Abstract
A large number of studies have demonstrated that the nucleus accumbens (NAC) is a critical site in the neuronal circuits controlling reward responses, motivation, and mood, but the neuronal cell type(s) underlying these processes are not yet known. Identification of the neuronal cell types that regulate depression-like states will guide us in understanding the biological basis of mood and its regulation by diseases like major depressive disorder. Taking advantage of recent findings demonstrating that the serotonin receptor chaperone, p11, is an important molecular regulator of depression-like states, here we identify cholinergic interneurons (CINs) as a primary site of action for p11 in the NAC. Depression-like behavior is observed in mice after decrease of p11 levels in NAC CINs. This phenotype is recapitulated by silencing neuronal transmission in these cells, demonstrating that accumbal cholinergic neuronal activity regulates depression-like behaviors and suggesting that accumbal CIN activity is crucial for the regulation of mood and motivation.
Keywords: acetylcholine, neurotransmission, s100a10, antidepressant
The nucleus accumbens (NAC) has been identified as a critical site in the neuronal circuits controlling reward responses, motivation, and mood (1–3). For example, in major depressive disorder (MDD): (i) NAC activity is reduced in response to positive stimuli (4) and (ii) MDD symptoms can be reversed by deep brain stimulation in the NAC (5, 6). Although these regional studies implicate the NAC, the cell type(s) responsible for the pathophysiology remain to be elucidated. In the case of MDD, it is crucial that we determine which neuronal cell types regulate depressive-like behaviors to eventually develop highly targeted antidepressants that could be both faster acting and have fewer off-site effects. MDD is a debilitating psychiatric condition characterized by anhedonia (defined as a loss of interest in pleasurable things), loss of motivation, negative affect, behavioral despair, and changes in cognition and basic drives such as eating and sleeping. Despite its complexity, central features of the disease, such as anhedonia and behavioral despair, can be modeled in rodents to probe the underlying neuronal circuitry.
The serotonin receptor (5HTR) interacting protein p11 is a small intracellular protein that regulates the localization of certain 5HTRs, including 5HTR1B and 5HTR4 (7, 8), at the cellular surface. Constitutive p11 knockout (KO) mice show both anhedonia (e.g., reduced sucrose preference) and increased behavioral despair (e.g., increased immobility) (7–9). Recently, we identified the NAC as a key brain region in which loss of p11 induces depression-like behaviors (9). The NAC is composed of several different cell types (10). Approximately 95% of the neurons in this region are medium spiny neurons (MSNs), the projection neurons of the NAC. The cholinergic interneurons have a characteristically large soma and make up less than 1% of the neurons in the striatum (10). Cholinergic interneurons primarily target MSNs via muscarinic and nicotinic acetylcholine receptors, although GABAergic interneurons also receive some cholinergic input.
In the current study, we identify cholinergic interneurons as a primary site of action for p11 in the NAC and show that manipulation of p11 in this single cell type regulates anhedonia and behavioral despair in rodent models of depression. The depression-like phenotype that we observe in mice lacking p11 in accumbal cholinergic interneurons is recapitulated by silencing neuronal transmission in these cells. This work identifies accumbal cholinergic interneurons as critical modulators of depression-like states and suggests that the activity of these neurons is crucial for the regulation of mood and motivation.
Results
Analysis of Accumbal Cell Types Expressing p11.
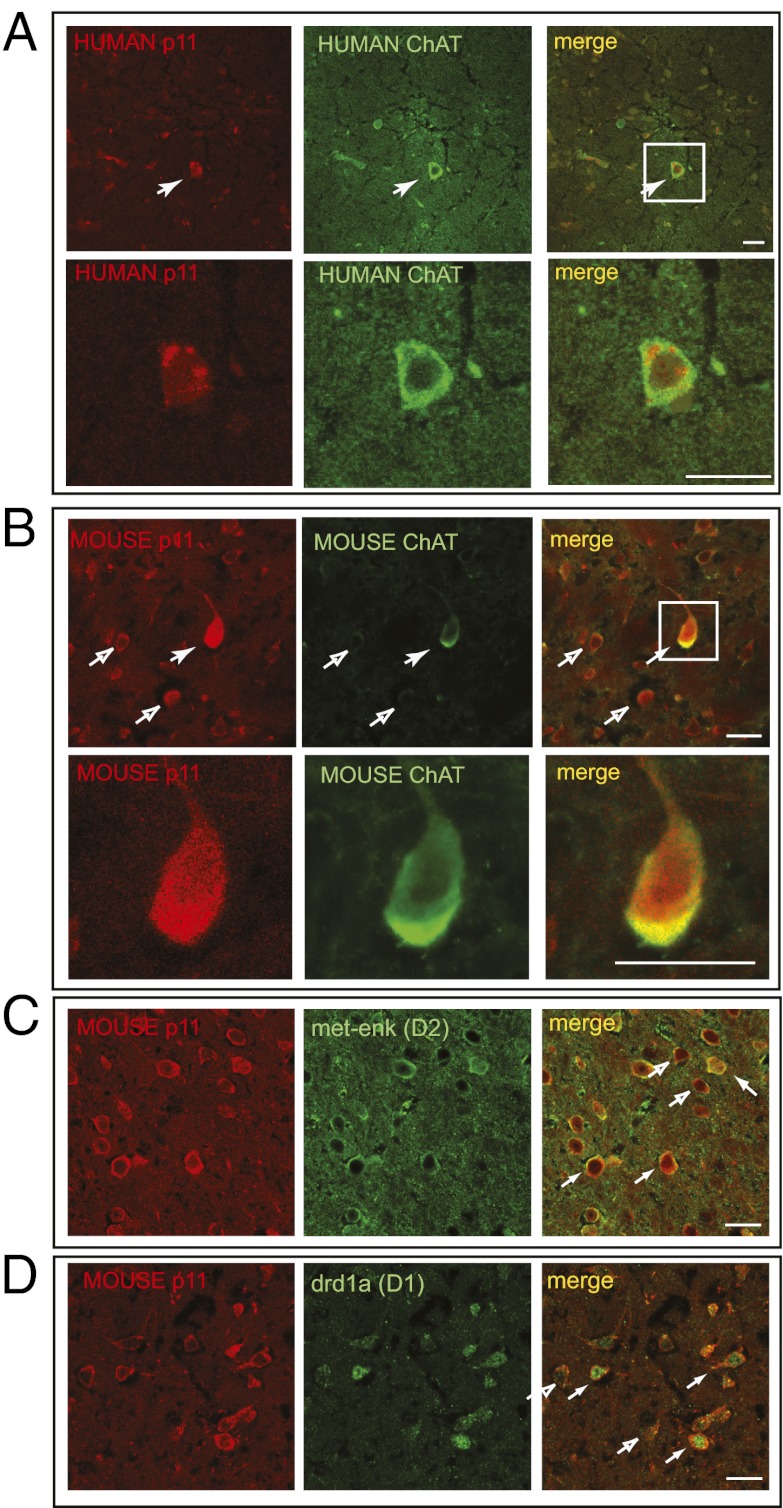
We reported that reduced p11 expression in the NAC leads to a depressive-like phenotype (9). Therefore, we sought to determine the cell types in the NAC that express p11. Immunohistochemical analysis of human NAC tissue indicated that p11 was highly expressed in cholinergic interneurons, which are identified by their expression of choline acetyltransferase (ChAT) (Fig. 1A). Similarly, in the mouse, ChAT neurons showed the highest levels of p11 in the NAC (Fig. 1B) compared with other cell types. We next used the translating ribosome affinity purification (TRAP) technique (11, 12) to isolate ribosome bound mRNA from multiple cell types in the NAC. We used bacTRAP mice expressing the EGFP-L10a transgene in cholinergic interneurons, dopamine receptor 1 (D1) expressing striatonigral MSNs, and D2-expressing striatopallidal MSNs. Subsequent quantification of p11 message levels by RT-quantitative PCR (qPCR) indicated that p11 expression in accumbal cholinergic interneurons was enriched 30-fold compared with noncholinergic cell types in the NAC [±9.09 SEM, F(1,4)=30.02, P < 0.01]. Both D1- and D2-receptor containing MSNs express p11 but at lower levels compared with the cholinergic interneurons (Fig. 1 C and D) [D1-MSNs vs. rest of the NAC: 1.3-fold ± 0.04 SEM, t(1,6) =3.611, P < 0.05; D2-MSNs vs. rest of the NAC: 1.25-fold ± 0.02 SEM, t(1,6) =2.979, P < 0.05].
Fig. 1.
Accumbal cholinergic interneurons express relatively high levels of p11. Colocalization of p11 (red) with choline acetyltransferase (green) in the human nucleus accumbens (A) or the nucleus accumbens of wild-type mice (B). The ChAT positive neuron from the 40× image (white box) is magnified below 4×. (C and D) Colocalization of p11 (red) with medium spiny neuron cell-type markers (green) in the nucleus accumbens of wild-type mice: met-enkephalin, a maker of D2-receptor–containing striatopallidal medium spiny neurons (C), and drd1a, a marker of D1-receptor–containing striatonigral medium spiny neurons (D). Filled arrows indicate a cell that coexpresses p11 and the cell-type marker (ChAT, met-enkephalin, or drd1a); open arrows indicate cells expressing p11 but not the marker. (Scale bars: 20 μm.)
Effect of Cell Type-Specific Deletion of p11 on Depression-Like Behavior.
Given the high levels of p11 expressed by cholinergic interneurons, we next examined whether these cells regulate depressive-like behavior, including anhedonia and behavioral despair. For this purpose, we used an intersectional strategy by using p11 conditional floxed mice (13) and four different BAC transgenic mice expressing CRE recombinase under different BAC promoters to target the different cell populations (14). First, ChAT-CRE–expressing mice were used to target cholinergic neurons, including but not limited to the NAC cholinergic interneurons. Second, dopamine D2-receptor (D2-) CRE mice targeted striatopallidal MSNs and ChAT neurons in the striatum but also expressed CRE in D2-containing neurons in the cortex, hypothalamus, and midbrain. However, the only point of intersection between the ChAT-CRE and D2-CRE mouse lines is striatal cholinergic interneurons. We took advantage of this intersection and compared behavioral results from these two lines. In contrast to the D2-CRE mice, adenosine 2a (A2a)-CRE is a striatopallidal MSN-specific line, targeting only those cells. Finally, dopamine D1-receptor (D1-) CRE expressing mice targeted striatonigral MSNs. Loss of p11 in the relevant cell type was confirmed by immunohistochemistry.
These cell type-specific p11 KO mice were tested in depression-related behavior tests. Anhedonia is modeled in rodents by measuring preference for palatable sucrose solution. In the current study, we assessed anhedonia and also performance in two widely used models of behavioral despair, the tail suspension test (TST) and the forced swim test (FST). Both the TST and the FST are models of depression that assess the response of an animal to an inescapable stressor (15). Behavioral analyses revealed that ChAT- and D2-p11KO mice had a reduced preference for sucrose (Fig. 2A), and increased immobility in the TST (Fig. 2B), consistent with the anhedonic and depressive-like phenotype observed in the constitutive p11KO mice (7). ChAT-p11KO mice also showed increased immobility in the FST (Fig. S1) compared with controls. In contrast, A2a-p11KO mice behaved similarly to controls in sucrose preference and TST (Fig. 2 A and B), indicating that the effects observed in the D2-p11KO mice can be attributed to the loss of p11 in the cholinergic interneurons, which express D2 receptors, and not because of p11 loss in the D2-expressing MSNs. D1-p11KO mice showed no difference in sucrose preference (Fig. 2A) and reduced immobility in the TST (Fig. 2B) compared with controls. All four transgenic mouse lines tested responded normally to the selective serotonin reuptake inhibitor (SSRI) antidepressant citalopram in the TST (Fig. 2B) and showed no differences in locomotor activity (Fig. S2).
Fig. 2.
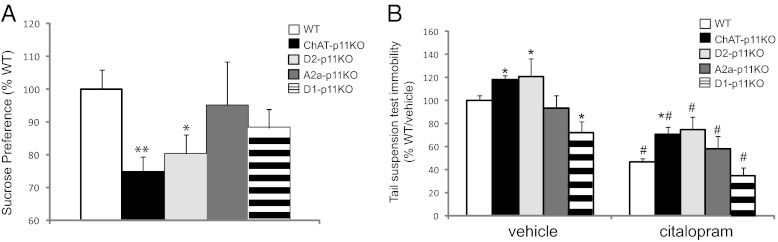
Cholinergic neurons mediate the effects of p11 on depression-like behaviors. (A) ChAT-, D2-, A2a-, and D1- p11 KO mice were tested for sucrose preference, a model for the hallmark depressive symptom of anhedonia [F(4,107) = 2.578, P < 0.05]. (B) Baseline immobility and the response to citalopram, an SSRI antidepressant, were measured in the tail suspension test in ChAT-, D2-, A2a-, or D1-p11 KO mice [genotype: F(4,111) = 8.604, P < 0.01; citalopram: F(1,111) = 64.5, P < 0.01; interaction: F(4,111) = 0.3805, n.s.]. All data are presented as means ± SEM. Statistically significant effects of genotype (*P < 0.05) or citalopram (#P < 0.05) are noted. n.s., not significant.
Silencing Neurotransmission in Cholinergic Interneurons Induces Depression-Like Behaviors.
To definitively identify accumbal ChAT neurons as regulators of depression-like behaviors and to determine how the activity of these neurons is involved in the behavioral outputs, we silenced neurotransmission in these cells by using virus-mediated delivery of two membrane-tethered toxins (t-toxins) against calcium voltage-gated channels. This approach has been used in other cell types and has been shown to effectively silence neurotransmission in cells expressing these t-toxins (16). Mice expressing CRE recombinase under the ChAT bac promoter were infected with the CRE-dependent AAV-t-toxins MPE and APC or with control AAV-PE virus. Expression of the t-toxins or control virus was determined by immunohistochemistry (Fig. 3A), and the number of ChAT-positive cells expressing the viruses was determined (Fig. 3 B and C). In mice infected with the control virus (AAV-PE), 77% of ChAT neurons expressed AAV-PE, as detected by EGFP/ChAT colocalization (Fig. 3B). In mice infected with the AAV-MPE/APC viruses, 78% of ChAT neurons expressed both AAV-MPE and APC, as detected by colocalization of EGFP, Cherry, and ChAT, and indicating the near-complete infection of NAC ChAT neurons by the viruses (Fig. 3C). All neurons expressing AAV-MPE/APC were ChAT positive, demonstrating that there was no ectopic expression of the t-toxin viruses in non-ChAT neurons. Mice infected with the t-toxins showed significantly reduced sucrose preference (Fig. 3D) and increased immobility in both the TST (Fig. 3E) and the FST (Fig. 3F) compared with controls. There was no effect of the t-toxins on total distance traveled in an open field (Fig. 3G), illustrating that the observed effects on immobility could not be attributed to changes in locomotor activity. Taken together, these data suggest that silencing neurotransmission in accumbal cholinergic interneurons mimics the effect of p11KO and induces a depression-like behavioral phenotype, suggesting that downstream neuronal activity of these neurons is necessary to maintain motivation and prevent behavioral despair.
Fig. 3.
Silencing accumbal cholinergic interneurons causes depression-like behaviors. (A) Immunohistochemical detection of EGFP (green), Cherry (red), and ChAT (blue) in the NAC of a ChAT-CRE positive mouse infected with the AAV-MPE/APC t-toxins mixture. Quantification of the number of ChAT neurons expressing AAV-PE control virus (B) or AAV-MPE/APC viruses (C) indicated near complete infection of ChAT neurons in the NAC by the viruses and no ectopic virus expression in non-ChAT neurons (Results). Mice infected with the AAV-t-toxins (AAV-MPE/APC) or with the AAV-control virus (AAV-PE) were tested in the sucrose preference test [t(16) = 2.259, P < 0.05] (D), the TST [t(18) = 4.095, P < 0.01] (E), the FST [t(18) = 2.565, P < 0.05] (F), or the open field test (G). All data are presented as means ± SEM. *P < 0.05, **P < 0.01.
Cell Type-Specific Overexpression of p11 Rescues KO Phenotype.
Given our previous studies implicating the NAC as the site where p11 loss exerts its effects on depression-related behaviors (9), and our current data showing that ChAT-p11KO and D2-p11KO mice are anhedonic and have increased behavioral despair, we took a second approach that permitted temporal, spatial, and cell type-specific p11 expression. For this purpose, we generated a virus that selectively overexpressed p11 only in neurons expressing CRE recombinase (Fig. 4 A and B). Thus, mice expressing CRE recombinase in ChAT neurons were bred with constitutive p11KO mice, and the NAC was infected with the CRE-dependent AAV. The NAC can be subdivided into core and shell regions, which share distinct anatomical and functional circuitry (17). Because cholinergic interneurons are an extremely sparse population of neurons (<1%) in the mouse NAC, we have not attempted to distinguish between core and shell regions in the current study. Behavioral analysis of these mice showed that p11 overexpression specifically in NAC ChAT neurons restored normal behavior in constitutive p11KO mice, reflected both in the sucrose preference test and the TST (Fig. 4 C and D). There was no effect of p11 overexpression on locomotor activity (Fig. 4E). In wild-type (WT) mice, p11 overexpression in NAC ChAT neurons had no effect on behavior (Fig. 4 C–E), which is consistent with previous results (9). Control virus injections (AAV-YFP) also did not influence behavioral responses in WT or p11KO mice (Fig. 4 C–E). Overexpression of p11 in ChAT neurons of the dorsal striatum (caudate/putamen; CPU) had no effect on the behavioral responses of p11KO mice tested in the sucrose preference test, TST, and open field (Fig. 4 F–H). These results demonstrate that the influence of p11 in ChAT neurons on depression-like behaviors is specific to cells located in the NAC and not generalized to ChAT neurons in the CPU.
Fig. 4.
Overexpression of p11 in NAc cholinergic interneurons of constitutive p11KO mice restores normal behavior. (A) Schematic of the virus used in these experiments (see text). Constitutive p11 KO mice were bred with ChAT-CRE mice to generate p11 KOs that expressed CRE in ChAT neurons. (B) Immunohistochemical detection of RFP (red), ChAT (blue), and p11 (green) in the NAC of a p11 KO mouse expressing (Upper) or not expressing (Lower) CRE in ChAT neurons. Arrows indicate ChAT-positive cholinergic interneurons overexpressing (Upper) or not over-expressing (Lower) p11. (C–E) Control virus (aav-YFP) or p11 overexpressing virus (aav-p11) was injected to the NAc of CRE-positive WT or p11 KO mice before behavioral testing in the TST [F(1,32) = 6.384, P < 0.05] (C), the sucrose preference test (F(1,32) = 5.681, P < 0.05) (D), or the open field test [main effect AAV: F(1,37) = 3.505, n.s.; main effect genotype: F(1,37) = 0.2654, n.s.] (E). All data are presented as means ± SEM. Significant effects of genotype (#P < 0.05) or p11 overexpression (*P < 0.05, **P < 0.01) are noted. (F–H) CRE-positive constitutive p11KO mice were injected with aav-p11 or aav-YFP into the dorsal striatum (CPU). No effect of p11 overexpression was observed in sucrose preference (main effect virus: F(1,26) = 0.3801, n.s.; main effect genotype: F(1,26) = 1.136, n.s.; interaction virus × genotype: F(1,26) = 0.1272, n.s.) (F), tail suspension test immobility (main effect virus: F(1,25) = 1.994, n.s.; main effect genotype F(1,25) = 1.521, n.s.; Interaction virus × genotype: F(1,25) = 3.874, n.s.) (G), or locomotor activity (main effect virus: F(1,26) ≤0.001, n.s.; main effect genotype: F(1,26) =1.244, n.s.; interaction virus × genotype: F(1,26) = 1.022, n.s.) (H). All data are presented as means ± SEM.
Discussion
Striatal cholinergic interneurons have been linked to addictive behaviors (18, 19), reward learning (19, 20), cognitive function (21), and feeding behaviors (22), all of which can be dysregulated in patients suffering from depression. The NAC, specifically, has been strongly linked to depressive symptoms (1, 4–6) and is a brain region where p11 levels modulate depressive-like behaviors (9). Using an intersectional loss-of-function approach, we demonstrate that deletion of p11 specifically in NAC cholinergic interneurons led to depression-like behaviors. This effect was reversed by subsequent overexpression of p11 in NAC ChAT neurons of constitutive p11KO mice, using a CRE-dependent AAV. Further, we demonstrate that ChAT interneurons play a key role in the NAC circuit regulating mood, because silencing neurotransmitter release from these cells using membrane-tethered toxins resulted in a depression-like phenotype. Taken together, these results show that NAC cholinergic interneurons are crucial for the generation of depression-related behavior, identifying this cell type as an important substrate in the circuitry that regulates mood, motivation, and reward responses.
NAC and Its Involvement in Mood Circuitry.
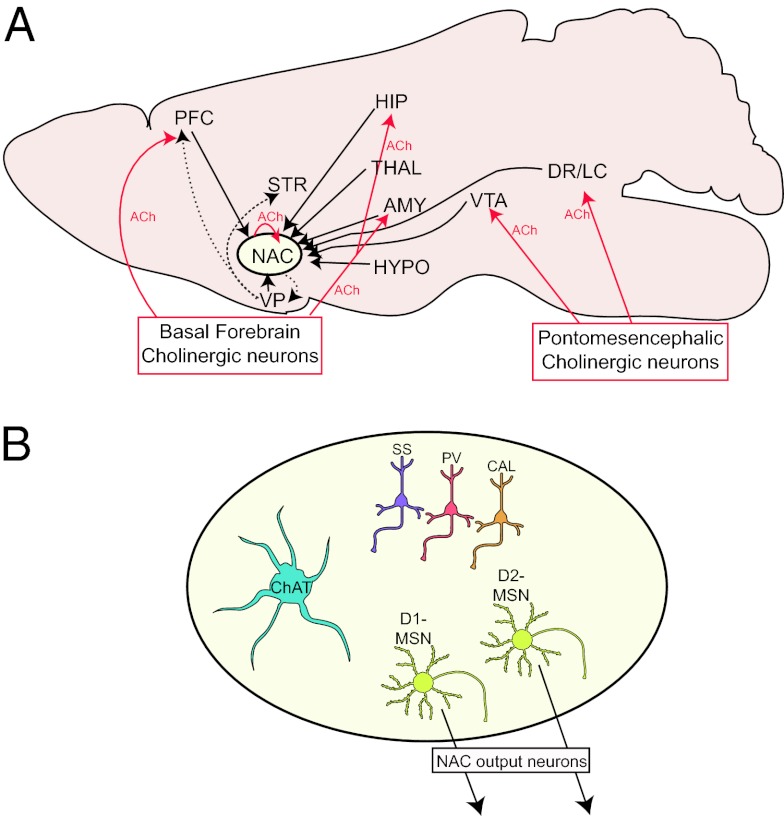
The NAC is a component of a dispersed circuit that modulates mood and motivation. Human imaging studies and rodent models have demonstrated that its activity is linked to depressive-like states (1, 5, 23, 24). The NAC receives dopaminergic inputs from the ventral tegmental area (VTA), serotoninergic inputs from the raphe, and glutamatergic inputs from regions such as the amygdala, hippocampus, thalamus, and infralimbic prefrontal cortex (PFC), all of which have been linked to depression and/or antidepressant action (Fig. 5A). Although MSNs are the primary projection neurons of the NAC, local cholinergic interneurons function to regulate their activity (10) via AChRs expressed on the MSN cell surface. While cholinergic interneurons account for only a small percentage of cells in the NAC (∼1%), they exert a robust influence over accumbal function. Our results emphasize this point in that deletion of p11 specifically from these cells was sufficient to induce a depression-like phenotype, likely a result of decreased activity of the ChAT cells, because silencing the cells with membrane tethered-toxins also resulted in depressive-like behavioral responses. Such an effect may have altered behavior as a result of altered MSN activity, because it has been shown that photoinhibition of NAC cholinergic interneurons enhances MSN spiking in vivo (19).
Fig. 5.
Circuitry that may mediate depressive-like behaviors and the actions of antidepressants. (A) A simplified summary of the inputs (solid black arrows) and outputs (dashed black arrows) of the NAC (for more detailed anatomy, see Groenwegen et al., 1999). Red arrows indicate cholinergic inputs to these areas, including basal forebrain and pontomesencephalic cholinergic neurons. In the NAC, cholinergic interneurons are the only source of acetylcholine, acting locally within the NAC. (B) In the NAC, there are at least six different cell types. Drd1a containing medium spiny neurons (D1-MSN) and Drd2 containing medium spiny neurons (D2-MSN) are the primary output neurons of the NAC. Cholinergic interneurons (ChAT) and three types of GABAergic interneurons expressing somatostatin/nNOS (SS), parvalbumin (PV), or calretinin (CAL), also reside in the NAC, acting locally to control the activity of the MSNs. AMY, amygdala; DR/LC, dorsal raphe/locus coeruleus; HIP, hippocampus; HYPO, hypothalamus; NAC, nucleus accumbens; PFC, prefrontal cortex; STR, striatum; THAL, thalamus; VP, ventral pallidum; VTA, ventral tegmental area.
It is notable that some depression-related behaviors (e.g., sucrose preference) may be explained by disruptions in reward learning, a feature of MDD in humans (25). Silencing neurotransmission in accumbal ChAT neurons reduces sucrose preference (Fig. 3A) and cocaine-induced reward learning (19).
We identify NAC cholinergic interneurons as a cell type that is responsible for inducing both anhedonia and behavioral despair in rodent models of depression. It has been suggested that acetylcholine (ACh) plays an important role in depression and its treatment (26, 27), and cholinergic interneurons are the sole source of acetylcholine in the NAC. However, a cholinergic hypothesis of depression suggests that increasing cholinergic transmission is prodepressive and that anticholinergics may function as antidepressants, supported in part by the observation that tricyclic antidepressants have central anticholinergic properties (26). This hypothesis is further supported by the more recent finding that scopolamine, a centrally acting antimuscarinic agent, is a potent antidepressant in humans (28). When considering the cellular circuitry that may underlie these effects, it is important to note that (i) there are several sources of ACh in the brain, e.g., basal forebrain and pontomesencephalic cholinergic neurons, which exert widespread and complex cholinergic modulation of many brain areas including the PFC, hippocampus, amygdala, VTA, and dorsal raphe (Fig. 5A) and (ii) the cellular mechanisms underlying the actions of anticholinergics (e.g., scopolamine) are not yet understood.
Within the accumbens, cholinergic interneurons are the only source of ACh, acting locally to regulate the activity of other interneurons and NAC efferents. Silencing neurotransmission in accumbal ChAT neurons would prevent release of acetylcholine that modulates downstream cellular responses to glutamate, dopamine, and other transmitters within the NAC, ultimately affecting the output of the NAC through the activity of D1- or D2-containing MSNs (Fig. 5B). However, there is new evidence that striatal cholinergic interneurons also modulate fast glutamatergic neurotransmission (29), which would also be silenced by the methods used in the current study. Therefore, although the cholinergic hypothesis outlined above suggests that anticholinergics are antidepressants, we find that silencing cholinergic interneurons locally in the NAC is prodepressive in rodent models of the disease. One simple explanation is that the effects of scopolamine and other anticholinergics are due to ACh activity in brain areas outside the NAC. Within the NAC, and given the complexity of the local microcircuitry and the diverse inputs and outputs, our identification of a single cell type that regulates anhedonia and behavioral despair is an important step forward in mapping the functional cellular circuitry dysregulated in MDD and related diseases.
Because silencing neurotransmission in ChAT neurons mimics the behavioral phenotype observed when p11 is deleted from these cells, we hypothesize that the removal of p11 disrupts ChAT neuron activity and/or neurotransmission. This effect may be achieved by changes in the cellular response to serotonin because the expression of serotonin receptors at the cell surface is regulated by p11 levels (7). It is also likely that other pathways may contribute to the altered physiological responses, because p11 is known to bind other transmembrane ion channels and signaling proteins (30). Here, we clearly identify accumbal ChAT neurons as a cellular substrate for regulation of depression-like behavior by p11. Future molecular and pharmacological studies, including TRAP molecular profiling will be necessary to validate this hypothesis.
Anatomical Dissociation Between Roles of p11 in Mediating Depressive-Like States and Antidepressant Responses.
Current antidepressant therapies have relatively low treatment response rates and are accompanied by a host of unpleasant side effects. These treatments are based on drugs developed more than 50 y ago. It is therefore crucial to incorporate the significant progress that has been made toward our understanding the neural circuitry mediating MDD into developing novel treatments that specifically target the underlying cell types. Previous work has shown that constitutive p11KO mice, which lack p11 in the whole brain and body, have both a depressive-like phenotype and are less sensitive to antidepressant treatment (7). p11 is normally expressed in a variety of cell types throughout the central nervous system (31). The current study demonstrates that p11 in NAC cholinergic interneurons is sufficient for regulating anhedonia and behavioral despair, identifying these cells as modulators of mood.
It is notable that ChAT-p11KO mice retain their ability to respond to SSRI antidepressant treatment. This result suggests an anatomical dissociation between p11’s involvement in regulating depression-like states and antidepressant responses. In fact, this notion is consistent with recent evidence showing that corticostriatal p11-expressing neurons, which have a strong molecular response to an SSRI also mediate behavioral antidepressant responses (32). Loss of p11 in these cells does not induce a depression-like phenotype (32). Our results are also consistent with the observation that CAMK2α-p11KO mice, which lack p11 in most forebrain neurons (including the corticostriatal p11 cells and striatal MSNs, but not striatal ChAT neurons), show no response to SSRI antidepressants (13). These mice also do not show a depression-like phenotype, presumably because p11 levels in the accumbal cholinergic interneurons are preserved.
The complexity of the neural circuitry underlying mood points toward distinct sites for pathophysiology and treatment. For example, our findings implicate NAC cholinergic interneurons in mediating depression-related behaviors, but the p11-expressing corticostriatal cells underlying antidepressant responses send projections predominantly to dorsal striatum with few efferents to the NAC (32). Although there appear to be no direct connections between these two cell types, they are both significant components of parallel basal ganglia loops, which have independent but integrated roles in cognitive and motivational features of behavior (33–35). These observations present definitive evidence that the cell types generating behavioral phenotypes and those required for effective therapy are distinct. Such an anatomical dissociation between disease and treatment is common for complex circuit-based diseases. In the case of Parkinson’s disease, the dopaminergic cells in substantia nigra degenerate, but current effective therapies target downstream dopamine-receptive cells (36). The present study combined with recent results from the cortex (32) sets the precedent that an in-depth analysis of multiple cell types will be required to understand both the genesis of the depressive behavioral phenotype and the mechanisms required for effective treatment.
Methods
Animals.
Male C57BL/6 mice (8–10 wk old; Charles River Laboratories) were used for experiments in Fig. 1. For all other experiments, male transgenic mice and WT littermates (8–24 wk old) were used. Mice were housed 2–5 per cage with ad libitum access to food and water. p11 constitutive KO mice were generated and maintained at the Rockefeller University (7). Cell type-specific p11 KO mice were generated at The Rockefeller University by breeding floxed p11 mice (13) with mice expressing the CRE recombinase under ChAT (GM60), Drd2 (ER44), Adora2a (KG139), or Drd1a (EY262) promoters (CRE mice provided by GENSAT). For AAV experiments, ChAT-CRE mice were bred with WT or p11 constitutive KO mice. For bacTRAP experiments, ChAT-, D1-, or D2- bacTRAP mice were used (12). Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use committees.
Immunohistochemistry.
Immunohistochemistry was performed using standard procedures (see SI Methods).
Behavioral Analysis.
Sucrose preference was performed as described (7). The open field locomotor test, TST, and FST were performed as described (8). Citalopram hydrobromide (Sigma) was dissolved in sterile saline and was delivered acutely (20 mg/kg, i.p.) 30 min before the tail suspension test.
AAV Vector Construction and Virus Production.
p11 viruses.
The CRE inducible overexpressing vectors were obtained by subcloning either the mouse p11 coding sequence or a flag-tagged YFP downstream of an RFP-STOP cassette flanked by two loxP sequences (ATAACTTCGTATAGCATACATTATACGAAGTTAT) into an AAV vector containing a CMV/Chicken β-actin hybrid promoter followed by composite chicken β-actin/rabbit β-globin intron (gift from Matthew J. During, Ohio State University, Columbus, Ohio). In the absence of the CRE recombinase, RFP is expressed. In the presence of the CRE recombinase, the loxP sequences are recombined, RFP-STOP cassette is excised, and p11 or flag YFP is expressed. Virus stocks were prepared by packaging the vector plasmid into AAV serotype 2 particles using a helper-free plasmid transfection system. The viral vectors were purified by using heparin affinity chromatography and dialyzed against PBS. AAV titers were determined by qPCR with primers to a fragment of the AAV backbone and adjusted to 1011 genomic particles per milliliter.
Vector construction and AAV production of the tethered peptide toxin antagonists of Cav2.1 and Cav2.2 calcium channels (MPE/APC t-toxins).
T-toxin expression cassettes (named MPE and APC) contained the sequences encoding the MVIIA conotoxin (from Conus magus) fused to the PDGF-receptor transmembrane domain followed by EGFP (MPE), or the AgaIVA agatoxin (from Agenelopsis aperta), followed by the TM domain and mCherry (APC). The no-toxin control contains the transmembrane domain of the PDGF receptor and EGFP (PE) (16). Double floxed AAV constructs were generated by insertion of the inverted t-toxin expression cassettes MPE or APC or the no-toxin control PE between double lox 2722 and lox P incompatible sites (DFI). The t-toxin double floxed cassettes were further subcloned into the pENN-AAV-CB7 vector. AAV Vector production of the AAV2/8 serotype was performed by University of Pennsylvania vector core. The titers (genome copies per milliliter) of the AAVs were as follows: 6.62e12 for AAV2/8.CB7.CI.DFI-MPE, 7e12 for AAV2/8.CB7.CI.DFI-APC, and 8.44e12 for AAV2/8.CB7.CI.DFI-PE. The MPE and APC virus stocks were combined (1:1) immediately before use, and 1 μL of the combined MPE/APC (t-toxin) stock or no-toxin control was injected bilaterally into the NAC by using standard stereotaxic surgical procedures. Stereotaxic virus injections were performed under ketamine and xylazine anesthesia as described (9).
bacTRAP and RT-qPCR.
Experiments were performed as described (12). ChAT-, D1-, or D2-bacTRAP mice, expressing EGFP-tagged L10a ribosomal protein under the choline acetyltransferase promoter, were backcrossed to C57BL/6 mice. Six to nine male mice from each line were pooled for each sample, and experiments were performed in triplicate. Striatum were dissected and homogenized, cleared by centrifugation, and polysomes were immunoprecipitated by using antibodies for EGFP. mRNA samples were collected by using the Absolutely RNA Nanoprep kit (Stratagene) according to the manufacturer’s instructions. RNA was quantified, and quality was checked by Bioanalyzer. Amplification and cDNA synthesis was performed by using the WT-Ovation RNA Amplification system (Nugen) according to the manufacturer’s instructions. Samples were run in triplicate and normalized to β-actin by using TaqMan assays (Applied Biosystems). Assays used were as follows: S100A10 (Mm00501457_m1) and β-actin (Mm00607939_s1).
Statistical Analysis.
Comparisons were made by two-tailed unpaired t test or ANOVA using Prism 5 software (GraphPad). In experiments composed of more than two groups, data were first analyzed by two-way ANOVA followed by post hoc Bonferroni test. Statistical significance was set at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Sebastian Auer, James A. Belarde, and Sarah Meller for their technical assistance. This work has been supported by US Army Medical Research Acquisition Activity Grants W81XWH-09-1-0401 (to J.L.W.-S.), W81XWH-09-1-0402 (to P.G.), W81XWH-09-1-0108 (to P.G.), and W81XWH-09-0381 (to M.G.K.); National Institutes of Health (NIH)/National Institute of Mental Health Grant MH090963 (to P.G., N.H., and E.F.S.); Howard Hughes Medical Institute (HHMI) (to N.H. and E.F.S.); American Recovery and Reinvestment Act NIH/National Institute on Drug Abuse Grant 1RC2DA028968 (to P.G., N.H., and E.F.S.); National Institute of Aging Grant AG09464 (to P.G.); The JPB Foundation (to P.G. and M.G.K.); The Fisher Center for Alzheimer’s Research Foundation (to P.G.); The Simons Foundation (to N.H. and P.G.); The Helmholtz Association 31-002 (to I.I.-T.); and The Sonderforschungsbereich SFB 665 (to I.I.-T.). N.H. is an HHMI Investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209293109/-/DCSupplemental.
References
- 1.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Schlaepfer TE, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 7.Svenningsson P, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 8.Warner-Schmidt JL, et al. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander B, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2:54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci USA. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cryan JF, Holmes A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 16.Auer S, et al. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- 17.Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witten IB, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajnal A, Székely M, Gálosi R, Lénárd L. Accumbens cholinergic interneurons play a role in the regulation of body weight and metabolism. Physiol Behav. 2000;70:95–103. doi: 10.1016/s0031-9384(00)00236-5. [DOI] [PubMed] [Google Scholar]
- 23.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 27.Mineur YS, Picciotto MR. Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: A randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svenningsson P, Greengard P. p11 (S100A10)—an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61:442–450. doi: 10.1016/j.neuropharm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt EF, et al. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bornstein AM, Daw ND. Multiplicity of control in the basal ganglia: Computational roles of striatal subregions. Curr Opin Neurobiol. 2011;21:374–380. doi: 10.1016/j.conb.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 35.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Limousin P, Martinez-Torres I. Deep brain stimulation for Parkinson’s disease. Neurotherapeutics. 2008;5:309–319. doi: 10.1016/j.nurt.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.