Abstract
The nerve damage occurring as a consequence of glucose toxicity in diabetes leads to neuropathic pain, among other problems. This pain dramatically reduces the quality of life in afflicted patients. The progressive damage to the peripheral nervous system is irreversible although strict control of hyperglycemia may prevent further damage. Current treatments include tricyclic antidepressants, anticonvulsants, and opioids, depending on the severity of the pain state. However, available therapeutics have drawbacks, arguing for the need to better understand the pathophysiology of neuropathic pain and develop novel treatments. Here we demonstrate that stabilization of a class of bioactive lipids, epoxygenated fatty acids (EpFAs), greatly reduces allodynia in rats caused by streptozocin-induced type I diabetes. Inhibitors of the soluble epoxide hydrolase (sEHI) elevated and stabilized the levels of plasma and spinal EpFAs, respectively, and generated dose-dependent antiallodynic effects more potently and efficaciously than gabapentin. In acute experiments, positive modulation of EpFAs did not display differences in insulin sensitivity, glucose tolerance, or insulin secretion, indicating the efficacy of sEHIs are not related to the glycemic status. Quantitative metabolomic analysis of a panel of 26 bioactive lipids demonstrated that sEHI-mediated antiallodynic effects coincided with a selective elevation of the levels of EpFAs in the plasma, and a decrease in degradation products coincided with the dihydroxy fatty acids in the spinal cord. Overall, these results argue that further efforts in understanding the spectrum of effects of EpFAs will yield novel opportunities in treating neuropathic pain.
Keywords: analgesic drug, soluble epoxide hydrolase inhibitor, cytochrome P450, glucose homeostasis, motor depressant
Recent Center for Disease Control data indicates that 23.6 million Americans of all ages have diabetes (http://www.cdc.gov/Features/dsDiabetes/). Diabetic neuropathy will manifest in up to half of the diabetic population as the most frequently encountered secondary condition of diabetes. Estimated care costs exceed $150 billion annually (1, 2). The nerve dysfunction as a consequence of diabetes is debilitating and can lead to life-threatening autonomic neuropathy, cardiac diseases, and CNS atrophy (3, 4). Dying back of distal peripheral nerve fibers because of glucose toxicity and the ensuing spinal sensitization are thought to be the underlying causes of pain (5–9). Although hyperglycemia is the initiating cause for diabetic neuropathy, aggressive glycemic control is effective in impeding the progression of neuronal loss and pain, but not in reversing neuropathy (10). Treatments include tricyclic antidepressants, anticonvulsants, serotonin-norepinephrin reuptake inhibitors, opiates, and recently lidocaine and capsaicin have found wider acceptance as add-on treatments (11, 12). A survey of patients with peripheral neuropathic disorders including diabetic neuropathy in the United Kingdom indicated that a significant number of them were surprisingly taking nonsteroidal anti-inflammatory drugs (NSAIDs) and selective cox inhibitors, despite the lack of apparent benefit from these drugs for neuropathic indications (13). Indeed, animal models of diabetic neuropathy are insensitive to NSAIDs (14). Because of the limited efficacy and side effects of current therapeutics, new agents with novel mechanisms of action are needed to provide more effective pain management. To this end, we investigated the feasibility of a unique approach, stabilization of epoxygenated fatty acids (EpFAs) by inhibiting soluble epoxide hydrolase (sEH, E.C. 3.3.2.10) in a streptozocin (STZ)-induced type I diabetes model of neuropathic pain.
Stabilization of natural EpFAs by inhibition of sEH is antiallodynic and antihyperalgesic in models of inflammatory pain without affecting motor function (15–17). EpFAs are produced from parent linoleic (LA), arachidonic (AA), eicosapentaenoic (EPA), and docosahexaenoic (DHA) acids by multiple isozymes of the cytochrome P450 enzymes (18). These molecules not only have selective and direct antinociceptive effects but also modulate the AA cascade, a major biological pathway (16, 19, 20). The EpFAs participate in physiological events, such as setting of the vascular tone, and they seem to have more important functions in pathophysiological processes (21–23). Stabilization of the EpFAs using sEH inhibitors (sEHI) results in beneficial effects in a number of disease models, demonstrating antihyperalgesic, anti-inflammatory, and other effects (15, 21, 24). EpFAs transcriptionally down-regulate cyclooxygenase-2 in addition to redirecting the flow of AA among the three branches of the cascade (16, 19, 25, 26). Given that glucose itself can influence nociceptive thresholds, we asked if the antinociceptive effects of inhibiting sEH are independent from affecting the glycemic status of rats (27). The observed biological activity is surprising in this model of pain, which is insensitive to NSAIDs. The EpFAs and sEHI have a significant control over the AA cascade and modulate nociceptive behavior in response to inflammatory algogens, such as LPS and the prostanoid PGE2. Here we demonstrate that EpFAs and sEHI reduce neuropathic pain without decreasing peripheral or central prostanoid levels. Our findings highlight an unexpected property of EpFAs and sEH in regulating nociceptive behavior outside of their roles in the inflammatory processes.
Results
Positive Modulation of EpFAs Is Effective in Blocking Type I Diabetes-Induced Neuropathic Pain-Related Behavior.
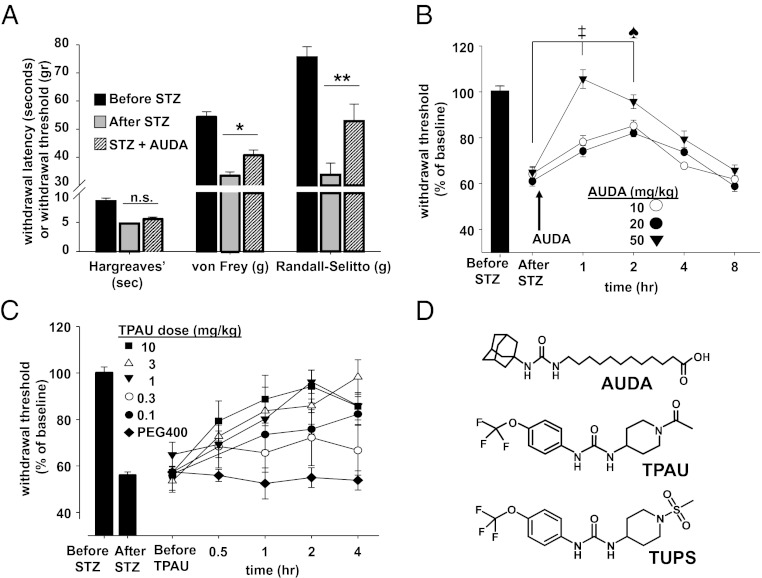
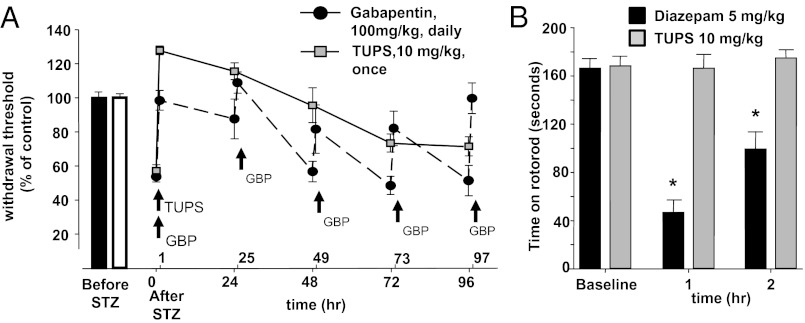
STZ is a commonly used former antibiotic selectively toxic to the insulin-producing β-cells of the pancreas. Consistent with previous observations, STZ treatment of rats led to a dramatic rise in blood glucose levels and decrease in pain thresholds that were 40–50% lower than that before administration of STZ (28). The efficacy of sEH inhibitors was then tested using three different sensory measures: heat hyperalgesia, mechanical hyperalgesia, and mechanical allodynia (Fig. 1A). Thermal escape latency was not affected by the sEHI 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) (IC50 = 11 nM on recombinant rat sEH), although hyperalgesia and allodynia were largely reduced in a dose- and time-dependent manner (Fig. 1 A and B). Although AUDA is a very potent sEH inhibitor, more modern sEH inhibitors have higher oral bioavailablity and have been altered to reduce plasma protein binding, increase water solubility, decrease logP, and circumvent the rapid inactivation of AUDA by β-oxidation and P450 hydroxylation on the adamantine. To test the hypothesis that inhibition of the sEH, rather than a compound-dependent effect, is responsible for the observed antinociception, we determined the efficacy of two other structurally different sEH inhibitors with longer plasma half-life. For this purpose we focused on the electronic von Frey assay as a sensitive measure of allodynia. The sEHI, 1-trifluoromethoxyphenyl-3-(1-acetylpiperidin-4-yl) urea (TPAU) (IC50 = 79 nM on recombinant rat sEH), led to a time- and dose-dependent decrease in pain-related behavior, largely restoring nociceptive threshold in the high-dose groups (3 and 10 mg/kg) (Fig. 1C). The third structurally different sEHI, 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS) (IC50 = 9 nM on recombinant rat sEH), was also highly effective in reducing allodynia but, unlike TPAU, elevated the mechanical withdrawal threshold (MWT) of rats above their pre-STZ baseline values (Fig. 2A). Thus, TUPS was compared with gabapentin, a standard therapeutic that is commonly prescribed to patients suffering from intense neuropathic pain. TUPS (10 mg/kg) was more efficacious than a 10-fold higher-dose gabapentin, but displayed comparable efficacy to morphine (3 mg/kg) (Fig. S1). This high efficacy and longer duration of activity of TUPS supports its utility as a probe to investigate EpFAs (29). To test the hypothesis that sustained inhibition of sEH will attenuate allodynia in a prolonged manner, we monitored the MWT of animals for over 4 d following a single dose of TUPS. Compared with TUPS, gabapentin (100 mg⋅kg⋅d), was administered daily for 4 consecutive days to maintain a pain-free status. Notably, TUPS at 10-fold lower dose was equally efficacious to daily repeated injections of gabapentin (Fig. 2A). Over the course of this experiment, the MWT of TUPS-treated rats was above the pre-STZ baseline level for at least 48 h, after which the effect gradually diminished. However, even after 96 h these rats had significantly less pain-related withdrawal response compared with their pre-TUPS baseline (Fig. 2A) (diabetic rat before sEHI vs. 96 h after sEHI treatment, P = 0.04, paired t test). Unlike diazepam, TUPS did not affect sensorimotor performance of rats on a revolving rod with steady speed (Fig. 2B).
Fig. 1.
Stabilization of EpFAs is antinociceptive in the type I diabetes-induced neuropathic pain model. (A) The sEHI, AUDA (n = 6 per group, 10 mg/kg s.c.), reduced mechanical allodynia and mechanical hyperalgesia but not the transient heat hyperalgesia elicited by STZ-induced type I diabetes (paired t test *P = 0.04, **P = 0.002). Heat hyperalgesia quantified by Hargreaves’ assay, allodynia by the von Frey assay, and mechanical hyperalgesia by the Randall–Selitto assay. Data are reported as grams of force or time in seconds required to elicit a withdrawal. In all subsequent panels and figures, data are reported as “percent of baseline” threshold. (B) Dose and time dependency of effect of AUDA (n = 6 per group, one-way ANOVA followed by Student Newman–Keuls test: ‡, P = 0.001, ♠, P = 0.03 vs. after STZ baseline, subcutaneous administration). (C) Dose- and time-dependent efficacy of a structurally different sEHI, TPAU compared with vehicle control (PEG400, subcutaneous administration). (D) Chemical structures of different classes of small molecule inhibitors of sEH used in this study. Although these molecules share the same urea pharmacophore, each probe has different chemical properties and in vitro potency values (IC50) on recombinant rat sEH; 11, 79, and 9 nM for AUDA, TPAU, and TUPS, respectively. Potencies on rat sEH are determined using the fluorescent substrate, CMNPC, α-cyanocarbonate. All data in this and following figures are presented as mean ± SE of mean.
Fig. 2.
Positive modulation of EpFAs is highly efficacious against diabetic neuropathic pain without sensorimotor impairment. (A) Mean antiallodynic effect of TUPS on diabetic rats over the course of 4 d. TUPS (10 mg/kg i.p.) at a 10-fold lower dose is comparable to Gabapentin (100 mg/kg i.p.), which requires repeated administration to maintain pain relief (n = 6 per group, one-way ANOVA followed by Student Newman–Keuls test, drugs vs. vehicle P < 0.001 for all pair-wise comparisons). TUPS remained efficacious across all time points. Error bars in some groups are smaller than the point shown. (B) Inhibition of sEH does not affect the time rats maintain position on a constant-speed rotating rod (TUPS, 10 mg/kg i.p., n = 5, paired t test vs. baseline, P = 0.76). Diazepam was used as a positive control (5 mg/kg i.p., n = 6, paired t test vs. baseline, *P < 0.05). Compounds were administered 60 min before testing and a cutoff time of 180 s was imposed.
Upon administration, TUPS concentration in blood rose rapidly and for 96 h remained well above the in vitro IC50 value of 9 nM for TUPS, determined using recombinant rat sEH enzyme (Fig. S2). Blood inhibitor levels correlated well with bioactivity (Spearman rank order correlation P = 0.002). Overall, these observations support the hypothesis that inhibition of sEH is effective in reducing diabetes-induced neuropathic pain without affecting gross motor skills.
Acute Inhibition of sEH Does Not Influence Glycemic Status of Type I Diabetic Rats.
Hyperglycemia directly affects nociceptive thresholds on rat peripheral nerves and dorsal root ganglia (27). Similarly, in both type II diabetes patients or in healthy human volunteers, intravenous infusion of glucose significantly decreases pain thresholds (30). Given that sEH is implicated in β-islet function, we asked if inhibition of sEH changed pain-related behavior by modulating the glycemic status of rats (31, 32). To investigate the acute effect of inhibiting sEH, glucose metabolism was examined at the same time course as the nociceptive assays. TPAU (3 mg/kg s.c.) was administered 30 min before the metabolic tests in all groups. In the insulin tolerance test, no significant differences between STZ+PEG400 and STZ+TPAU groups were detected (Fig. S3A). In the glucose tolerance test, both STZ+PEG400 and STZ+TPAU groups (all fasted) showed comparable ability to clear glucose from the peripheral circulation, suggesting that acute sEH inhibition did not alter glucose tolerance (Fig. S3B). There were no differences between animals treated with either vehicle or sEHI in insulin secretion following glucose administration (Fig. S3C). Rats that were not diabetic but treated with either vehicle or sEHI did not demonstrate any significant differences in glucose levels in response to insulin or following glucose administration (Fig. S3). These findings support the hypothesis that the observed antinociception was not related to modulation of the glycemic status of the rats by inhibition of sEH. Rather, these pain-reducing effects are acute and seem to be a direct effect on the sensory nociceptive system.
Inhibition of sEH Positively Modulates the Ratio of Epoxy to Dihydroxy Fatty Acids in the Blood and Spinal Cord of Type I Diabetic Rats.
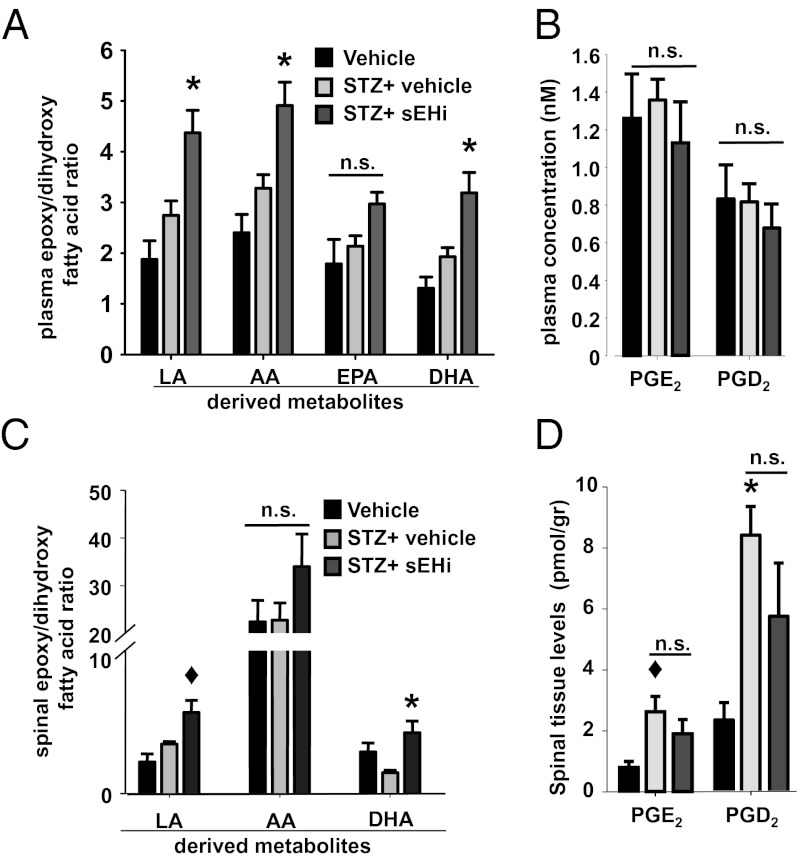
To investigate if treatment with sEHI modulated the levels of bioactive lipids, as would be expected from the mechanism of action of sEH inhibition, the levels of 88 lipid metabolites were analyzed by LC-MS/MS and 26 were found above their limit of quantification (Tables S1 and S2). Three groups of rats were analyzed (nondiabetic, type I diabetic, and type I diabetic+sEHI). The same volume of vehicle was administered to the two groups that did not receive sEHI. The metabolites reported here are organized into four lipid classes based on the parent fatty acids; LA, AA, EPA, and DHA. Type I diabetic status did not lead to significant changes in the plasma levels of bioactive lipid metabolites compared with nondiabetic rats (expressed as the ratio of epoxy to dihydroxy fatty acids, epoxide/diol ratio) (Fig. 3A and Table S1). In the liver, the major suspected source of plasma EpFAs, total cytochrome P450 activity was unchanged in diabetic rats compared with controls, although sEH activity was induced threefold by diabetes. Despite higher activity of sEH in the diabetic rats, the plasma dihydroxy fatty acids were not different from nondiabetic animals (Fig. 3). Inhibition of sEH led to significant increases in the plasma epoxide/diol ratio of most parent fatty acid classes compared with diabetic and healthy control rats, consistent with earlier findings (Table S1). In contrast to a LPS-elicited acute inflammatory model, in the current neuropathy model, plasma levels of two major prostanoids, PGE2 and PGD2, were not significantly elevated by diabetes and inhibition of sEH did not significantly decrease their levels, demonstrating a distinct mechanism of action (Fig. 3B).
Fig. 3.
Inhibition of sEH modulates levels of EpFAs and dihydroxy fatty acids in plasma and spinal cord tissue. (A) Type I diabetes does not change the plasma levels of substrates and products of sEH (pre-STZ n = 6, post-STZ n = 18 and post-STZ+TPAU n = 6, one-way ANOVA, P = 0.1 for LA and AA metabolites, P = 0.07 for EPA metabolites and P = 0.09 for DHA metabolites). TPAU significantly alters the mean ratio of summed epoxy to dihydroxy fatty acids in diabetic rats, with the exception of EPA metabolites (Kruskal–Wallis one-way ANOVA followed by Dunn’s all pair-wise comparison, *P < 0.02). Table S1 displays the identity and detected quantity of the metabolites analyzed from plasma. (B) Plasma levels of two major prostanoids show no significant change between groups (one-way ANOVA, P = 0.62). (C) Inhibition of sEH leads to selective changes in the levels of spinal EpFAs and their degradation products, dihydroxy fatty acids expressed as ratios (pre-STZ n = 3, post-STZ n = 4, and post-STZ+TUPS n = 6, one-way ANOVA followed by Student Newman–Keuls test; ◆, P = 0.03 for post-STZ vs. post-STZ+TUPS for LA metabolites and *, P = 0.02 for DHA metabolites). (D) Spinal prostanoids are elevated by diabetes but not reduced by inhibition of sEH (one-way ANOVA followed by Student Newman–Keuls test, ◆, P = 0.03 for vehicle vs. post-STZ PGE2 and *, P = 0.006 for vehicle vs. post-STZ PGD2).
In the spinal cord tissue, a drastically different profile of bioactive lipids was observed (Fig. 3C and Fig. S5). In diabetic rats, key proinflammatory cyclooxygenase products were elevated (Fig. 3D). Only the levels of LA metabolites, particularly leukotoxin, increased with diabetes; AA and DHA metabolite EpFAs remained constant (Fig. S5). This finding highlights an unexpected asymmetry in bioactive lipid metabolism, given that not all AA metabolites were similarly elevated. EPA-derived metabolites were below the limit of detection. The levels of sEH degradation product dihydroxy fatty acids from LA, AA, and DHA were largely elevated by diabetes, leading to a significant shift in the spinal epoxide/diol fatty acid ratio in favor of dihydroxy fatty acids (see Fig. S5 for concentrations). This increase was not dependent on changes in sEH activity (Fig. S4). Whereas inhibition of sEH ameliorated these changes by normalizing the levels of dihydroxy fatty acids, sEH inhibition was without effect on the spinal levels of prostanoids (Fig. 3D). The broad changes in lipid mediators demonstrate an imbalance in spinal bioactive lipids with diabetes. This imbalance may contribute to allodynia in these animals. Overall, these findings argue in favor of the hypothesis that antinociceptive mechanisms of sEH inhibition are dichotomous and context-dependent for inflammatory versus neuropathic pain.
Discussion
The cytochrome P450-mediated oxygenation of fatty acids leads to the production of EpFAs, a class of bioactive lipids that have exceedingly short half-lives (33). Levels of these molecules are regulated by synthesis, degradation, and membrane incorporation (17, 34, 35). However, preventing their hydrolysis by inhibiting sEH offers advantages because this greatly enhances the in vivo epoxide/diol ratio (17, 24). A number of biological activities have been attributed to EpFAs; their effects on the functioning of the nervous system are newly described (22, 23, 34). Initial reports of the antinociceptive effects of inhibiting sEH preceded knowledge about the expression and distribution of cytochrome P450 enzymes and the sEH in the central and peripheral nervous systems, particularly in neurons (36, 37). Clearly, these components are present in both the CNS and the peripheral nervous system. Even though the EpFAs do not fulfill the criteria of being neurotransmitters, nor are they known ligands for major ion channels, they strongly regulate nociceptive signaling peripherally, in the spinal cord, and possibly in the brain (20, 22).
In this study we characterized the acute effect of inhibiting sEH on type I diabetes-induced mechanical allodynia, which was largely effective in eliminating pain behavior. The efficacy of the sEH inhibitor AUDA on two measures of pain-related behavior is consistent with earlier observations where AUDA reduced inflammation-elicited pain behavior (15). We pursued this observation using two potent and metabolically stable inhibitors, TPAU and TUPS. Overall, three structurally different inhibitors of sEH reversed or eliminated mechanical allodynia in a dose-dependent manner. These effects were time- as well as dose-dependent, indicating a specific interaction between the inhibition of sEH and the observed decrease in pain-related behavior. Strikingly, TUPS reduced allodynia at least 10-fold more efficaciously than gabapentin, a calcium channel trafficking disruptor drug prescribed to patients suffering diabetic neuropathy (38). Moreover, the efficacy of TUPS was longer in duration than gabapentin, which was administered daily to maintain a pain-free state (39). Compared with TUPS, morphine was more efficacious but produced a higher magnitude of analgesia with a shorter duration of efficacy (Fig. S1). TUPS, in contrast, did not lead to sedation or sensorimotor impairment. Despite this favorable bioactivity profile in rodents and drug-like physicochemical properties, pharmacokinetics with high area-under-the-curve values in monkey (40), it remains to be seen if inhibitors of sEH will be efficacious in human and outperform some of the current pain medications prescribed to diabetic patients.
Glucose homeostasis was not altered by acute inhibition of the sEH within the time-scale of these experiments, but nociceptive parameters were improved (i.e., the complete elimination of pain-related behavior). Three important parameters of glucose homeostasis—insulin sensitivity, glucose tolerance, and glucose-stimulated insulin secretion—remained unchanged. TPAU did not display changes in metabolic parameters related to glucose homeostasis in either diabetic or healthy rats. It was recently reported that chemical inhibition or targeted genetic deletion of sEH prevented hyperglycemia and promoted insulin secretion (32). Distinctively, metabolic experiments here were conducted in an acute manner: 30 min following inhibitor administration in rats. In contrast, at least 3 wk were required to observe significant changes in metabolic parameters in sEH−/− or sEH+/+ mice receiving a potent sEH inhibitor following STZ administration. Thus, the acute antiallodynic effects are possibly mediated through a different mechanism.
Notably, the efficacy of TUPS correlated with the plasma levels of this compound between 4 and 96 h following administration. This observation supports the hypothesis that the antinociceptive activity is maintained by sEH inhibitor-mediated stabilization of EpFAs. In the early time points following TUPS administration we observed analgesia, which was not correlated to plasma inhibitor levels. This observation suggests that a threshold level of EpFAs or epoxide/diol ratio needs to be reached to initiate antinociceptive activity and that it is reached before maximal plasma concentration of TUPS. Consequently, a sudden and rapid rise in the epoxide/diol ratio results in an initial strong effect after which (i.e., 4 h) the antinociceptive effect becomes dependent on the plasma level of sEH inhibitor, and thus inhibition of sEH, in a linear manner (Fig. S2C). These observations also argue that EpFAs have a specific, possibly receptor-mediated mechanism of action. It is possible that in an environment where EpFAs are scarce, even a slight increase in EpFAs may tip the balance toward antinociception.
The plasma levels of EpFAs in diabetic+vehicle and diabetic+TPAU treated rats were investigated using the same dose of TPAU (3 mg/kg) as in metabolic studies and behavioral assays. Although type I diabetes did not elicit major changes in the plasma levels of 13 independent EpFAs, TPAU elevated the levels of these metabolites; the levels of their corresponding dihydroxy metabolites were significantly decreased by sEH inhibition (Table S1). Among those decreased, EpFA metabolites of AA, EPA, and DHA possess direct antinociceptive effects peripherally in a model of inflammatory pain (20). Observed antiallodynic activity corresponded well to enhanced plasma epoxide/diol ratio. A full-scale target engagement study was not intended here. However, highly correlative results on plasma inhibitor, metabolite levels, and the antinociceptive effects indicate a significant level of target engagement. It is plausible that the sEH inhibitors are effective because they elevate the levels of numerous antinociceptive molecules. Given that these EpFAs are directly antihyperalgesic when injected into an inflamed paw, it is reasonable that a portion of the observed antinociception is mediated through peripheral mechanisms (20). The mode of action of EpFAs and sEHI in the diabetic neuropathic pain model does not seem to involve their ability to modulate NFĸB and the ensuing down-regulation of COX1/2 because no changes in plasma levels of PGE2 and PGD2 are observed (25). Consistent with this argument, sEHI do not down-regulate induced spinal COX-2 in diabetic rats (Fig. 3D). Thus, our findings demonstrate that the mechanism of action of sEHI in this rodent model differs from inflammatory models.
In the spinal cord, type I diabetes led to a complex profile of bioactive lipids. Key proinflammatory cyclooxygenase products were elevated, consistent with earlier findings indicating up-regulation of the COX-2 gene. Although LA-derived EpFAs were elevated, surprisingly the levels of AA- and DHA-derived EpFAs remained unaffected. In contrast, levels of the dihydroxy fatty acids in general were increased, leading to a significant shift in the ratio of epoxy to dihydroxy fatty acids (Fig. S6). This change was in the absence of a change in spinal sEH activity, thus may be driven by lipolysis. The asymmetric changes highlight the complexity of spinal regulation of bioactive lipids under pathological conditions. It remains to be seen if the dihydroxy fatty acids are biomarkers of spinal pathology. Spinal tissue levels of EpFAs were also investigated using the more potent and antinociceptive inhibitor TUPS. Inhibition of sEH consistently normalized the ratio of epoxy to dihydroxy fatty acids that was greatly reduced in diabetic animals. This normalization was primarily a result of a significant reduction of dihydroxy fatty acid levels by inhibition of sEH. If dihydroxy fatty acids in the spinal cord have pronociceptive roles, reduction of their levels by sEH inhibition should contribute to sEHI-mediated antinociception. Alternatively, the lack of increase in most spinal EpFAs following sEH inhibition may indicate that the primary site of action of sEH inhibitors in this model is not the spinal cord. TUPS was not only antiallodynic but also analgesic. This level of antinociception observed with TUPS administration is difficult to explain solely by normalization of the spinal ratio of epoxy to dihydroxy fatty acids. Although supraspinal sites of action cannot be ruled out, peripheral antinociceptive effects of EpFAs may have a governing role in this model.
Nevertheless, the observed changes in lipid metabolites are indicative of spinal lipid dysregulation in diabetic rats and may correlate to human patients with type I diabetes. Such a dysregulation is likely to influence nociceptive signaling, given the known properties of these bioactive lipids. It remains to be seen if these changes selectively occur in diabetes or if similar dysregulation transpires under other neuropathic conditions. If so, one can then begin to identify lipid biomarkers of painful conditions and target these molecules to reduce pain. Targeting spinal lipid dysregulation may become a valuable strategy for treating neuropathic pain as more selective molecular probes become available. Thus, our findings open a venue for searching and investigating unstudied lipid targets in the spinal cord.
In conclusion, by monitoring changes in bioactive lipids in this type I diabetes model, we demonstrated the presence of dysregulated lipid homeostasis and asymmetric metabolism in the spinal cord. Positive modulation of EpFAs by inhibition of sEH dose-dependently attenuated diabetes-elicited neuropathic pain, although the site of action of EpFAs in this model was not addressed. More importantly, we demonstrated that the antinociceptive effects are acute and occur through a mechanism that is unrelated to regulation of glucose homeostasis. Inhibitors of sEH elevated plasma EpFAs and decreased plasma and spinal dihydroxy fatty acids. The biological effects were well correlated with the changes in lipid metabolites and plasma levels of the inhibitors. We report that the efficacy of sEH inhibitors is superior to that of gabapentin in this rat model of diabetic neuropathy. Despite producing analgesia, the inhibition of sEH did not affect sensorimotor function. Although it is not clear if sEH inhibitors will be efficacious in human patients, our findings in rodent models suggest that inhibition of sEH may become a viable strategy to prevent and alleviate neuropathic pain in diabetes patients.
Materials and Methods
Details of the experimental protocols are given in the SI Materials and Methods.
Diabetic Pain Model, Behavioral and Metabolic Tests, and Treatments.
Age-matched males Sprague-Dawley rats weighing 250–300 g were obtained from Charles River Laboratories and maintained under standard conditions. All studies were conducted in line with federal regulations and were approved by the Institutional Animal Care and Use Committee at the University of California at Davis. Diabetes was instigated by a single intravenous injection of STZ (55 mg/kg) as described by Aley and Levine (28). Nociceptive measurements were taken at the same time of the day for all groups. Pain behavior was measured using von Frey, Randall–Selitto, and modified Hargreaves’ tests (15, 16). Prediabetic baseline mechanical withdrawal thresholds varied between 70 and 80 g of force for both mechanical tests. The baseline withdrawal thresholds before STZ administration were taken as 100% response and all mean nociceptive thresholds were converted to percentage values. All data are presented as mean ± SE of mean. Glucose was measured in blood collected from the tail using a portable glucometer (Home Aide Diagnostics). Serum insulin was determined by ELISA using rat insulin as a standard (Crystal Chem). Metabolic tests were performed as described in detail in the SI Materials and Methods. Results are expressed as milligram per deciliter of glucose or nanogram per milliliter insulin. Inhibitors were administered after completely dissolving in vehicle PEG400 (1 mL/kg). Oxylipin analysis from plasma and tissues are described in the SI Materials and Methods. Gabapentin and morphine were dissolved in saline. Drugs were administered by subcutaneous or intraperitoneal routes, as specified. Blood samples (∼5 μL) from tail vein were obtained on a daily basis immediately after pain measurements and TUPS was quantified using LC-MS/MS.
Statistical Analyses.
Data were analyzed by parametric and nonparametric one-way ANOVA followed by post hoc tests, as indicated in the figure legends, using the SigmaPlot analysis package (Systat Software). Results are depicted as mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.); NIEHS Superfund Basic Research Program P42 ES004699; NIEHS Grant T32ES007059 (to K.M.W.); and a fellowship from the Schormueller Foundation (Berlin, Germany) of the German Society of Food Chemists (to N.H.S.). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation. Research in the F.G.H laboratory is funded by Research Grant 1-2009-337 from the Juvenile Diabetes Research Foundation and National Institutes of Health Grant R01 DK090492.
Footnotes
Conflict of interest statement. B.I., K.M.W., S.H.H., C.M., and B.D.H are coinventors on patents related to inhibitors of the soluble epoxide hydrolase and pain by the University of California.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208708109/-/DCSupplemental.
References
- 1.Calcutt NA. Tolerating diabetes: An alternative therapeutic approach for diabetic neuropathy. ASN Neuro. 2010;2:e00042. doi: 10.1042/AN20100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:928–930, 932, 934–944. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 4.Said G. Diabetic neuropathy—A review. Nat Clin Pract Neurol. 2007;3:331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 5.Sima AAF, et al. Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. N Engl J Med. 1988;319:548–555. doi: 10.1056/NEJM198809013190905. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Ann Neurol. 1986;19:450–457. doi: 10.1002/ana.410190505. [DOI] [PubMed] [Google Scholar]
- 7.Brown MJ, Asbury AK. Diabetic neuropathy. Ann Neurol. 1984;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- 8.Gilliatt RW, Willison RG. Peripheral nerve conduction in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1962;25:11–18. doi: 10.1136/jnnp.25.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: Behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- 10.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay TJ, Rodgers BC, Savath V, Hettinger K. Treating diabetic peripheral neuropathic pain. Am Fam Physician. 2010;82:151–158. [PubMed] [Google Scholar]
- 12.Attal N, et al. EFNS Task Force EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 13.Gore M, Dukes E, Rowbotham DJ, Tai K-S, Leslie D. Clinical characteristics and pain management among patients with painful peripheral neuropathic disorders in general practice settings. Eur J Pain. 2007;11:652–664. doi: 10.1016/j.ejpain.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Ahlgren SC, Levine JD. Mechanical hyperalgesia in streptozotocin-diabetic rats. Neuroscience. 1993;52:1049–1055. doi: 10.1016/0306-4522(93)90551-p. [DOI] [PubMed] [Google Scholar]
- 15.Inceoglu B, et al. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inceoglu B, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chacos N, et al. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 18.Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci USA. 1981;78:5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JY, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morisseau C, et al. Naturally occurring mono epoxides of EPA and DHA are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner K, Inceoglu B, Gill SS, Hammock BD. Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: Novel mediators of pain reduction. J Agric Food Chem. 2011;59:2816–2824. doi: 10.1021/jf102559q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelzer KR, et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelzer KR, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobretsov M, Hastings SL, Romanovsky D, Stimers JR, Zhang JM. Mechanical hyperalgesia in rat models of systemic and local hyperglycemia. Brain Res. 2003;960:174–183. doi: 10.1016/s0006-8993(02)03828-3. [DOI] [PubMed] [Google Scholar]
- 28.Aley KO, Levine JD. Rapid onset pain induced by intravenous streptozotocin in the rat. J Pain. 2001;2:146–150. doi: 10.1054/jpai.2001.21592. [DOI] [PubMed] [Google Scholar]
- 29.Inceoglu B, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley GK, Mooradian AD, Levine AS, Morley JE. Mechanism of pain in diabetic peripheral neuropathy. Effect of glucose on pain perception in humans. Am J Med. 1984;77:79–82. doi: 10.1016/0002-9343(84)90439-x. [DOI] [PubMed] [Google Scholar]
- 31.Luria A, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo P, et al. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334:430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones PD, et al. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem. 2005;343:66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267:3686–3690. [PubMed] [Google Scholar]
- 36.Jones PD, Tsai H-J, Do ZN, Morisseau C, Hammock BD. Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett. 2006;16:5212–5216. doi: 10.1016/j.bmcl.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliff JJ, et al. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrich J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10:276–281. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- 40.Ulu A, et al. Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. Br J Pharmacol. 2012;165:1401–1412. doi: 10.1111/j.1476-5381.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.