human and monkey cerebral cortex at Croatian Institute for Brain Research is supported by grants from the Ministry of Science, Education and Sport of the Republic of Croatia.
Footnotes
The authors declare no conflict of interest.
See companion article on page 11366.
References
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci USA. 2012;109:11366–11371. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel KM, et al. Birth weight, schizophrenia, and adult mental disorder: Is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923–930. doi: 10.1001/archgenpsychiatry.2010.100. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgeois JP, Jastreboff PJ, Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: Evidence for intrinsic regulation of synaptic overproduction. Proc Natl Acad Sci USA. 1989;86:4297–4301. doi: 10.1073/pnas.86.11.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu X, et al. Rapid metabolic evolution in human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:6181–6186. doi: 10.1073/pnas.1019164108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan KY, et al. Species-dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell. 2012;149:899–911. doi: 10.1016/j.cell.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. The “psychic” neuron of the cerebral cortex. Ann N Y Acad Sci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- 11.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petanjek Z, Judas M, Kostović I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- 13.Kostović I, Judas M. Transient patterns of cortical lamination during prenatal life: Do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Clowry G, Molnár Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217:276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 16.Antonow-Schlorke I, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numata S, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: Update 2012. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]