Abstract
Endangered species recovery programs seek to restore populations to self-sustaining levels. Nonetheless, many recovering species require continuing management to compensate for persistent threats in their environment. Judging true recovery in the face of this management is often difficult, impeding thorough analysis of the success of conservation programs. We illustrate these challenges with a multidisciplinary study of one of the world’s rarest birds—the California condor (Gymnogyps californianus). California condors were brought to the brink of extinction, in part, because of lead poisoning, and lead poisoning remains a significant threat today. We evaluated individual lead-related health effects, the efficacy of current efforts to prevent lead-caused deaths, and the consequences of any reduction in currently intensive management actions. Our results show that condors in California remain chronically exposed to harmful levels of lead; 30% of the annual blood samples collected from condors indicate lead exposure (blood lead ≥ 200 ng/mL) that causes significant subclinical health effects, measured as >60% inhibition of the heme biosynthetic enzyme δ-aminolevulinic acid dehydratase. Furthermore, each year, ∼20% of free-flying birds have blood lead levels (≥450 ng/mL) that indicate the need for clinical intervention to avert morbidity and mortality. Lead isotopic analysis shows that lead-based ammunition is the principle source of lead poisoning in condors. Finally, population models based on condor demographic data show that the condor’s apparent recovery is solely because of intensive ongoing management, with the only hope of achieving true recovery dependent on the elimination or substantial reduction of lead poisoning rates.
Keywords: wildlife, ecotoxicology, hunting, demography, vulture
Endangered species recovery efforts aim to identify the threats that led to population declines, eliminate those threats, steward and monitor recovering populations, and ultimately, delist the species after self-sustaining populations are achieved (1). Legislated policies related to species recovery efforts (e.g., the US Endangered Species Act and the Canadian Species at Risk Act) explicitly or implicitly assume that intensive management efforts will be of finite duration, with successful actions leading to delisting of legally protected species and cessation of most or all management action (2, 3). However, recovery efforts over the past several decades have led to an emerging realization that many endangered populations may require ongoing management to successfully sustain viable populations in the wild (1, 4). Nonetheless, a crucial distinction exists between endangered species for which necessary actions to promote self-sustaining recovery are clear and attainable but perhaps, controversial or politically charged and those species for which they are truly intractable, such as permanent loss of suitable habitat. Recognizing when an endangered species and its management needs are in one vs. the other of these categories requires careful analysis of the factors limiting recovery. Here, we present such an analysis for one of the most iconic and threatened species in North America, the California condor (Gymnogyps californianus).
The California condor has been a symbol of environmental tragedy and triumph for over 30 years. On the brink of extinction in 1982, with a world population of only 22 individuals (5), the recovery of North America’s largest bird was highly uncertain (6). Captive breeding programs were established, leading to the reintroduction of condors in the wild. Today, the condor’s recovery is recognized by the public as a success (7), with a population of nearly 400 birds at the end of 2010, approximately one-half of which are free-flying.
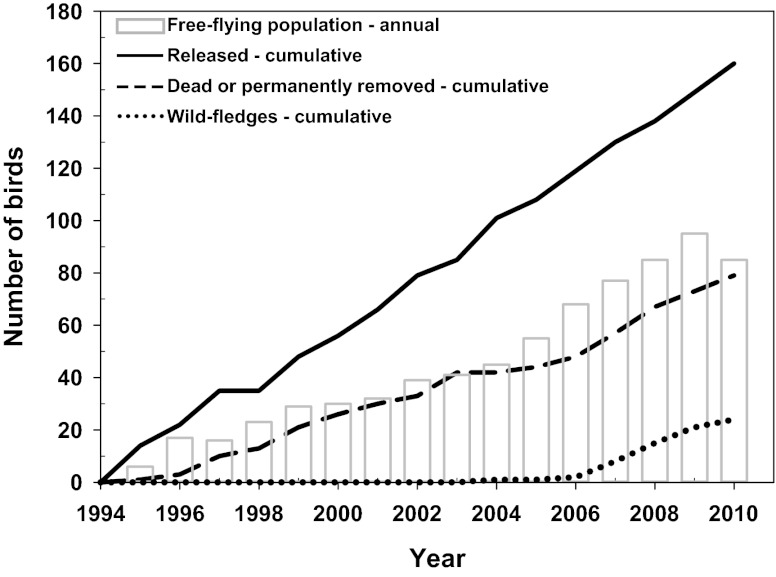
However, if recovery is defined as establishment of a self-sustaining population, the condor’s situation is less clear. Intensive and continuing management interventions currently involve all birds being closely monitored by radio and/or global positioning system transmitters, regular provision of food, vaccination against West Nile virus, removal of trash from and around active condor habitat (e.g., nesting sites), and finally, semiannual recapture of nearly every bird for physical checkups and if indicated by health status, extensive stays in captive facilities (7, 8). Furthermore, although the free-flying condor population within California has gone from 0 to almost 100 individuals over the past two decades, this increase is largely because of the release of captive-raised birds. In California, there have been 160 original releases, but only 24 chicks have fledged in the wild (Fig. 1).
Fig. 1.
Free-flying (i.e., wild) condor population growth in California by year shows that the number of birds has increased since 1994, when there were no free-flying condors. However, the cumulative number of birds that have died or been permanently removed from the wild far exceeds the cumulative number of chicks that have fledged in the wild. Data courtesy of the US Fish and Wildlife Service.
One of the greatest threats to condors and a major factor demanding such intensive ongoing management is lead poisoning (7, 9, 10). Evidence of elevated lead exposure in California condors began to emerge in the mid-1970s (11), and lead poisoning may have been a factor for their near-extinction in the 1980s (7). As a result, lead exposures are monitored by field crews by semiannual blood measurements for the majority of free-flying condors within California. However, the degree to which lead poisoning impacts condor population health has not been fully understood, in part because these intensive management practices partially compensate for the population-level impacts of lead, thus obscuring the seriousness of this problem.
The California condor illustrates the complexity and consequences of endangered species planning when significant environmental hazards are not adequately mitigated. Here, we (i) present data on the frequency, magnitude, and sources of lead exposure and related health effects in condors free-flying in California and (ii) develop a demographic model to estimate future condor population growth in the presence or absence of current management efforts with and without the impacts of continued lead exposure.
Results and Discussion
Rates of Lead Exposure and Consequences to Individual Health.
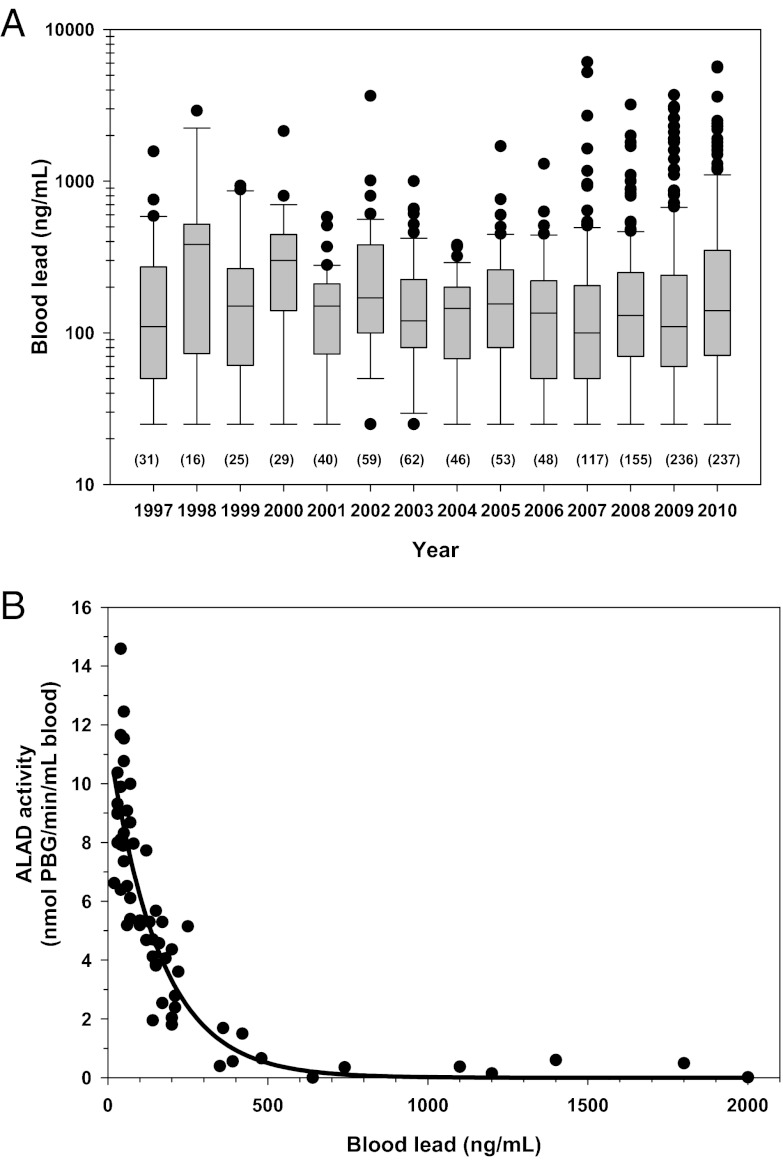
Blood lead levels measured between 1997 and 2010 (n = 150 birds, n = 1,154 independent blood samples) show that free-flying condors in California were chronically exposed to lead, with the median blood lead level each year exceeding the proposed 100 ng/mL blood lead exposure threshold for condors (12) (Fig. 2A); the 100 ng/mL lead level is approximately threefold higher than the average background blood lead level of prerelease condors with no history of lead exposure (mean = 30.3 ± 9.7 ng/mL, n = 22). The annual prevalence of lead exposure in condors in California, defined as the number of birds with a blood lead level ≥ 100 ng/mL divided by the total number of birds sampled that year, ranged from 50% to 88% (median = 71%).
Fig. 2.
California condor blood lead levels and sublethal exposure effects show that the population is negatively impacted by chronic lead exposure. (A) Blood lead levels of condors in California by year. Box indicates median, upper and lower bounds are 75th and 25th percentiles, whiskers represent 10th and 90th percentiles, (number of samples), y axis is log scale, lowest value presented is 25 ng/mL (one-half the measurement detection limit). (B) ALAD activity vs. blood lead levels in free-flying condors in California. Data show that there is a significant decreasing exponential relationship between blood ALAD activity and blood lead level [the equation is ALAD activity (nanomoles porphobilinogen per minute per milliliter blood) = 11.6 × e(−0.006 × blood lead in nanograms per milliliter), R = 0.891, P < 0.001, n = 60].
Even more notable than exposure rates in condors is the prevalence of birds in need of clinical treatment for lead poisoning. We use a blood lead level ≥ 450 ng/mL as a threshold indicative of clinical lead poisoning and need for chelation therapy, consistent with the recommended threshold of the Centers for Disease Control and Prevention for chelation therapy in lead-poisoned children (13). From 1997 to 2010, the median annual California condor lead poisoning prevalence rate, defined as the number of lead-poisoned birds divided by the number of birds sampled that year, was 20% (range = 0–44%) (Fig. S1A). Accordingly, ∼20% of free-flying birds in California each year were in need of treatment for lead poisoning, and cumulatively over the years 1997–2010, 48% of the free-flying condor individuals in California presented with a blood lead level indicating the need for chelation treatment (i.e., 88 birds with a blood lead level ≥ 450 ng/mL of a total of 184 birds released or wild-fledged) (Fig. S1A). Many birds were repeatedly poisoned within and across years.
Although we use a blood lead level of 450 ng/mL to conservatively estimate the prevalence of clinical lead poisoning and associated management effort to treat lead-poisoned birds, some condors with lead levels < 450 ng/mL have been treated with chelation therapy, in part because treatment protocols varied between California release sites over this time. Lead affects multiple organ systems across both avian and mammalian species at blood lead values below 450 ng/mL (14–16), although there are no well-established lead poisoning treatment guidelines for species other than humans. Therefore, we considered additional blood lead thresholds to more broadly reflect health risks as well as the clinical resources needed to manage lead poisoned condors. We found that, between 1997 and 2010, the average annual probability that a condor would have a blood lead equal to or greater than 300, 350, 400, and 500 ng/mL was 31%, 28%, 22%, and 17%, respectively (Fig. S1B). By any measure, the lead poisoning rates in condors are of epidemic proportions and require substantial effort to mitigate.
California condor lead poisoning prevalence rates seem to exceed the rates of co-occurring scavenging raptors.[Even greater contrasts are drawn if condor lead exposure rates are compared with rates in young children, which is the segment of the human population considered at greatest risk for health effects from lead (SI Results and Discussion).] For example, the work by Kelly et al. (17) reported that the highest blood lead level observed in golden eagles (n = 55) and turkey vultures (n = 71) in California between 2007 and 2009 was 1,100 and 440 ng/mL, respectively, with 7% of golden eagle blood samples greater than 500 ng/mL. Across the years 1997–2010, the highest blood lead level recorded for condors in California was 6,100 ng/mL, with 12% of samples collected (i.e., n = 137 of 1154) exceeding 500 ng/mL. The higher risk of lead exposure in condors may be because of their extensive use of large mammal carcasses (18); condors have apparently always relied on large-bodied animals for food (19), including carcasses of both marine and large terrestrial mammals when available (20).
Blood lead monitoring likely underestimates the frequency and magnitude of California condor lead exposure.
Managing the risk of morbidity and mortality from lead poisoning in condors requires information on the magnitude, frequency, and duration of lead poisoning events. Blood lead monitoring reflects only recent exposures, because lead is relatively rapidly cleared from condor blood (estimated elimination half-life ∼ 13 d) (21). Thus, biannual blood lead monitoring may capture only ∼10% of a condor’s annual exposure history, and it is unlikely to capture the peak magnitude of an exposure event. By comparison, analysis of lead in sequential feather segments has proven useful in evaluating a condor’s lead exposure history over a period of several months (22) (Fig. S2A), and thus, it can be used to better determine the magnitude and duration of lead poisoning events.
We have shown previously that the ratio between a condor’s blood lead (nanograms per milliliter) and feather lead (micrograms per gram) concentrations is ∼200:1 (22). Here, we use this relationship to investigate the correlation between a bird’s measured blood lead level and the magnitude of the bird’s estimated peak lead exposure estimated from sequential feather samples associated with that measured blood lead level. We used cases of paired blood–feather analyses (n = 10 pairs) and found that measured blood lead levels underestimate the estimated blood lead level at the peak of exposure by a range of 1.4- to 14.4-fold (geometric mean = 4.3-fold, SE = 1.3) (Table S1 and Fig. S2B).
Lead levels in sequential feather segments also provide information on the duration of time that a bird has an elevated blood lead level. Analyses of condor feathers (n = 18) indicate that sampled birds were lead-exposed (i.e., estimated blood level ≥ 100 ng/mL) for ∼75% of the duration of feather growth and that they were clinically lead-poisoned (blood lead ≥ 450 ng/mL) for ∼34% of the duration of feather growth (Table S1). Assuming a single feather captures 3 mo of exposure history (22), a condor is clinically lead poisoned for 1 mo after an exposure event. Sequential feather analyses corroborate the blood lead data in showing that condors are chronically lead-poisoned, and they also illustrate that the magnitude of lead exposure is likely much higher than indicated by periodic blood monitoring.
Condors are experiencing sublethal impacts of lead exposure.
The health impacts of lead exposure on wildlife are most commonly expressed in terms of mortality, although it is well-known that sublethal lead exposures can impact multiple organ systems in vertebrate species (14–16). However, there have been no previous efforts to document sublethal impacts of lead exposure in condors. To address this, we measured blood δ-aminolevulinic acid dehydratase (ALAD) enzyme activity and blood lead concentrations in condors over 2007–2009 (n = 34 condors, n = 60 samples). Blood ALAD activity is a well-established and sensitive biomarker of sublethal lead toxicity in humans and wildlife, including avian species (23, 24); ALAD is an essential enzyme in the heme biosynthetic pathway, and reduced activity is associated with adverse health effects (25).
We found a significant negative exponential relationship between blood ALAD activity and blood lead level over lead levels from 20–2,000 ng/mL (R = 0.891, P < 0.001), such that ALAD activity was inhibited by ≥60% at blood lead levels ≥ 200 ng/mL (Fig. 2B). Blood monitoring data from free-flying condors show that ∼30% of samples collected in a given year had lead concentrations ≥ 200 ng/mL (Fig. 2A), whereas feather lead levels from lead-exposed condors indicate that ∼50% of their time in the wild is spent with blood levels ≥ 200 ng/mL (Table S1). Furthermore, these data indicate that condors are suffering up to 90% ALAD inhibition at blood lead levels below the level that would warrant chelation treatment (<450 ng/mL). Our results show that condors are experiencing chronic sublethal effects from lead exposure, and compared with published studies (16, 23, 24), they suggest that condors are as sensitive to sublethal lead effects as are other vertebrate species.
Sources of Lead Exposure.
California condors are obligate scavengers, and the principle source of lead exposure to condors is believed to be the ingestion of lead ammunition fragments embedded within carcasses of animals shot with lead ammunition (7). This belief is supported by circumstantial evidence implicating lead ammunition as the primary source of condor lead poisonings (12) and our initial study using stable lead isotopic analyses of condor blood (n = 24 condors) and ammunition (n = 18) (26). However, lead poisoning of condors by ammunition has remained a topic of debate (27, 28).
Here, we used stable isotopic analysis to substantially expand our prior work in identifying the sources of lead to condors (22, 26). Lead isotopic analysis to identify sources and pathways of lead exposure to humans (29–31) and wildlife (32, 33) is well-established. Given that lead isotopic compositions may not be singularly unique to a particular lead source, we considered several factors in applying lead isotope analyses as a tool to evaluate lead exposure sources (26, 29, 34), including (i) knowledge of plausible sources of lead to condors as well as the lead concentrations and lead loadings in those sources; (ii) measured isotopic ratios of plausible lead sources within the condor’s environment; and (iii) information about the behavioral feeding habits of condors and consideration of plausible exposure pathways.
Direct evidence links lead-containing fragments/ammunition with lead-poisoned birds.
Directly linking an observed feeding and/or recovery of ingested ammunition fragment(s) from a lead-poisoned condor is uncommon, largely because condors can fly over 200 km and traverse their entire range in a single day (35), but their feeding episodes can last less than 1 h (36). Since 2007, in part because of increased efforts by condor biologists and veterinary staff, there have been six cases where a lead-containing metal fragment (or in one case, buckshot) was recovered from a lead-poisoned bird or a condor was observed feeding on a carcass that had been shot with lead-based ammunition. In all six of these cases, isotopic analysis showed that the fragments/ammunition and condor blood had highly similar (difference ≤ 0.22%) lead isotope ratios (207Pb/206Pb) (Fig. S3), establishing that the recovered lead-containing fragment (or ammunition from the carcass on which the bird was observed feeding) (22) was the cause of the lead poisoning.
Majority of free-flying condors have a blood lead isotopic composition that is consistent with lead-based ammunition.
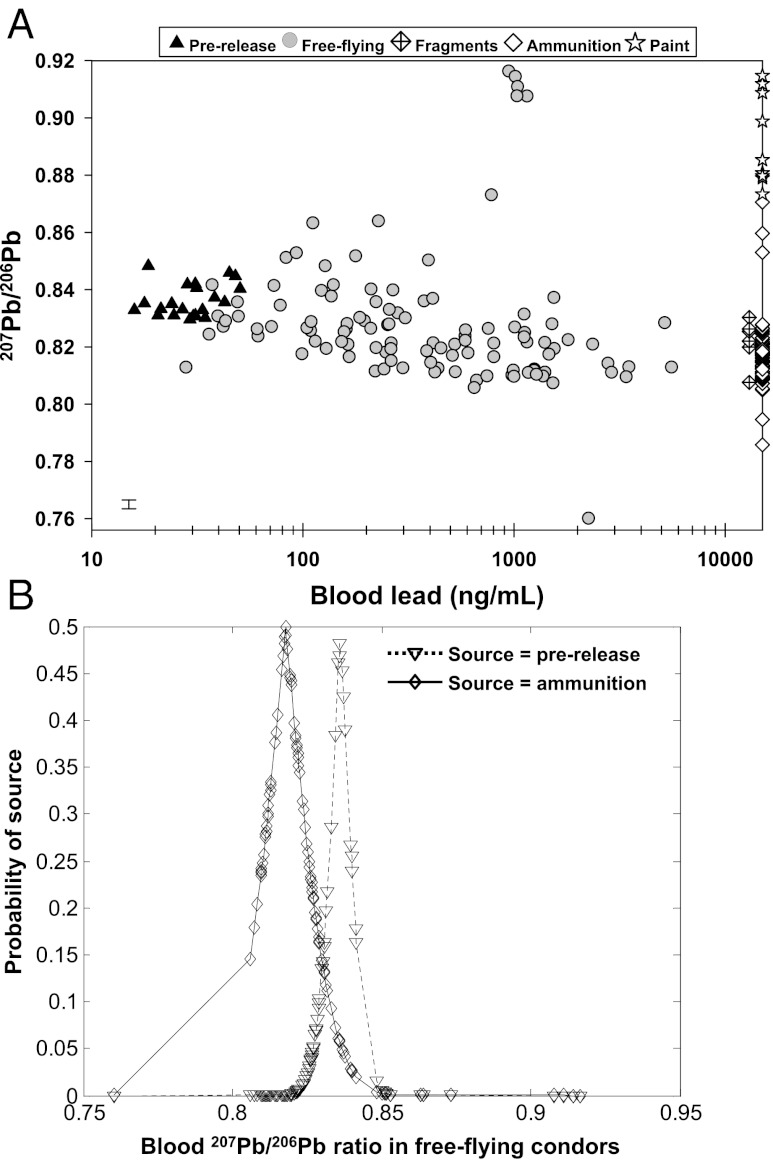
We have expanded previous analyses of condors and lead ammunition (26) by approximately fivefold to more fully examine the relationship between blood lead level and lead isotopic composition in prerelease condors (no history of elevated lead exposure; thus, reflecting background environmental lead), free-flying condors, and lead-based ammunition/lead-containing fragments (Fig. 3A). Consistent with previous findings (26), the 207Pb/206Pb ratios of prerelease condors fall within the range of background environmental lead in California [207Pb/206Pb = 0.8338–0.8453 (summarized in the work by Church et al. in ref. 26) and 207Pb/206Pb = 0.8306–0.8554 in lichens, collected in California between 1992 and 2006 (37)]† and are generally distinct from the 207Pb/206Pb ratios of lead-based ammunition from California. Indeed, only one ammunition sample has a 207Pb/206Pb ratio that falls within the range (but at the lower limit) of 207Pb/206Pb ratios of prerelease condors (Fig. 3A), substantiating that prerelease condors with background lead levels and no history of elevated lead exposure possess a different 207Pb/206Pb signature than the lead-based ammunition sampled from California. In contrast, free-flying condors tend to have 207Pb/206Pb ratios that are consistent with the 207Pb/206Pb ratio measured in ammunition/fragment samples, with an inverse trending relationship between a bird’s blood lead level and their 207Pb/206Pb ratio (SI Results and Discussion and Fig. 3A).
Fig. 3.
Ammunition is the principle source of lead exposure in condors in California. (A) Blood lead concentrations and 207Pb/206Pb ratios of free-flying (n = 110) and prerelease (n = 22) condors collected over 2002–2011 (Table S2A). The majority of free-flying condors have blood 207Pb/206Pb ratios consistent with lead-based ammunition (n = 70) and lead-containing fragments (recovered from lead-poisoned birds; n = 6) (Table S2B), with the exception of five to six birds that have a blood 207Pb/206Pb ratio that matches a subset (n = 3) of lead-based paint samples (n = 9) (Table S2C) collected from a fire lookout tower located in the birds’ habitat; x axis is log scale. Error bar (lower left) represents long-term analytical precision for the 207Pb/206Pb ratio measurements (0.2%, 2 relative SD). (B) Probability (one-tailed test) that the 207Pb/206Pb ratio in a free-flying condor in California is consistent with the 207Pb/206Pb ratios of lead-based ammunition or prerelease condors (thus reflecting background environmental lead). Birds that have a probability > 0.05 for a given lead source have a 207Pb/206Pb signature that is not significantly different from that source (assuming normally distributed values) (SI Materials and Methods).
In a unique case where a few condors were identified with lead poisoning coincident with observed roosting on or in the vicinity of an inactive fire lookout tower with deteriorating lead-based paint, the blood 207Pb/206Pb ratio of the lead-poisoned birds (n = 5) matched the 207Pb/206Pb ratio of lead-based paint collected from the fire lookout tower, strongly implicating the lead-based paint as the source of lead poisoning.‡ Importantly, the 207Pb/206Pb ratio of this lead paint was clearly distinct from lead ammunition and the 207Pb/206Pb ratio of prerelease birds (Fig. 3A).
We determined the one-tailed statistical probability that the 207Pb/206Pb ratio in a given free-flying condor blood sample came from the distribution of 207Pb/206Pb ratios seen in prerelease birds (i.e., background lead) and separately, that it came from the distribution of ratios measured in lead ammunition. The majority (79%) of free-flying condors had blood 207Pb/206Pb ratios that were not significantly different (P > 0.05) than lead-based ammunition, whereas only 27% had 207Pb/206Pb ratios that were consistent with the ratios of prerelease birds (Fig. 3B).
Fourteen condors (∼13% of free-flying birds) had a blood 207Pb/206Pb ratio that could not be explained by the background or lead-based ammunition isotope ratios, with the lead source for five of these birds most likely attributable to lead-based paint (described above). Thus, only nine of the total number of free-flying birds evaluated (n = 110) had a lead isotopic signature that could not be explained by the sources considered here (background, ammunition, or paint), underscoring the use of stable lead isotope tracer methods to assess lead poisoning sources in California condors when applied in conjunction with knowledge of behavioral feeding habits and plausible lead sources within the condor’s environment. Collectively, the case studies of lead-poisoned condors, the broad comparison of the lead isotopic composition of free-flying condors and lead-based ammunition, and the statistical model results substantiate that lead ammunition is the principle source of lead exposure in condors in California.
Lead Poisoning and the Prospects for Condor Recovery in California.
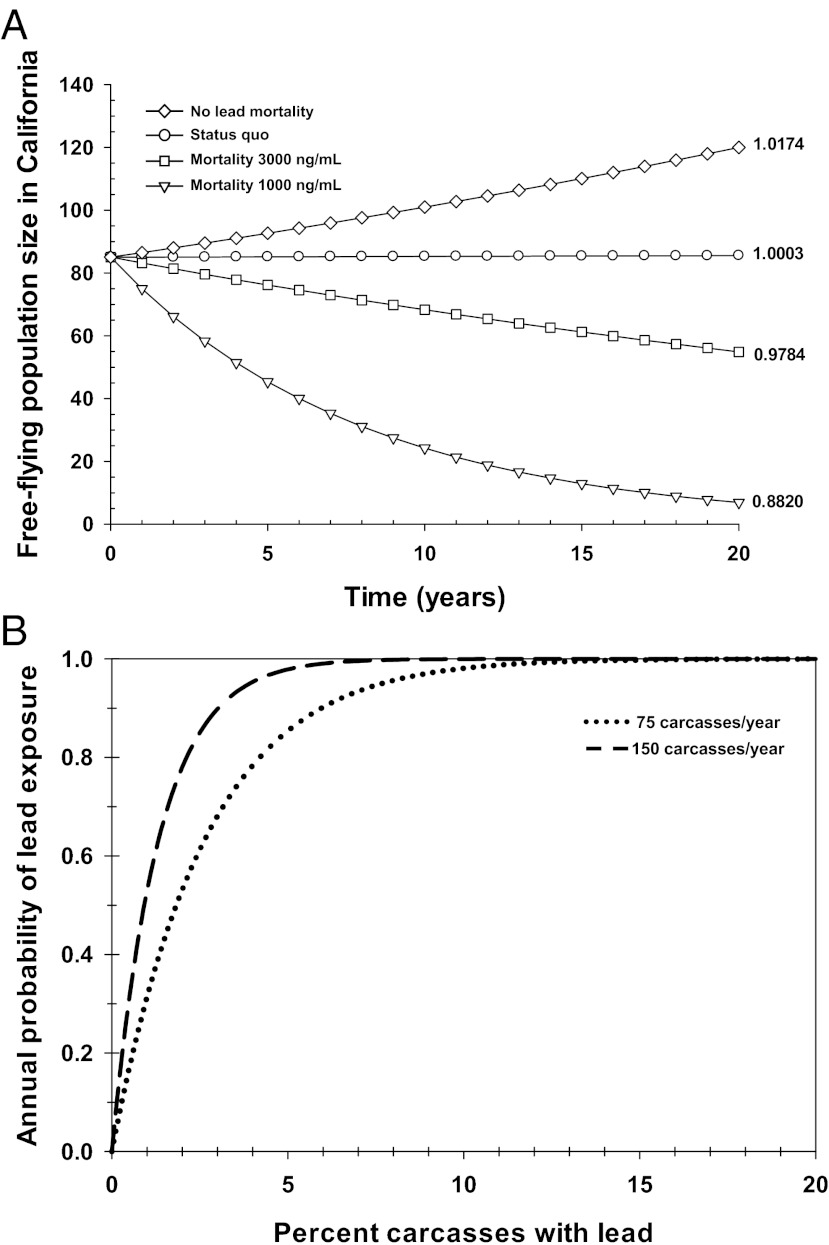
Although continued mortality because of anthropogenic causes occurs in most recovering populations, this added mortality must be low enough that populations can be, at a minimum, stable. The substantial level of management to limit condor mortality from lead exposure as well as the continuing release of condors from captive facilities into free-flying populations complicate any simple assessment of the sustainability of condor populations from census information. Therefore, we used demographic analyses to explore the self-sustainability of the free-flying condor population within California (i.e., without considering future releases of captive-reared birds) under four scenarios: (i) status quo, where current management interventions to mitigate lead poisonings are continued, (ii) cessation of management interventions to mitigate lead poisonings, with the result that birds die when blood lead levels are ≥3,000 ng/mL, (iii) cessation of management interventions to mitigate lead poisonings, with the result that birds die when blood lead levels are ≥1,000 ng/mL, and (iv) no lead-related mortalities, which we estimated from diagnosed mortality rates from lead poisoning published in the work by Rideout et al. (10). We base these population growth estimates on analysis of the survival and reproduction of free-flying condors in California over the past ∼20 y (assessing all birds since releases began) along with assumptions about how survival rates would be altered with changes in management. All estimates are optimistic in assuming no demographic or environmental stochasticity, in not counting permanent removal of birds to captivity as mortality, and in continuation of intensive management of reproductive efforts of free-flying birds.
With current levels of intensive management, the California condor population is predicted to be roughly stable (best estimate of annual growth = 1.0003) (Fig. 4A). Thus, without future releases of captive-reared birds, the population would take ∼1,800 y to meet the recovery goal of a noncaptive population of 150 individuals within California (9). Importantly, this estimate of population stability is dependent on the continuation in perpetuity of the current level of management interventions, including near daily monitoring and targeted trapping and treatment if individual behaviors indicate lead poisoning. In addition, accounting for parameter estimation uncertainty shows that even this stability is unclear, with a 53% probability of growth rates less than one under current conditions (Fig. S4 and Table S3).
Fig. 4.
Lead poisoning is prohibiting California condor population recovery and even low carcass contamination rates will result in high probabilities of lead poisoning. (A) Projected condor free-flying (i.e., wild) population size in California over 20 y under four scenarios of management and without consideration of captive-reared releases: (i) status quo (current interventions to mitigate lead poisoning continued), (ii) cessation of interventions to mitigate lead poisoning with mortality occurring at blood lead ≥ 3,000 ng/mL, (iii) cessation of interventions with mortality occurring at blood lead ≥ 1,000 ng/mL, or (iv) no lead-related mortalities. Projections start with 85 birds at year 0 (free-flying population in California in 2010). Numbers represent annual population growth rate for each population projection. (B) Annual probability that a condor encounters one or more lead-poisoned carcasses, assuming that a condor eats from 75 to 150 carcasses/y [1 − (1 − probability of contamination in a carcass)N, where N is the number fed on in 1 y]. California condors are estimated to feed on between 75 and 150 carcasses per year based on the estimate in the work by Snyder and Snyder (33) that a condor needs to feed every 2–3 d to maintain a healthy weight.
To predict condor population health with a reduction in management actions to prevent lead-related deaths, we reestimated survival rates assuming that mortality would occur for birds that reached or exceeded a blood lead level of either 3,000 or 1,000 ng/mL. These blood lead mortality thresholds most likely underestimate a condor’s true exposure, because lead levels in growing feathers suggest that birds with blood leads of 1,000–3,000 ng/mL will, on average, have suffered peak exposure blood lead levels approximately four times higher (Table S1 and Fig. S2). With the cessation of lead-related interventions, annual population growth rates declined between 2% and 12%—well below the level needed for a stable population (λ = 0.9784 and λ = 0.8820 for the 3,000 and 1,000 ng/mL blood lead thresholds, respectively) (Fig. 4A). Starting from current (2010) numbers, these estimates would result in a wild population of only 22 birds (the number that triggered complete capture of all wild condors in 1982) within 61 or 11 y, respectively. Incorporating parameter uncertainty does not change these conclusions; with the 1,000-ng/mL threshold, there is a 100% probability of decline, and with the 3,000-ng/mL threshold, there is a 90% probability of decline (Fig. S4). Thus, without continued management to prevent lead-related deaths, the wild condor population is predicted to again face the substantial threat of extinction in the coming decades.
We also predicted how condor population dynamics would respond to a lead-free environment. Based on the estimated fraction of observed mortality events caused by lead (i.e., 27%) (10), we increased juvenile and adult survivorship to reflect the absence of lead-related deaths and found that condor population growth increased by ∼2% per year [λ = 1.0174 vs. 1.0003 (Fig. 4A); with only a 15% chance of λ < 1 (Fig. S4)]. Similar models in which we only eliminated the fraction of high-lead exposure events that can be best explained by ammunition yield qualitatively identical results (SI Results and Discussion). It is noteworthy that this estimated rate of lead-caused mortality is based on the actual deaths that occurred despite intensive management interventions to mitigate lead poisonings; if lead was truly removed as an environmental hazard, the increase in condor health and survival should be substantially greater than modeled here.
Conclusion
Our data show that the prevalence of lead poisoning in California condors is of epidemic proportion and that the principle source of lead poisoning is lead-based ammunition. Restricting the use of lead ammunition is a complicated political process and illustrates the challenge of merging political and conservation-oriented goals. For example, if restrictions were in place that resulted in only 1% of carcasses containing lead, the annual probability that a condor would feed on one or more contaminated carcasses would only be reduced to 31–53% (Fig. 4B). When considering the need for long-lived birds to avoid lead poisoning for many years, the necessity for extremely low carcass contamination rates is even clearer: if only 0.5% of carcasses are contaminated with lead, the probability that, over 10 y, a condor will feed on a contaminated carcass is still 85–98%. Thus, very low carcass contamination rates are required to avoid high probabilities of lead poisoning within the condor population.
These results are especially pertinent given recent regulatory efforts in California to mitigate the lead exposure hazard to California condors by partial bans of lead ammunition use in condor habitat (39, 40). Although these regulations have been in place for only a few years, we looked for evidence that they had impacted the prevalence of lead poisoning in California condors. We compared blood lead levels in birds in 2006–2007 (preban) with levels in 2009–2010 (postban) and found no indication that blood lead levels had declined in 2009–2010 compared with 2006–2007 (Fig. 2A and Fig. S5A), suggesting that, at least thus far, the regulations to help reduce lead exposure in condors have not been effective.
Here, we describe a situation in which intensive ongoing management efforts conceal the lack of true recovery of a critically endangered species. Despite the recovery efforts for the California condor, this species is not on a trajectory to a self-sustaining wild population. Our demographic model clearly illustrates that, without reduced lead poisoning, the California condor will require extraordinary management efforts in perpetuity to avoid again declining to extinction in the wild. Additionally, our analyses show that, if the lead exposure hazard is removed and thus lead deaths are halted or severely reduced, California condors could once again achieve a sustainable wild population. Although we present work only on condors in California, the condor populations in Arizona and Baja California are also experiencing impacts from lead poisonings (7). Moreover, lead exposure is a pervasive problem for multiple species in a diversity of ecosystems (41–43), and we are only slowly coming to understand how many species are impacted by exposure because of ammunition in carcasses. Our work highlights the extent to which scavenging species are lead-exposed and also emphasizes that small reductions in exposure are unlikely to sufficiently protect the most vulnerable species.
Materials and Methods
Sample Collection.
California condor blood and feather samples were collected during standard monitoring events between the years 1997 and 2010 at the six California, United States release sites (Bitter Creek National Wildlife Refuge, Hopper Mountain National Wildlife Refuge, Lion Canyon, and Castle Crags in southern California and Pinnacles National Monument and Ventana Wildlife Society’s Big Sur Condor Sanctuary in central California). Ammunition samples were obtained through several means, including an ammunition exchange program in central California, donations from hunters, recovery from shot carcasses, or previously published information (26), whereas lead shot/fragments were recovered from lead-poisoned condors (Table S2B). Paint was collected from an inactive fire lookout tower and associated structures (SI Materials and Methods and Table S2C).
Sample Processing and Analysis.
To determine lead concentrations and isotopic compositions, whole-blood, feather, ammunition/fragment, and paint samples were processed and analyzed using established trace metal clean techniques (22, 31, 44, 45). ALAD activity in condor whole-blood samples was measured using a colorimetric assay based on previously described methods (46, 47) (SI Materials and Methods).
Blood Data Analysis.
Blood monitoring results collected between 1997 and 2010 (n = 1,154) were used for analysis; results from 1992 to 1996 were excluded because of limited samples sizes (<10 samples/y) (SI Materials and Methods, Fig. S5B, and Table S4). Blood lead samples below the commercial laboratory’s detection limit (50 ng/mL) were assigned a value of 25 ng/mL (i.e., one-half the detection limit) for data analyses. Independent (in terms of a lead exposure event) blood lead samples were used for analysis. In cases where multiple samples were collected over time from an individual bird, results from samples separated in time by >2 mo were used (i.e., approximately five half-lives of lead in condor blood) (21), unless the second sample was >100 ng/mL higher than the first sample, indicating that a lead exposure event occurred between collections. Samples taken while birds were under clinical care were excluded.
Isotopic Fitting Models.
We used the 207Pb/206Pb ratios for a total of 22, 110, and 76 samples from prerelease condors, free-flying condors, and ammunition/fragments, respectively, to determine the one-tailed statistical probability that the 207Pb/206Pb ratio in a given free-flying condor blood sample came from the distribution of 207Pb/206Pb ratios seen in prerelease birds, and separately, that the ratio came from the distribution of ratios measured in lead ammunition/fragments (SI Materials and Methods and Fig. S6).
Demographic Model.
To the greatest extent possible, we projected future population trends based on empirical data taken from 1994 to 2010 on 182 free-flying condors released in California, which together, included a total of 703 condor-years of information (SI Materials and Methods has details and descriptions of parameter uncertainty analyses).
Supplementary Material
Acknowledgments
We thank V. Bakker, M. Clark, C. Eng, D. Finkelstein, S. Flannagan, R. Franks, G. Grisdale, C. Johnson, T. Kelly, D. Lang, B. Massey, D. Moen, M. Nydes, J. Petterson, P. Raimondi, B. Rideout, R. Risebrough, S. Scherbinski, B. Sullivan, J. Theyerl, M. Tyner, A. Welch, and J. Wynne for their important contributions to this study. We also thank the staff of the Wildlife Disease Laboratories at the San Diego Zoo, the veterinary facility at the Los Angeles Zoo, the field crews from the Hopper Mountain National Wildlife Refuge Complex, Pinnacles National Monument, and the Ventana Wildlife Society. This work was supported by the National Park Service, the Western National Park Association, and the US Fish and Wildlife Service.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
†The lead isotopic composition of background environmental lead in California reflects multiple sources but is dominated by the persistent, albeit declining, contamination from leaded gas use, which was largely phased out in the late 1970s (37).
‡As of December 2011, lead-based paint on this inactive fire lookout tower has been remediated; tracking data confirm that condor association with fire lookout towers in central California is a rare occurrence (38).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203141109/-/DCSupplemental.
References
- 1.Scott JM, Goble DD, Haines AM, Wiens JA, Neel MC. Conservation-reliant species and the future of conservation. Conserv Lett. 2010;3:91–97. [Google Scholar]
- 2.US Fish and Wildlife Service . Report to Congress, Endangered and Threatened Species Recovery Program. Washington, DC: Government Printing Office; 1996. [Google Scholar]
- 3.Department of Justice Canada . Species at Risk Act. Ottawa, Canada: Minister of Justice; 2002. Available at http://laws-lois.justice.gc.ca/PDF/S-15.3.pdf. [Google Scholar]
- 4.Scott JM, et al. Recovery of imperiled species under the Endangered Species Act: The need for a new approach. Front Ecol Environ. 2005;3:383–389. [Google Scholar]
- 5.Snyder NFR, Snyder HA. The California Condor: A Saga of Natural History and Conservation. London: Academic; 2000. [Google Scholar]
- 6.Wilcove DS, May RM. Endangered species—the fate of the California condor. Nature. 1986;319:16. [Google Scholar]
- 7.Walters JR, et al. Status of the California condor (Gymnogyps Californianus) and efforts to achieve its recovery. Auk. 2010;127:969–1001. [Google Scholar]
- 8.Grantham J. In: Reintroduction of California Condors into Their Historic Range: The Recovery Program in California. California Condors in the 21st Century Series in Ornithology. Mee A, Hall LS, editors. Vol 2. Washington, DC: American Ornithologists Union, Nuttall Ornithological Club; 2007. pp. 123–138. [Google Scholar]
- 9.Kiff LF, Mesta RI, Wallace MP. Recovery Plan for the California Condor. Portland, OR: US Fish and Wildlife Service; 1996. [Google Scholar]
- 10.Rideout BA, et al. Patterns of mortality in free-ranging California Condors (Gymnogyps californianus) J Wildl Dis. 2012;48:95–112. doi: 10.7589/0090-3558-48.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Wiemeyer SN, Krynitsky AJ, Wilbur SR. Environmental contaminants in tissues, foods, and feces of California condors. In: Wilbur SR, Jackson JA, editors. Vulture Biology and Management. Berkeley, CA: Univ California Press; 1983. pp. 427–439. [Google Scholar]
- 12.Cade TJ. Exposure of California condors to lead from spent ammunition. J Wildl Manage. 2007;71:2125–2133. [Google Scholar]
- 13.Centers for Disease Control and Prevention . Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Bethesda: US Department of Health and Human Services, Public Health Service; 2002. [Google Scholar]
- 14.Bellinger DC. The protean toxicities of lead: New chapters in a familiar story. Int J Environ Res Public Health. 2011;8:2593–2628. doi: 10.3390/ijerph8072593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey PM, Burger J, Gochfeld M, Reuhl KR. Developmental lead exposure disturbs expression of synaptic neural cell adhesion molecules in herring gull brains. Toxicology. 2000;146:137–147. doi: 10.1016/s0300-483x(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 16.Telisman S, et al. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly TR, et al. Impact of the California lead ammunition ban on reducing lead exposure in golden eagles and turkey vultures. PLoS One. 2011;6:e17656. doi: 10.1371/journal.pone.0017656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins PW, Snyder NFR, Emslie SD. Faunal remains in California Condor nest caves. Condor. 2000;102:222–227. [Google Scholar]
- 19.Emslie SD. Age and diet of fossil california condors in grand canyon, Arizona. Science. 1987;237:768–770. doi: 10.1126/science.237.4816.768. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain CP, et al. Pleistocene to recent dietary shifts in California condors. Proc Natl Acad Sci USA. 2005;102:16707–16711. doi: 10.1073/pnas.0508529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry DM, Maurer J. Assessment of Lead Contamination Sources Exposing California Condors. Sacramento, CA: California Deptartment of Fish and Game; 2003. [Google Scholar]
- 22.Finkelstein ME, et al. Feather lead concentrations and (207)Pb/(206)Pb ratios reveal lead exposure history of California Condors (Gymnogyps californianus) Environ Sci Technol. 2010;44:2639–2647. doi: 10.1021/es903176w. [DOI] [PubMed] [Google Scholar]
- 23.McBride TJ, Smith JP, Gross HP, Hooper MJ. Blood-lead and ALAD activity levels of Cooper's Hawks (Accipiter cooperii) migrating through the southern Rocky Mountains. J Raptor Res. 2004;38:118–124. [Google Scholar]
- 24.Work TM, Smith MR. Lead exposure in Laysan albatross adults and chicks in Hawaii: Prevalence, risk factors, and biochemical effects. Arch Environ Contam Toxicol. 1996;31:115–119. doi: 10.1007/BF00203915. [DOI] [PubMed] [Google Scholar]
- 25.Felitsyn N, McLeod C, Shroads AL, Stacpoole PW, Notterpek L. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem. 2008;106:2068–2079. doi: 10.1111/j.1471-4159.2008.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Church ME, et al. Ammunition is the principal source of lead accumulated by California condors re-introduced to the wild. Environ Sci Technol. 2006;40:6143–6150. doi: 10.1021/es060765s. [DOI] [PubMed] [Google Scholar]
- 27.Platt JR. Scientific American. New York: Nature America, Inc.; 2009. Fight to protect California condors from lead ammunition moves to Arizona. [Google Scholar]
- 28.Di Paola M. California Condors Survive Lead-Poisoned Gut Piles: Commentary. New York: Bloomberg News; 2010. [Google Scholar]
- 29.Gwiazda RH, Smith DR. Lead isotopes as a supplementary tool in the routine evaluation of household lead hazards. Environ Health Perspect. 2000;108:1091–1097. doi: 10.1289/ehp.001081091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturges WT, Barrie LA. Lead-206-207 isotope ratios in the atmosphere of North America as tracers of USA and Canadian emissions. Nature. 1987;329:144–146. [Google Scholar]
- 31.Smith DR, Osterloh JD, Flegal AR. Use of endogenous, stable lead isotopes to determine release of lead from the skeleton. Environ Health Perspect. 1996;104:60–66. doi: 10.1289/ehp.9610460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelstein ME, Gwiazda RH, Smith DR. Lead poisoning of seabirds: Environmental risks from leaded paint at a decommissioned military base. Environ Sci Technol. 2003;37:3256–3260. doi: 10.1021/es026272e. [DOI] [PubMed] [Google Scholar]
- 33.Outridge PM, Evans RD, Wagemann R, Stewart REA. Historical trends of heavy metals and stable lead isotopes in beluga (Delphinapterus leucas) and walrus (Odobenus rosmarus rosmarus) in the Canadian Arctic. Sci Total Environ. 1997;203:209–219. [Google Scholar]
- 34.Church ME, et al. Ammunition is the principal source of lead accumulated by California condors re-introduced to the wild—reply. Environ Sci Technol. 2008;42:1809–1811. doi: 10.1021/es0715795. [DOI] [PubMed] [Google Scholar]
- 35.Meretsky VJ, Snyder NFR. Range use and movements of California condors. Condor. 1992;94:313–335. [Google Scholar]
- 36.Snyder NFR, Snyder HA. Introduction to the Californnia Condor. Berkeley, CA: Univ California Press; 2005. [Google Scholar]
- 37.Flegal AR, Gallon C, Hibdon S, Kuspa ZE, Laporte LF. Declining-but persistent-atmospheric contamination in central California from the resuspension of historic leaded gasoline emissions as recorded in the lace lichen (Ramalina menziesii Taylor) from 1892 to 2006. Environ Sci Technol. 2010;44:5613–5618. doi: 10.1021/es100246e. [DOI] [PubMed] [Google Scholar]
- 38.George D, et al. Pinnacles National Monument California Condor Reestablishment, Annual Report 2012. Paicines, CA: Pinnacles Natl Monument, Natl Park Serv; 2012. [Google Scholar]
- 39.Ridley-Tree Condor Preservation Act . In Assembly Bill No. 821. Sacramento, CA: California State Assembly; 2008. Available at http://www.leginfo.ca.gov/bilinfo.html. [Google Scholar]
- 40.California Department of Fish and Game 2008. Methods Authorized for Taking Big Game, Section 353, Title 14, CCR (Office of Administrative Law, Sacramento, CA). Available at http://www.oal.ca.gov/Publications.htm.
- 41.Rogers TA, Bedrosian B, Graham J, Foresman KR. Lead exposure in large carnivores in the greater Yellowstone ecosystem. J Wildl Manage. 2011;9999:1–8. [Google Scholar]
- 42.Fisher IJ, Pain DJ, Thomas VG. A review of lead poisoning from ammunition sources in terrestrial birds. Biol Conserv. 2006;131:421–432. [Google Scholar]
- 43.Kenntner N, Crettenand Y, Funfstuck HJ, Janovsky M, Tataruch F. Lead poisoning and heavy metal exposure of golden eagles (Aquila chrysaetos) from the European Alps. J Ornithol. 2007;148:173–177. [Google Scholar]
- 44.Gwiazda R, Campbell C, Smith D. A noninvasive isotopic approach to estimate the bone lead contribution to blood in children: Implications for assessing the efficacy of lead abatement. Environ Health Perspect. 2005;113:104–110. doi: 10.1289/ehp.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gwiazda R, Woolard D, Smith D. Improved lead isotope ratio measurements in environmental and biological samples with a double focusing magnetic sector inductively coupled plasma mass spectrometer (ICP-MS) J Anal At Spectrom. 1998;13:1233–1238. [Google Scholar]
- 46.Fujita H. Measurement of δ-aminolevulinate dehydratase activity. In: Maines M, Costa LG, Hodgson E, Reed DJ, Sipes IG, editors. Current Protocols in Toxicology. New York: John, Wiley and Sons; 1999. pp. 8.6.1–8.6.11. [Google Scholar]
- 47.Scheuhammer AM. Erythrocyte delta-aminolevulinic acid dehydratase in birds. I. The effects of lead and other metals in vitro. Toxicology. 1987;45:155–163. doi: 10.1016/0300-483x(87)90101-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.