Abstract
Dendritic cells (DCs) are composed of multiple subsets that play a dual role in inducing immunity and tolerance. However, it is unclear how CD205+ conventional DCs (cDCs) control immune responses in vivo. Here we generated knock-in mice with the selective conditional ablation of CD205+ cDCs. CD205+ cDCs contributed to antigen-specific priming of CD4+ T cells under steady-state conditions, whereas they were dispensable for antigen-specific CD4+ T-cell responses under inflammatory conditions. In contrast, CD205+ cDCs were required for antigen-specific priming of CD8+ T cells to generate cytotoxic T lymphocytes (CTLs) mediated through cross-presentation. Although CD205+ cDCs were involved in the thymic generation of CD4+ regulatory T cells (Tregs), they maintained the homeostasis of CD4+ Tregs and CD4+ effector T cells in peripheral and mucosal tissues. On the other hand, CD205+ cDCs were involved in the inflammation triggered by Toll-like receptor ligand as well as bacterial and viral infections. Upon microbial infections, CD205+ cDCs contributed to the cross-priming of CD8+ T cells for generating antimicrobial CTLs to efficiently eliminate pathogens, whereas they suppressed antimicrobial CD4+ T-cell responses. Thus, these findings reveal a critical role for CD205+ cDCs in the regulation of T-cell immunity and homeostasis in vivo.
Keywords: knock-in mouse, innate immunity, adaptive immunity
Dendritic cells (DCs) are essential antigen-presenting cells (APCs) that consist of heterogeneous subsets, mainly classified as conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (1). DCs serve as sentinels, recognizing the presence of invading pathogens or virus-infected cells through various pattern-recognition receptors, including Toll-like receptors (TLRs) (1). DCs process such exogenous antigens intracellularly and present them to CD4+ T cells via MHC class II (MHC II) for induction of CD4+ effector T (Teff) cells (1–3). DCs also show an unusual specialization in their MHC class I (MHC I) presentation pathway to prime CD8+ T cells. Although most cells use MHC I molecules to present peptides derived from endogenously synthesized proteins, DCs have the capacity to deliver exogenous antigens to the MHC I pathway, a phenomenon known as cross-presentation, that underlies the generation of cytotoxic T lymphocyte (CTL) immunity (1–3). DCs thereby play a critical role in the link between innate and adaptive immunity. Conversely, DCs are also crucial for the induction of immunological tolerance under steady-state conditions, and the mechanisms involved include recessive tolerance mediated by deletion and anergy, and dominant tolerance by maintaining the homeostasis of self-reactive CD4+Foxp3+ naturally occurring regulatory T cells (nTregs) and de novo generation of antigen-specific CD4+Foxp3+ inducible Tregs (iTregs) (4–7).
Mouse cDCs in lymphoid organs are comprised of two major subsets, classified as CD8α+ cDCs and CD8α− cDCs. CD8α+ cDCs mainly reside in the T-cell zone, and CD8α− cDCs reside in the red pulp and marginal zone (2, 8). Series of in vitro and ex vivo studies reported that CD8α+ cDCs compared with CD8α− cDCs strongly generate T-helper cell type 1 (Th1) cells because of the potential high-level production of IL-12 (9). In addition, CD8α+ cDCs are more efficient in the phagocytic uptake of dead cells and in the cross-presentation of cell-bound or soluble antigens on MHC I to generate CTLs than other DC subsets (9).
CD205, an endocytic type I C-type lectin-like molecule that belongs to the mannose receptor family, is mainly expressed on CD8α+ cDCs and cortical thymic epithelium, as well as interdigitating DCs in cutaneous lymph nodes (LNs) derived from dermal DCs and epidermal Langerhans cells (LCs), usually at a higher level than seen on macrophages and B cells (10–13). CD205 may function as an endocytic receptor involved in the uptake of extracellular antigens. Although an endogenous ligand for CD205 has not been identified, an antigen-conjugated mAb specific for CD205 was internalized, processed in the endosomal compartment, and presented to both MHC II and MHC I for cross-presentation with high efficiency (10). Although these observations based on analyses in vitro and ex vivo provide the functions of CD205+ cDCs, their role in the immune system under physiological conditions remains unclear because of the lack of a system that selectively eliminates this cell subset in vivo.
To precisely evaluate the contribution of CD205+ cDCs to the immune system, we engineered knock-in mice that express the diphtheria toxin (DT) receptor (DTR) (12, 14, 15) under the control of the Cd205 gene, which allows the selective conditional ablation of CD205+ cDCs in vivo. Using these mice, we demonstrated the unique role of CD205+ cDCs in the control of T-cell responses and homeostasis in vivo.
Results
Inducible Ablation of CD205+ cDCs in Mice.
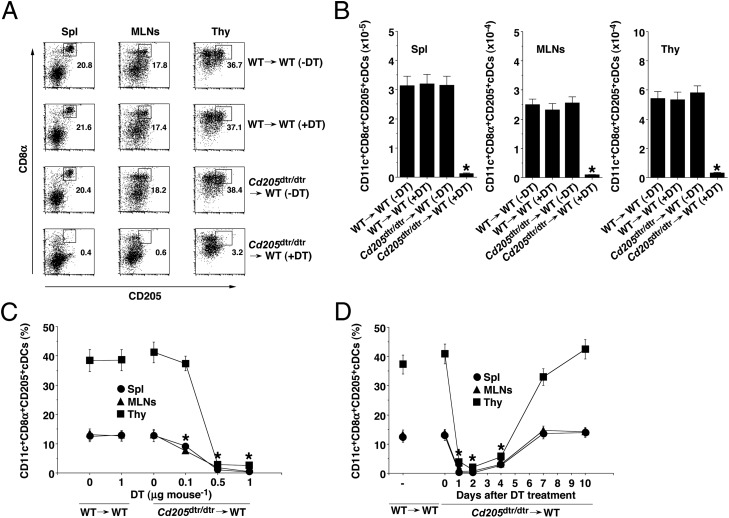
To allow the specific elimination of CD205+ cDCs in lymphoid tissues in vivo, we created Cd205dtr/dtr mice harboring cDNA encoding the human DTR fused to enhanced GFP (EGFP) and an internal ribosome entry site (12, 15) into the 3′ UTR of the Cd205 gene (Fig. S1). Cd205dtr/dtr mice were born at normal Mendelian frequencies and healthy. We observed that a single injection with DT at 0.5∼1 μg per mouse (about 25∼50 ng/g body weight) resulted in the death of Cd205dtr/dtr mice in 10 d. To exclude the possible role of CD205+ cortical thymic epithelium (10) and radioresistant CD8α−CD205+ LCs (12), we generated bone marrow (BM) chimeric mice by reconstitution with BM from Cd205dtr/dtr mice into lethally irradiated recipient WT mice and used them for all of our experiments.
To validate the Cd205dtr/dtr→WT chimeras in terms of DT-induced elimination of CD205+ cDCs, Cd205dtr/dtr→WT chimeras received a single injection of DT, and we monitored 2 d later the subsequent frequency among CD11c+ DCs and the absolute number of CD205+ cDCs. Treatment with DT at 0.5 μg per mouse almost completely ablated CD8α+CD205+ cDCs and CD8α−CD205+ cDCs among CD11c+ DCs in various lymphoid tissues in Cd205dtr/dtr→WT chimeras, whereas this treatment had no effect on their frequency and absolute number in WT→WT chimeras (Fig. 1 A and B and Fig. S2). The DT-mediated ablation of CD205+ cDCs occurred in a dose-dependent manner (Fig. 1C), and near-complete elimination persisted for 2 d, after which their proportions were gradually restored by 7 d (Fig. 1D). On the other hand, Cd205dtr/dtr→WT chimeras exhibited increased proportions of CD11c+CD8α− cDCs and CD11c+BST2+ pDCs in spleen (Spl) (Figs. S3A and S4), but not in mesenteric LNs (MLNs) (Fig. S5), until 4 d after the injection with DT, but their cell numbers were returned to the basal levels by day 7 (Fig. S3B).
Fig. 1.
Conditional ablation of CD205+ cDCs in Cd205dtr/dtr mice. (A and B) WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) were injected with DT (0.5 μg/mouse), and Spl, MLNs, and thymus (Thy) were obtained 2 d after the injection. (A) The frequency of CD11c+CD8α+CD205+ cDCs was analyzed by flow cytometry. Data are represented by a dot plot, and numbers represent the proportion of CD8α+CD205+ cDCs among CD11c+ cells in each quadrant. (B) The absolute number of CD11c+CD8α+CD205+ cDCs was analyzed by flow cytometry. (C) WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) were injected with various doses of DT, and Spl, MLNs, and Thy were obtained 2 d after the injection. The frequency of CD11c+CD8α+CD205+ cDCs was analyzed by flow cytometry. Data are the percentage of positive cells among CD11c+ cells. (D) WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) were injected with DT (0.5 μg/mouse), and Spl, MLNs, and Thy were obtained at the indicated days after the injection. The frequency of CD11c+CD8α+CD205+ cDCs was analyzed by flow cytometry. Data are the percentage of positive cells among CD11c+ cells. *P < 0.01 compared with WT→WT chimeras. Data are the mean ± SD, and the results are representative of six independent experiments.
We also addressed whether other leukocyte populations are affected by the DT treatment in Cd205dtr/dtr→WT chimeras. There was no change in the frequency of CD4+ T cells, CD8+ T cells CD3+CD49b+ NKT cells, CD3−CD49b+ NK cells, and CD11b+CD11c− macrophages in Spl (Fig. S4) and MLNs (Fig. S5) or in the content of thymocytes (Fig. S6) in DT-treated Cd205dtr/dtr→WT chimeras. In addition, analysis of T-cell receptor (TCR) Vβ use revealed that there were no major differences in the formation of the TCR repertoire of thymic and peripheral CD4+ T cells and CD8+ T cells between WT→WT chimeras and DT-treated Cd205dtr/dtr→WT chimeras (Fig. S7). However, DT-induced ablation of CD205+ cDCs led to a slight reduction in the frequency of splenic B220+ B cells (Fig. S4), although not MLN B220+ B cells (Fig. S5). We also observed that DT had no effect on the viability of splenic B220+ B cells in vitro, indicating that this reduction might not be because of the direct cytotoxicity of DT.
Taken together, Cd205dtr/dtr→WT chimeras provide a unique means of specifically probing the impact of CD205+ cDCs on innate and adaptive immune responses.
Ablation of CD205+ cDCs Has Distinct Effects on Antigen-Specific CD4+ T-Cell Responses in Vivo.
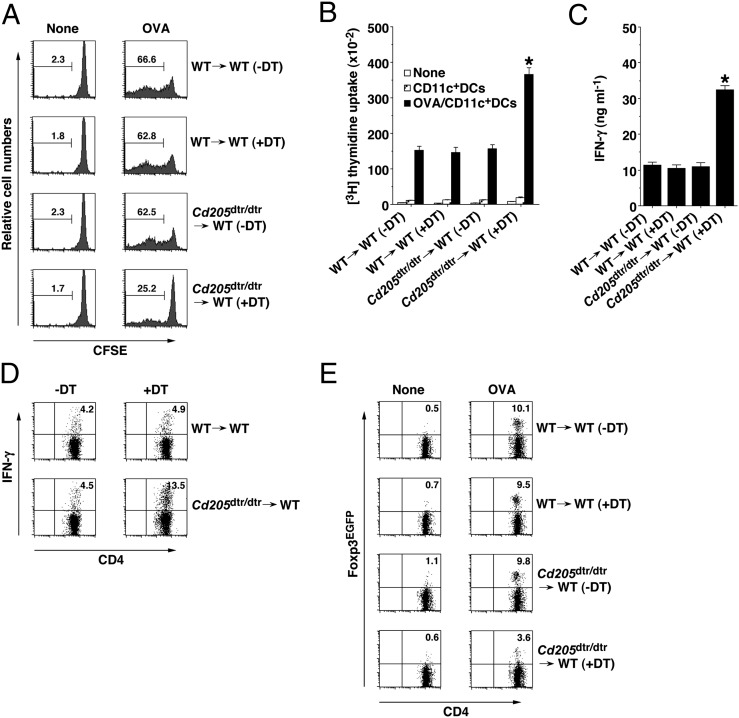
To address the role of CD205+ cDCs in the antigen-specific priming of CD4+ T cells in vivo, we adoptively transferred carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled ovalbumin (OVA)-specific TCR transgenic OT-II CD4+ T cells (15) into chimeric mice, and analyzed their division in Spl 3 d after the systemic injection of soluble OVA protein under steady-state conditions (Fig. 2A). Although the immunization of WT→WT chimeras with OVA protein led to a potent antigen-specific division of OT-II CD4+ T cells, DT-treated Cd205dtr/dtr→WT chimeras showed imapired antigen-specific responses.
Fig. 2.
Ablation of CD205+ cDCs influences antigen-specific CD4+ T-cell responses in vivo. (A) CD45.1+OT-II CD4+ T cells were transferred into WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse), and then the mice were immunized with or without OVA protein. Antigen-specific division of CD45.1+OT-II CD4+ T cells was analyzed at 3 d after the immunization by flow cytometry. Data are represented by a histogram, and numbers represent the proportion of CFSE dilution among gated CD45.1+OT-II CD4+ T cells in each quadrant. (B–D) WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse) were immunized with CFA plus OVA protein. At 14 d after the immunization, Spl CD4+ T cells were cultured with WT CD11c+ DCs in the presence or absence of OVA protein for the measurements of proliferative response by [3H]thymidine incorporation (B) and production of IFN-γ by ELISA (C). *P < 0.01 compared with WT→WT chimeras. Data are the mean ± SD. (D) Intracellular expression of IFN-γ in the cultured CD4+ T cells was analyzed by flow cytometry. Data are represented by a dot plot, and numbers represent the proportion of IFN-γ+ cells among gated CD4+ T cells in each quadrant. (E) CD45.1+OT-II CD4+Foxp3EGFP- T cells were transferred into WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse), and then the mice were immunized with or without OVA protein. Expression of Foxp3EGFP among gated CD45.1+OT-II CD4+ T cells in Spl was analyzed at 8 d after the immunization by flow cytometry. Data are represented by a dot plot, and numbers represent the proportion of Foxp3EGFP+ cells among gated CD45.1 OT-II CD4+ T cells in each quadrant. The results are representative of three independent experiments.
To clarify the influence of the ablation of CD205+ cDCs on antigen-specific CD4+ T-cell responses in vivo under inflammatory conditions, we examined the antigen-specific recalled response of splenic CD4+ T cells from chimeric mice 14 d after subcutaneous immunization with OVA protein emulsified in complete Freund’s adjuvant (CFA). CD4+ T cells from DT-treated Cd205dtr/dtr→WT chimeras showed a more vigorous proliferation on restimulation with OVA protein than did CD4+ T cells from WT→WT chimeras (Fig. 2B). Furthermore, CD4+ T cells from DT-treated Cd205dtr/dtr→WT chimeras markedly enhanced the production of IFN-γ as well as the frequency of IFN-γ–producing cells (Th1 cells) compared with the cells from WT→WT chimeras (Fig. 2 C and D, and Fig. S8 A and B).
We also investigated the role of CD205+ cDCs in the antigen-specific differentiation of CD4+Foxp3− T cells into CD4+Foxp3+ iTregs in peripheral tissues. Chimeric mice were adoptively transferred with OT-II CD4+Foxp3EGFP- T cells, and we monitored the generation of OT-II CD4+Foxp3EGFP+ iTregs in Spl 8 d after systemic immunization with OVA protein (Fig. 2E). An injection with OVA protein efficiently generated OT-II CD4+Foxp3EGFP+ iTregs in WT→WT chimeras under steady-state conditions. However, such antigen-specific peripheral generation of OT-II CD4+Foxp3EGFP+ iTregs was severely diminished in DT-treated Cd205dtr/dtr→WT chimeras.
Ablation of CD205+ cDCs Impairs Antigen-Specific CD8+ T-Cell Responses in Vivo.
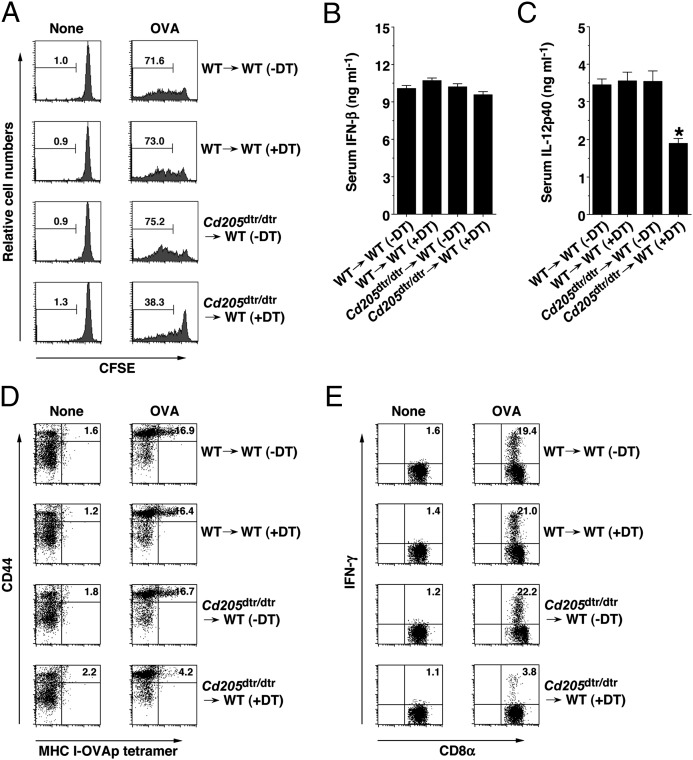
To address the impact of the absence of CD205+ cDCs on the cross-presentation of soluble antigen for the priming of CD8+ T cells in vivo, chimeric mice that had been adoptively transferred with CFSE-labeled OVA-specific OT-I CD8+ T cells (15) were immunized with soluble OVA protein, and their antigen-specific division was measured in Spl 3 d after immunization (Fig. 3A). DT-treated Cd205dtr/dtr→WT chimeras showed a marked reduction of antigen-specific division of OT-I CD8+ T cells under steady-state conditions compared with WT→WT chimeras.
Fig. 3.
Ablation of CD205+ cDCs reduces antigen-specific CD8+ T-cell responses in vivo. (A) CD45.1+OT-I CD8+ T cells were transferred into WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse), and then the mice were immunized with or without OVA protein. Antigen-specific division of CD45.1+OT-I CD8+ T cells was analyzed at 3 d after the immunization by flow cytometry. Data are represented by a histogram, and numbers represent the proportion of CFSE dilution among gated CD45.1+OT-I CD8+ T cells in each quadrant. (B–E) WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse) were immunized with or without OVA protein in combination with poly (I:C) plus anti-CD40 mAb. (B and C) Serum levels of IFN-β (B) and IL-12p40 (C) were measured at 3 h after injection by ELISA. *P < 0.01 compared with WT→WT chimeras. Data are the mean ± SD (D and E) At 6 d after the immunization, splenocytes were analyzed for the generation of MHC I-OVA tetramer+CD44highCD8+ T cells (D) and for intracellular IFN-γ–producing CD8+ T cells (E) by flow cytometry. Data are represented by a dot plot, and numbers represent the proportion of MHC I-OVA tetramer+CD44high cells (D) and IFN-γ+ cells (E) among gated CD8+ T cells in each quadrant. The results are representative of three independent experiments.
To directly prove the influence of the lack of CD205+ cDCs on the generation of CTLs through the cross-presentation of soluble antigen in vivo, chimeric mice were immunized with OVA protein combined with poly (I:C) (a TLR3 ligand) and agonistic anti-CD40 mAb, and we quantified the generation of antigen-specific CD8+ T cells in Spl based on binding with the MHC I-OVA tetramer and OVA-specific intracellular expression of IFN-γ at 6 d later. Immunization of WT→WT chimeras resulted in a marked elevation in serum concentrations of IFN-β and IL-12p40 3 h after the immunization (Fig. 3 B and C and Fig. S8C), and the prominent generation of MHC I-OVA tetramer+CD44highCD8+ T cells and CD8+IFN-γ+ T cells (Fig. 3 D and E). However, DT-treated Cd205dtr/dtr→WT chimeras showed decreased production of serum IL-12p40 (Fig. 3C) and the impaired generation of OVA-specific CTLs (Fig. 3 D and E).
Ablation of CD205+ cDCs Controls T-Cell Homeostasis in Vivo.
To determine the impact of the ablation of CD205+ cDCs on T-cell homeostasis, we measured the frequency and number of CD4+Foxp3+ nTregs and CD4+ Teff cells in chimeric mice (Fig. S9). DT-treated Cd205dtr/dtr→WT chimeras exhibited a lower frequency and absolute number of thymic CD4+Foxp3+ Tregs than WT→WT chimeras, and the population of CD4+Foxp3+ Tregs in Spl, as well as the lamina propria of the small intestine, was higher in DT-treated Cd205dtr/dtr→WT chimeras than in WT→WT chimeras. On the other hand, DT-treated Cd205dtr/dtr→WT chimeras had an increased proportion and absolute number of Th1 cells and IL-17-producing CD4+ T cells (Th17 cells) compared with WT→WT chimeras.
Ablation of CD205+ cDCs Alters Host Defense Against Bacterial Infection in Vivo.
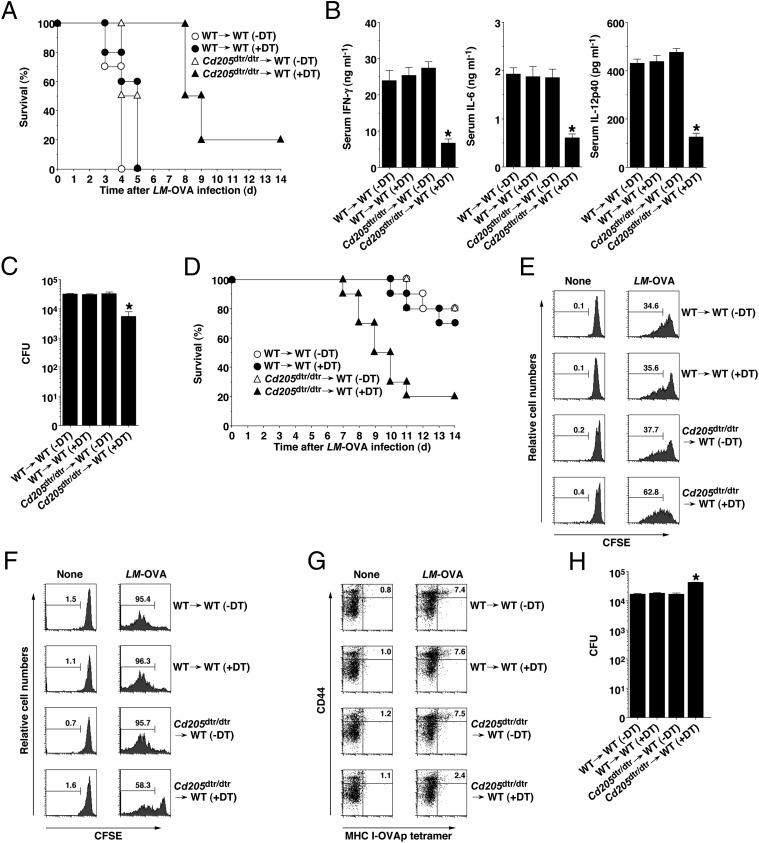
CD11c+ DCs have been suggested to control host defenses against intracellular bacterial infections (14, 16, 17). We therefore examined host immune responses to an infection with Listeria monocytogenes expressing OVA (LM-OVA) in the absence of CD205+ cDCs. Similar to microbial septic shock (18), a high dose of LM-OVA (1 × 107 CFU/mouse) was lethal to WT→WT chimeras (Fig. 4A), and was associated with the marked production of serum proinflammatory cytokines 24 h after infection (Fig. 4B and Fig. S8C). In contrast, DT-treated Cd205dtr/dtr→WT chimeras showed resistance for 5 d after infection (P < 0.01) (Fig. 4A), and significantly less production of proinflammatory cytokines and splenic bacterial burden than WT→WT chimeras (Fig. 4 B and C). However, their survival rates gradually declined 8 d after infection (Fig. 4A). On the other hand, DT-treated Cd205dtr/dtr→WT chimeras were more susceptible to bacterial infection than WT→WT chimeras 7 d after infection with a low dose of LM-OVA (5 × 105 CFU/mouse) (P < 0.01) (Fig. 4D).
Fig. 4.
Ablation of CD205+ cDCs controls host defenses against bacterial infections in vivo. (A–C) WT→WT chimeras (n = 10) and Cd205dtr/dtr→WT chimeras (n = 10) that had been treated with DT (0.5 μg/mouse) were infected with LM-OVA (1 × 107 CFU/mouse). (A) Survival was monitored for 14 d. (B) Serum levels of IFN-γ, IL-6, and IL-12p40 were measured at 24 h after infection by ELISA. (C) Bacterial burden in the Spl was determined as CFU 2 d after infection. *P < 0.01 compared with WT→WT chimeras. Data are the mean ± SD. (D) WT→WT chimeras (n = 10) and Cd205dtr/dtr→WT chimeras (n = 10) that had been treated with DT (0.5 μg/mouse) were infected with LM-OVA (5 × 105 CFU/mouse), and survival was monitored for 14 d. (E and F) CD45.1+OT-II CD4+ T cells (E) or CD45.1+OT-I CD8+ T cells (F) were transferred into WT→WT chimeras (n = 6) and Cd205dtr/dtr→WT chimeras (n = 6) that had been treated with DT (0.5 μg/mouse), and then the mice were uninfected or infected with LM-OVA (5 × 105 CFU/mouse). Antigen-specific division of CD45.1+OT-II CD4+ T cells or CD45.1+OT-I CD8+ T cells was analyzed at 3 d after infection by flow cytometry. Data are represented by a histogram, and numbers represent the proportion of CFSE dilution among gated CD45.1+OT-II CD4+ T cells or CD45.1+OT-I CD8+ T cells in each quadrant. (G and H) WT→WT chimeras (n = 10) and Cd205dtr/dtr→WT chimeras (n = 10) that had been treated with DT (0.5 μg/mouse) were uninfected or infected with LM-OVA (5 × 105 CFU/mouse). (G) At 6 d after infection, splenocytes were analyzed for the generation of MHC I-OVA tetramer+CD44highCD8+ T cells by flow cytometry. Data are represented by a dot plot, and numbers represent the proportion of MHC I-OVA tetramer+CD44high cells among gated CD8+ T cells in each quadrant. (H) Bacterial burden in the Spl was determined as CFU 6 d after infection. *P < 0.01 compared with WT→WT chimeras. Data are the mean ± SD, and the results are representative of three independent experiments.
To clarify the role of CD205+ cDCs in the antibacterial priming of CD4+ T cells or CD8+ T cells in vivo, antigen-specific division of CFSE-labeled OT-II CD4+ T cells or OT-I CD8+ T cells transferred into chimeric mice was measured in Spl 3 d after infection with a low dose of LM-OVA (5 × 105 CFU/mouse). DT-treated Cd205dtr/dtr→WT chimeras exhibited the enhanced antigen-specific division of OT-II CD4+ T cells following infection with LM-OVA (Fig. 4E), but they showed a decreased antigen-specific response of OT-I CD8+ T cells compared with WT→WT chimeras (Fig. 4F).
We also examined the influence of the ablation of CD205+ cDCs on the generation of antibacterial CTLs. DT-treated Cd205dtr/dtr→WT chimeras had a reduced capacity to generate MHC I-OVA tetramer+CD44highCD8+ T cells in Spl compared with WT→WT chimeras 6 d after infection with a low dose of LM-OVA (5 × 105 CFU/mouse) (Fig. 4G). In addition, DT-treated Cd205dtr/dtr→WT chimeras showed a higher bacterial burden in Spl 6 d after infection a low dose of LM-OVA (5 × 105 CFU/mouse) than WT→WT chimeras (Fig. 4H).
Ablation of CD205+ cDCs Impairs Host Defense Against Viral Infection in Vivo.
The importance of CD11c+CD8α+ cDCs in antiviral immunity has been mainly demonstrated by ex vivo analysis examining the initiation of CD8+ T-cell responses through the cross-presentation of viral antigens (9). To determine the contribution of CD205+ cDCs to the cross-presentation of viral antigens for the activation of CD8+ T cells in vivo, we evaluated the host protective immunity in chimeric mice infected with HSV-1. The infection of WT→WT chimeras with HSV-1 elicited the production of IFN-β and IL-12p40 6 h later (Figs. S8C and S10 A and B), and the efficient generation of MHC I-HSV-1 gB tetramer+CD44highCD8+ T cells and HSV-1 gB-specific CD8+IFN-γ+ T cells in Spl 5 d after infection (Fig. S10 C and D). In contrast, DT-treated Cd205dtr/dtr→WT chimeras showed a significant reduction in the production of IL-12p40, but not IFN-β (Fig. S10 A and B), but they had a marked reduction of anti–HSV-1 CTLs following infection (Fig. S10 C and D). In addition, a viral burden in Spl was detected in DT-treated Cd205dtr/dtr→WT chimeras, but not in WT→WT chimeras, 5 d after infection (Fig. S10E).
Discussion
In this article, we are unique in reporting the use of a DTR-based knock-in system to generate mice that conditionally lack CD8α+CD205+ cDCs and CD8α−CD205+ cDCs to address the role for these cells in the control of innate and adaptive immunity. It had been shown that mice with mutations to several transcription factor genes, including Id2 and Irf8, lack CD8α+ cDCs but have additional immune defects (9, 19). Furthermore, mice deficient in the transcription factor Batf3 reportedly also constitutively lack CD8α+ cDCs as well as CD103+CD11b− cDCs, but not other DC subsets, in lymphoid and peripheral tissues (3, 20). Therefore, we demonstrated that the almost complete and specific conditional ablation of CD8α+CD205+ cDCs and CD8α−CD205+ cDCs provides for the study of their function in the regulation of immune responses in vivo. Previous studies have shown some controversies regarding the influence of the constitutive versus conditional elimination of CD11c+ DCs or LCs on immune responses, implying that their chronic ablation allows for the emergence of compensatory mechanisms, which can confound any primary deficits (6, 7, 21, 22). Therefore, our system allowing inducible ablation of CD205+ cDCs could take an advantageous means to analyze their spaciotemporal role for the control of immune responses.
The observations in Batf3-deficient mice showed that the constitutive lack of CD8α+ cDCs had no effect on CD4+ T-cell responses (3). We showed that the antigen-specific division of splenic CD4+ T cells was impaired under the conditional ablation of CD8α+CD205+ cDCs following systemic antigenic stimulation alone. Therefore, CD8α+CD205+ cDCs could contribute to the antigen-specific priming of splenic CD4+ T cells in vivo under steady-state conditions. Conversely, the conditional ablation of CD8α+CD205+ cDCs and CD8α−CD205+ cDCs in the lymphoid tissues and possibly in the skin (12) promoted antigen-specific activation of splenic CD4+ T cells for proliferation and Th1-differentiation after subcutaneous antigenic inflammatory stimulation as well as systemic microbial infection. Furthermore, the conditional ablation of CD205+ cDCs reduced the antigen-specific peripheral generation of CD4+Foxp3+ iTregs. In vitro and ex vivo observations have shown that CD11c+CD8α+ cDCs had the potential to produce CD4+Foxp3+ iTregs compared with CD11c+CD8α− cDCs because of the endogenous production of TGF-β, and the in vivo delivery of antigen to CD11c+CD8α+CD205+ cDCs caused a more marked generation of CD4+Foxp3+ iTregs than that to CD11c+CD8α− cDCs (4, 8). Therefore, CD205+ cDCs could be critical APCs for converting CD4+Foxp3− T cells into CD4+Foxp3+ iTregs to control the immune tolerance. On the other hand, CD11c+CD8α− cDCs are reportedly more effective than CD11c+CD8α+ cDCs in the activation of the antigen-specific CD4+ T cells, possibly because of their superior capacity in producing MHC class II-peptide complexes (2). Taking these data together, the enhanced antigen-specific CD4+ T-cell responses by the conditional ablation of CD205+ cDCs after inflammatory antigenic stimulation could be because of the impaired generation of antigen-specific CD4+Foxp3+ iTregs as well as the increased proportions of the activated CD11c+CD8α− cDCs.
Similar to the results in Batf3-deficient mice (3), the conditional ablation of CD8α+CD205+ cDCs led to the impaired generation of splenic CTLs following application with antigen plus maturation stimuli. It had been reported that type I IFN, but not IL-12, is required for the generation of CTLs (23). Taking these data together, we find that CD8α+CD205+ cDCs could play a prerequisite role in the cross-presentation of exogenous antigen for the generation of CTLs regardless of sufficient type I IFN production.
CD11c+ DCs have been implicated in the control of the generation and homeostasis of CD4+Foxp3+ nTregs and CD4+ Teff cells (5, 6, 24). Conditional ablation of CD8α+CD205+ cDCs reduced the production of thymic CD4+Foxp3+ nTregs. Therefore, the self-reactive interaction of CD4+Foxp3− thymocytes with thymic CD8α+CD205+ cDCs and their costimulation through CD28 and CD80/CD86 (25) could be required for the development of thymic CD4+Foxp3+ nTregs. On the other hand, the conditional ablation of CD8α+CD205+ cDCs and CD8α−CD205+ cDCs increased population of both CD4+Foxp3+ nTregs and CD4+ Teff cells in peripheral and mucosal lymphoid tissues, possibly because of the indirect effect through the enhanced proportions of CD11c+CD8α− cDCs. Collectively, these findings suggest that CD205+ cDCs are critical for the regulation of the equilibrium of CD4+Foxp3+ nTregs and CD4+ Teff cells to maintain peripheral and mucosal immune homeostasis.
Although CD11c+CD8α+ cDCs might be required for efficient bacterial entry into the Spl (17), the relevance of this process to the subsequent immune responses remains to be understood. The conditional ablation of CD8α+CD205+ cDCs diminished the splenic colonization of bacteria, and that led to the limitation of lethal inflammation after infection with a high dose of bacteria. Therefore, splenic CD8α+CD205+ cDCs could provide a specific nitch for the bacterial spreading and replication in the Spl that initiate undesirable lethal inflammatory responses during the early phase of an extensive bacterial infection. Conversely, the conditional ablation of CD8α+CD205+ cDCs caused a decrease in the survival of the infected mice that accompanied the abortive generation of antibacterial CTLs and the enhanced splenic bacterial burden after infection with a low dose of bacteria. Taken together, our findings suggest that splenic CD8α+CD205+ cDCs are involved in the initiation of microbial septic shock after the massive bacterial infection, although they are required for the protection against the bacterial organ failure mediated by the cross-priming of CD8+ T cells for generating antibacterial CTLs to efficiently eliminate pathogenic microbes under the lack of lethal bacterial inflammation.
Published reports suggest that dermal CD11c+CD103+ cDCs are the dominant migratory APCs cross-presenting viral antigens to CD8+ T cells after an infection in the skin or lungs (3, 20, 26, 27). Our results show that the conditional ablation of splenic CD8α+CD205+ cDCs abrogated the generation of antiviral CTLs after systemic viral infection independent of type I IFN production. These findings led us to hypothesize that CD8α+CD205+ cDCs located in T-cell areas of Spl predominantly phagocytose virus-infected cells to cross-present viral antigens for the optimal generation of antiviral CTL-responses to eliminate the virus during a systemic infection.
Collectively, our findings demonstrate that CD205+ cDCs play a critical role in controlling T-cell immunity and homeostasis as well as inflammation in vivo. A series of studies have suggested that human blood BDCA3+ DCs are homologous to mouse CD8α+CD205+ cDCs in terms of their specific gene signature and functionally equivalent for cross-presentation (28). Therefore, further elucidation of CD205+ cDCs might provide insights into immune regulation and pathology, and aid therapeutic interventions for infectious diseases as well as autoimmune and inflammatory disorders.
Materials and Methods
Mice.
The following mice were used in this study: C57BL/6 mice (Charles River Laboratories); B6.OT-I TCR transgenic mice harboring OVA-specific CD8+ T cells (15); and B6.OT-II TCR transgenic mice harboring OVA-specific CD4+ T cells (15). B6.CD45.1+ OT-I mice and B6.CD45.1+ OT-II mice were bred in-house by crossing B6.OT-I mice and B6.OT-II mice with CD45.1+ B6 mice (15). Foxp3EGFPCD45.1+ OT-II mice were also generated by crossing B6.CD45.1+ OT-II mice with B6.Foxp3EGFP mice (15, 29). All mice were bred and maintained in specific pathogen-free conditions in the animal facility at RIKEN Research Center for Allergy and Immunology in accordance with institutional guidelines.
Immunization.
Details of the immunization are described in SI Materials and Methods.
Bacteria, Virus, and Infection.
Details of the bacteria, virus, and infection are described in SI Materials and Methods.
Statistical Analysis.
Data are expressed as the mean ± SD. The statistical significance of the values obtained was evaluated by ANOVA and the Kaplan–Meier log-rank test. A P value of <0.01 was considered significant.
Supplementary Material
Acknowledgments
We thank Y. Kawaguchi (The Institute of Medical Science, University of Tokyo) for WT HSV-1 (strain F); all members of the Central Facility at the RIKEN Research Center for Allergy and Immunology for technical help in cell sorting; and S. Haraguchi for secretarial assistance. This work was supported by a Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (to Katsuaki Sato) and the Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (Katsuaki Sato).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202208109/-/DCSupplemental.
References
- 1.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 2.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 3.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 5.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darrasse-Jèze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 12.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takagi H, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Zammit DJ, Cauley LS, Pham QM, Lefrançois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelson BT, et al. CD8α+ dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita S, et al. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood. 2006;107:3656–3664. doi: 10.1182/blood-2005-10-4190. [DOI] [PubMed] [Google Scholar]
- 19.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birnberg T, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen CL, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukaya T, et al. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood. 2010;116:2266–2276. doi: 10.1182/blood-2009-10-250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 27.Henri S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villadangos JA, Shortman K. Found in translation: The human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–1134. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol. 2008;180:1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.