Abstract
Background
Evidence regarding the relationship of cardiovascular health (CVH) defined by the American Heart Association (AHA) and specific cardiovascular outcomes is lacking, particularly among Hispanics. This study sought to evaluate the relationship between the number of ideal CVH metrics and cardiovascular risk, overall and by event subtype, in a multiethnic community-based prospective cohort.
Methods and Results
2981 subjects (mean age 69±10 years, 54% Caribbean Hispanic, 25% black, 21% white) free of myocardial infarction (MI) and stroke at baseline in the Northern Manhattan Study were prospectively followed (median follow-up 11 years). The relationship between the number of ideal CVH metrics and the risk of cardiovascular disease (CVD), including MI, stroke and vascular death was investigated. Overall, a strong gradient relationship was observed between the adjusted hazard ratios for CVD and the number of ideal CVH metrics: 0.73 (95% CI: 0.60–0.89), 0.61 (0.50–0.76), 0.49 (0.38–0.63) and 0.41 (0.26–0.63), respectively, for those having 2, 3, 4, and 5–6 ideal CVH metrics compared with those having 0–1 ideal CVH metrics (P for trend <0.0001). Similar graded relationships were found between the number of ideal CVH metrics and the adjusted incidence rate for each specific outcome and among whites, blacks, and Caribbean Hispanics.
Conclusions
Our findings demonstrated a steep gradient relationship between ideal CVH and individual CVD endpoints, including stroke, which was similar for whites, blacks and Caribbean Hispanics. This evidence supports the application of the AHA ideal cardiovascular health metrics for CVD risk assessment and health promotion for all Americans regardless of race-ethnic background.
Keywords: epidemiology, myocardial infarction, race/ethnicity, stroke, Cardiovascular health
Heart disease and stroke present major global public health problems accounting for the leading causes of death, disability, and health-care costs.1 Although mortality has declined for cardiovascular disease (CVD) in the last several decades,2–6 the aging of the population and the increasing prevalence of obesity and diabetes are projected to increase the burden of these conditions.7 Moreover, substantial disparities in the mortality and incidence of CVD across race-ethnic subgroups have been documented with greater burdens among blacks and Hispanics.8–10 The health-care costs of CVD are projected to triple, from $273 billion in 2010 to $818 billion in 2030, unless prevention strategies are more successfully implemented.10
In 2010, the American Heart Association (AHA) set its 2020 impact goal to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular diseases and stroke by 20%.11 Based on the wealth of evidence regarding seven modifiable CVD risk factors (smoking, body mass index, physical activity, diet, blood pressure, total cholesterol, fasting glucose), the AHA has defined ideal cardiovascular health (CVH) metrics as ideal, intermediate and poor. A greater emphasis has been placed upon promoting CVH by focusing on lifestyle and diet modifications to move from poor and intermediate to ideal health. Efforts to promote and achieve ideal CVH will be even more challenging in minority populations.
The definition of ideal CVH metrics has largely been derived from evidence collected in white cohorts.11 To date, only two studies have reported the prevalence of the 7 ideal CVH metrics newly-defined by AHA and risk of CVD events associated with ideal CVH in bi-ethnic (whites and blacks) community-based cohorts (Heart Strategies concentrating on Risk Evaluation (Heart SCORE) and Atherosclerosis Risk in Communities Study (ARIC)).12, 13 Neither of the two reports evaluated the relationship of ideal CVH and individual CVD outcome events, such as stroke. While Hispanics are the fastest growing and largest minority group in the United States, no studies have addressed ideal CVH in this population. The Northern Manhattan Study (NOMAS) is a prospective community-based cohort study among a multi-ethnic population with a significant representation of Caribbean Hispanics. In this analysis, we sought to evaluate the gradient between the number of ideal CVH factors and risk of CVD events, overall and by individual event type, and to determine how well these ideal CVH metrics predict CVD in whites, blacks and Caribbean Hispanics living in the same community.
Methods
Study Population
NOMAS is a longitudinal study of 3298 subjects recruited through random digit dialing in a community between 1993 and 2001 and followed annually since 1993. NOMAS was designed to identify novel determinants of stroke and cardiovascular diseases in three race-ethnic groups. The study was approved by the institutional review boards of Columbia University and the University of Miami, and written informed consent was obtained from all participants. Details of enrollment were described previously.14, 15 Briefly, the enrollment of subjects into the study was based on the following criteria: 1) ≥40 years of age; 2) no history of stroke; 3) Northern Manhattan resident for ≥3 months in a household with a telephone. The overall response rate from the random-digit dialing (telephone screen completion rate 91%) and in-person enrollment (75% of contacted telephone screen subjects enrolled) was 68%. Of the 3298 stroke-free subjects, 238 with a history of myocardial infarction (MI) at entry and 79 subjects who were not classified as Hispanic, white, or black were excluded from the present analysis. This led to a final study population of 2981 participants.
Ascertainment of Baseline Characteristics
All subjects had a thorough baseline evaluation including comprehensive medical history, physical examination, review of medical records, and fasting blood samples. Race-ethnicity was defined through self-identification from a series of questions modeled after the US census. Hispanics were asked to identify their country of origin. Our Hispanic group was mainly comprised of Caribbean Hispanics from the Dominican Republic, Puerto Rico, Cuba and other Latin American countries. White subjects were those who classified themselves as white without any Hispanic origin, and black subjects were those who classified themselves as black without any Hispanic origin.
Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System.16 Smoking was based on self-reported age of starting smoking and age of quitting smoking. Leisure-time physical activity was measured using a questionnaire based on the National Health Interview Survey.17 Diet was assessed through a structured in-person interview using questions adapted from the NCI Food Frequency questionnaire.18 Blood pressure, height, weight, and fasting glucose were measured using standard methods as described previously.6, 7 Fasting total cholesterol was measured using a Hitachi 705 automated spectrophotometer (Boehringer Mannheim, Mannheim, Germany).
Classification of Cardiovascular Health
In accordance with AHA definitions,11 7 CVH factors were classified into ideal, intermediate, or poor as the following: 1) smoking: ideal (never or quit >1 year), intermediate (quit ≤1 year) and poor (current); 2) body mass index (BMI): ideal (<25 kg/m2), intermediate (25 to <30 kg/m2) and poor (≥30 kg/m2); 3) physical activity: ideal (≥150 minutes/week moderate intensity or ≥75 minutes/week vigorous intensity or equivalent combination), intermediate (1–149 minutes/week moderate intensity or 1–74 minutes/week vigorous intensity or equivalent combination) and poor (no moderate and vigorous activity); 4) diet: ideal (4–5 healthy components), intermediate (2–3 healthy components) and poor (0–1 healthy component) based on 5 health dietary metrics (≥4.5 cups fruits and vegetables/day, ≥two 3.5 oz servings fish/week, ≥three 1-oz equivalent servings fiber-rich whole grains/day, <1500 mg sodium/day, and ≤450 kcal sugar-sweetened beverages/week); 5) total cholesterol: ideal (untreated and <200 mg/dL), intermediate (treated to <200 mg/dL or 200–239 mg/dL) and poor (≥240mg/dL); 6) blood pressure: ideal (untreated and <120/<80 mmHg), intermediate (treated to <120/<80 mmHg or 120–139/80–89 mmHg) and poor (≥140/90 mmHg); and 7) fasting plasma glucose: (untreated and <100 mg/dL), intermediate (treated to <100 mg/dL or 100–125/mg/dL) and poor (≥126 mg/dL).
Ascertainment of Incident CVD Events
NOMAS participants have been followed annually by telephone interview with an average annual contact rate of 99%.19 A positive screen for any potential cardiac or neurological event was followed up by an in-person assessment to determine whether a vascular outcome occurred. All admissions and discharges to New York-Presbyterian Hospital were prospectively screened to detect hospitalizations and outcomes that may not have been captured by telephone interview. The 3 outcomes of primary interest were any stroke, MI, and any vascular death. Stroke was verified and classified by at least two study neurologists as described in prior reports.14 MI was adjudicated by study team cardiologists according to previously published criteria.19 For those who developed both MI and stroke, we counted the first event of MI and stroke and had the second one censored at the occurrence time of the first event. Causes of death were determined from death certificates, medical records of hospitalizations, family interviews, and primary care physicians. Vascular causes of death included stroke, MI, heart failure, cardiac arrhythmia, and other vascular causes regardless of their status of the incident MI and stroke.
Statistical Analysis
F test was used to examine the differences in age, whereas chi-square test was used to compare the frequencies of categorical variables and ideal CVH metrics among the racial/ethnic groups. Direct standardization method was used to calculate the age- and sex-adjusted prevalence of ideal CVH metrics based on the distribution of age (5-year band) and sex in the total cohort as the standard. Logistic regression models were employed to compare the prevalence of ideal CVH metrics across race-ethnic groups after adjustment for age and sex.
For each participant, the time (years) at risk for a cardiovascular event was computed from the date of enrollment to the occurrence of incident cardiovascular event, death, loss to follow-up, or the most recent follow-up date, whichever came first. To examine the relationship between the number of ideal CVH metrics and cardiovascular risk, we classified 2981 NOMAS participants into five groups: 0–1, 2, 3, 4, and 5–6 ideal CVH metrics present at baseline. We collapsed 0 with 1 and 5 with 6 ideal metrics due to relatively few subjects who had 0 (2.3% of total cohort) or 6 (0.5% of total cohort) ideal health metrics. Poisson regression models were used to calculate the adjusted incidence of CVD events, which were directly standardized to the distribution of age and sex in total cohort. Cox proportional hazards models were employed to estimate the cumulative hazard function and multivariable-adjusted hazards ratio (HR) and the 95% confidence interval (CI), after adjusting for age, sex and race/ethnicity. Overall C statistics were computed to evaluate the discriminatory capability of the models with the number of ideal CVH metrics.20 Analyses were done for the incidence of CVD (MI, stroke, or vascular death) and for stroke or MI individually. Separate analyses were done for all cause mortality, vascular and non-vascular death.
We calculated interactions with race-ethnicity and performed stratified analyses by race-ethnicity to explore the gradient relationship between the number of ideal CVH metrics and CVD and across race-ethnic groups. In addition, regression analyses were performed to examine the gradient relationship between the number of 4 ideal cardiovascular health behaviors (smoking, BMI, physical activity, and diet) and CVD risk and between the number of 3 ideal cardiovascular health factors (blood pressure, total cholesterol, and fasting plasma glucose) and CVD risk. All data analyses were performed with SAS statistical software version 9.2 (SAS Institute Inc, Cary, NC).
Considering that the standard deviation in the number of ideal CVH metrics was 1.08 and the overall cumulative risk was 7.0% for MI, 8.5% for stroke, 13.7% for vascular death, and 24.0% for CVD, we had approximately 80% power (alpha=0.05) to detect a corresponding HR of 0.84 (MI), 0.85(stroke), 0.88 (vascular death) and 0.91(CVD) for per one number increase in ideal health metrics for the whole sample (n=2981), of 0.78, 0.80, 0.84, 0.88 for Hispanics (n=1617), of 0.70, 0.72, 0.77 and 0.82 for blacks (n=745), and of 0.67, 0.70, 0.75 and 0.81 for whites.21
Results
Sample Characteristics and Prevalence of Ideal Cardiovascular Health at Baseline
Table 1 presents baseline sample characteristics and prevalence of ideal cardiovascular health for the total cohort, whites (20.8%), blacks (25.0%), and Caribbean Hispanics (54.2%). Among the 2981 participants, 63.7% were women and the mean age was 69±10 years with 36.3% < 64 years and 26.4 >75 years. On average, Caribbean Hispanics were 6 years younger than blacks and 7 years younger than whites. Overall, no person had all 7 ideal CVH factors, only 4.4% of the cohort had 5 or 6 CVH factors, and the majority of the cohort (62.4%) had only 2 or 3 ideal factors. A significantly greater prevalence of having 5–6 ideal CVH factors was seen among whites (7.7%) compared to blacks (4.3%) and Caribbean Hispanics (3.2%) and the disparity remained similar after adjustment for age and sex (Supplemental Figure 1–2).
Table 1.
Age, Sex and Cardiovascular Health Status at Baseline in the NOMAS Cohort
| Characteristic | Total Cohort (N=2981) | Race-ethnicity
|
P-value for χ2/F test | P-value adjusted for age and sex † | |||||
|---|---|---|---|---|---|---|---|---|---|
| White (N=619) | Black (N=745) | Caribbean Hispanic (N=1617) | |||||||
|
|
|
|
|||||||
| Age in years, Mean (SD) | 69 (10) | 73 (10) | 72 (10) | 66(9) | <.0001 | ||||
| Female, % | 63.7 | 60.7 | 67.2 | 63.3 | 0.04 | ||||
| Cardiovascular health metrics | |||||||||
| Smoking, % | <.0001 | <.0001 | |||||||
| Ideal (never or quit >1year) | 80.5 | 85.3 | 74.9 | 81.3 | |||||
| Intermediate ( quit < 1year) | 2.1 | 1.3 | 2.2 | 2.4 | |||||
| Poor (current) | 17.3 | 13.4 | 23.0 | 16.3 | |||||
| Body mass index, % | <.0001 | <.0001 | |||||||
| Ideal (<25 kg/m2) | 30.7 | 47.2 | 30.3 | 24.6 | |||||
| Intermediate (25–<30 kg/m2) | 41.8 | 35.0 | 39.0 | 45.7 | |||||
| Poor (≥30 kg/m2) | 27.5 | 17.9 | 30.7 | 29.7 | |||||
| Physical activity, % | <0001 | <.0001 | |||||||
| Ideal (≥75 min/wk vigorous or ≥150 min/wk moderate or equivalent combination) | 33.3 | 45.2 | 37.6 | 26.8 | |||||
| Intermediate (1–74 min/wk vigorous or 1–149 min/wk moderate or equivalent combination) | 24.9 | 24.2 | 28.5 | 23.6 | |||||
| Poor (no moderate and vigorous activity) | 41.7 | 30.5 | 34.0 | 49.6 | |||||
| Diet*, % | 0.66 | 0.62 | |||||||
| Ideal (4–5 healthy components) | 0.4 | 0.2 | 0.5 | 0.5 | |||||
| Intermediate (2–3 healthy components) | 24.7 | 23.2 | 25.9 | 24.8 | |||||
| Poor (0–1 healthy component) | 74.9 | 76.6 | 73.6 | 74.7 | |||||
| Blood pressure, % | <.0001 | <.0001 | |||||||
| Ideal (untreated and <120/<80 mmHg) | 5.9 | 9.1 | 5.3 | 5.0 | |||||
| Intermediate (treated to <120/<80mmHg or 120–139/80–89 mmHg) | 56.8 | 60.6 | 51.4 | 57.9 | |||||
| Poor (≥140/90 mmHg) | 37.3 | 30.3 | 43.4 | 37.2 | |||||
| Total cholesterol, % | 0.003 | <.0001 | |||||||
| Ideal (untreated and <200 mg/dL) | 42.1 | 35.3 | 46.0 | 43.0 | |||||
| Intermediate (treated to <200 mg/dL or 200–239 mg/dL) | 40.7 | 45.5 | 37.8 | 40.2 | |||||
| Poor (≥240mg/dL) | 17.2 | 19.2 | 16.2 | 16.8 | |||||
| Plasma glucose, % | 0.008 | 0.04 | |||||||
| Ideal (untreated and <100 mg/dL) | 63.1 | 66.9 | 61.5 | 62.3 | |||||
| Intermediate (treated to <100 mg/dL or 100–125/mg/dL) | 21.1 | 22.1 | 21.3 | 20.5 | |||||
| Poor (≥126 mg/dL) | 15.9 | 11.0 | 17.2 | 17.2 | |||||
| Number of ideal health metrics, % | <0.0001 | <.0001 | |||||||
| 0 | 2.3 | 1.5 | 3.2 | 2.2 | |||||
| 1 | 16.7 | 11.5 | 17.0 | 18.6 | |||||
| 2 | 32 | 26.3 | 29.7 | 35.2 | |||||
| 3 | 30.4 | 31.0 | 30.6 | 30.0 | |||||
| 4 | 14.3 | 22.0 | 15.2 | 10.9 | |||||
| 5 | 3.9 | 6.9 | 3.8 | 2.8 | |||||
| 6 | 0.5 | 0.8 | 0.5 | 0.4 | |||||
308 subjects were missing baseline diet data and the proportion of subjects with missing diet data was 9.5%, 11.9%, and 9.9% in whites, blacks and Caribbean Hispanics, respectively (p=0.24 for chi-square test with df of 2).
P values were based on Wald chi-square test with df of 2 in the multiple binary (ideal vs. other for individual metrics or 5–6 vs. 0–4 for the number of ideal health metrics) logistic regression after adjustment for age and sex.
Among the 4 health behaviors, the prevalence of ideal non-smoking behavior was high overall, although lowest among blacks. Ideal BMI was present in 30.7% of the total cohort, greatest among whites (47.2%), lower among blacks (30.3%) and lowest among Caribbean Hispanics (24.6%). Similarly, ideal physical activity was present in 33.3% of the total cohort, greatest among whites (45.2%), lower among blacks (37.6%) and lowest among Caribbean Hispanics (26.8%). However, only 0.4% of the total cohort met the ideal diet definition and the diet component of ideal CVH was equally poor across all three race-ethnic groups.
For each of the 3 health factors, the prevalence of ideal blood pressure was low overall and lower among blacks (5.3%) and Caribbean Hispanics (5.0%) compared to whites (9.1%). The prevalence of ideal fasting glucose was 63.1% overall, greatest among whites (66.9%), lower among blacks (61.5%) and Caribbean Hispanics (62.3%). The prevalence of ideal total cholesterol was lower among whites (35.3%) and greater among blacks (46.0%) and Caribbean Hispanics (43.0%).
Ideal CVH and CVD Risk, Overall and by Race/Ethnicity
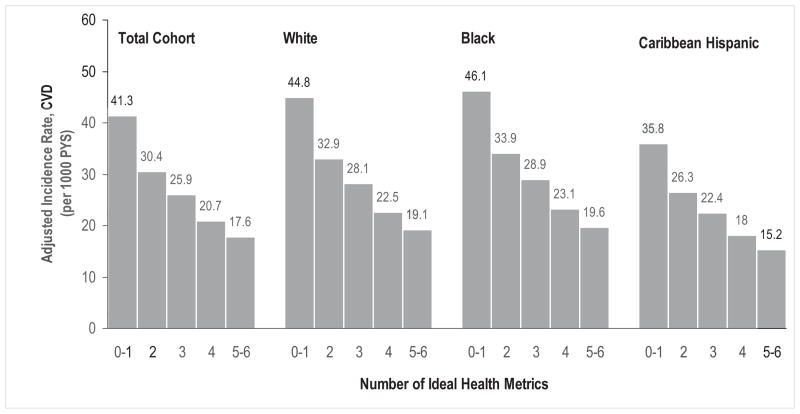
During a median follow-up of 11 years, 722 individuals developed an incident CVD event, including stroke, MI, and vascular deaths. The overall incidence rate of CVD was 24.0 per 1000 person-years in the total cohort. The age and sex-adjusted incidence rate (crude rate) of CVD was 28.8 (32.7), 31.6 (31.9), and 25.1 (17.8) per 1000 person-years, respectively, in whites, blacks and Caribbean Hispanics. After adjustment for age, sex and race-ethnicity, the overall CVD incidence rate in the total cohort was lower among those with greater number of ideal CVH metrics, with an adjusted incidence rate of 41.3, 30.4, 25.9, 20.7 and 17.6 per 1000 person-years for those having 0–1, 2, 3, 4, and 5–6 ideal CVH metrics, respectively. A similar trend of lower adjusted incidence rates with an increase in the number of ideal CVH metrics was observed in whites (from 44.8 to 19.1 per 1000 person-years), blacks (from 46.1 to 19.6 per 1000 person-years) and Caribbean Hispanics (from 35.8 to 15.2 person-years) (Figure 1). Cox regression analysis did not detect a significant interaction between race-ethnicity and the number of ideal CVH metrics in the HRs for CVD risk (Wald chi-square=9.50, df=8, p=0.30) and showed a clear gradient relationship between the hazard ratios of CVD events and the number of ideal CVH metrics overall and across race-ethnic groups (all p values for trend ≤ 0.001). Subjects with 5–6 ideal CVH metrics had a much lower risk compared to those with 0–1 ideal CVH metric for CVD in the total cohort (HR: 0.41, 95% CI: 0.26–0.63), whites (0.48, 0.25–0.92), blacks (0.31 0.12–0.78), and Caribbean Hispanics (0.33, 0.14–0.77). The discriminatory capabilities of Cox regression models with the number of ideal CVH metrics were similar for overall or each subgroup, with a C statistic ranging from 0.63 to 0.68 (Table 2).
Figure 1.
Incidence rates of cardiovascular disease (CVD) by the number of ideal health metrics in total cohort, whites, blacks, and Caribbean Hispanics. Incidence rates of cardiovascular disease by the number of ideal health metrics were adjusted for age, sex and race-ethnicity if applicable, NOMAS (Northern Manhattan Study), 1993–2011.
Table 2.
Hazard Ratios (HR) of Cardiovascular Events by the Number of Ideal Cardiovascular Health Metrics
| Event | No. of Ideal Health Metrics | Total Cohort
|
White
|
Black
|
Caribbean Hispanic
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No case | HR (95% CI)† | No. case | HR (95% CI)‡ | No. case | HR (95% CI)‡ | No. case | HR (95% CI)‡ | |||
| CVD* | 0–1 | 165 | Reference | 30 | Reference | 54 | Reference | 81 | Reference | |
| 2 | 228 | 0.73 (0.60–0.89) | 54 | 0.66 (0.42–1.04) | 76 | 0.79 (0.56–1.13) | 98 | 0.69 (0.52–0.93) | ||
| 3 | 212 | 0.61 (0.50–0.76) | 54 | 0.47 (0.30–0.74) | 62 | 0.54 (0.37–0.78) | 96 | 0.77 (0.57–1.03) | ||
| 4 | 93 | 0.49 (0.38–0.63) | 39 | 0.45 (0.27–0.72) | 30 | 0.53 (0.34–0.83) | 24 | 0.43 (0.27–0.68) | ||
| ≥5 | 24 | 0.41 (0.26–0.63) | 13 | 0.48 (0.25–0.92) | 5 | 0.31 (0.12–0.78) | 6 | 0.33 (0.14–0.77) | ||
| P for for trend trend | <0.0001 | 0.001 | <.0001 | 0.0002 | ||||||
| C-statistic (95% (95% CI) | 0.68 (0.60–0.75) | 0.67 (0.52–0.81) | 0.63 (0.49–0.77) | 0.67 (0.56–0.78) | ||||||
| Myocardial infarction | 0–1 | 48 | Reference | 11 | Reference | 9 | Reference | 28 | Reference | |
| 2 | 71 | 0.78 (0.54–1.12) | 25 | 0.86 (0.42–1.75) | 15 | 0.90 (0.39–2.08) | 31 | 0.66 (0.39–1.09) | ||
| 3 | 56 | 0.57 (0.38–0.84) | 18 | 0.45 (0.21–0.96) | 9 | 0.46 (0.18–1.18) | 29 | 0.70 (0.41–1.17) | ||
| 4 | 30 | 0.53 (0.33–0.85) | 15 | 0.52 (0.23–1.14) | 8 | 0.86 (0.32–2.28) | 7 | 0.38 (0.16–0.86) | ||
| ≥5 | 3 | 0.16 (0.05–0.52) | 2 | 0.22 (0.05–0.98) | 0 | - | 1 | 0.15 (0.02–1.11) | ||
| P for for trend | <0.0001 | 0.006 | 0.125 | 0.006 | ||||||
| C-statistic (95% CI) | 0.69 (0.55–0.81) | 0.67 (0.42–0.88) | 0.60 (0.37–0.81) | 0.69 (0.48–0.86) | ||||||
| Stroke | 0–1 | 64 | Reference | 7 | Reference | 22 | Reference | 35 | Reference | |
| 2 | 81 | 0.71 (0.51–0.99) | 12 | 0.71 (0.28–1.80) | 29 | 0.82 (0.47–1.43) | 40 | 0.68 (0.43–1.06) | ||
| 3 | 70 | 0.60 (0.42–0.84) | 13 | 0.59 (0.23–1.50) | 17 | 0.41 (0.22–0.78) | 40 | 0.77 (0.49–1.21) | ||
| 4 | 29 | 0.49 (0.31–0.76) | 7 | 0.44 (0.15–1.23) | 11 | 0.55 (0.26–1.14) | 11 | 0.48 (0.25–0.96) | ||
| ≥5 | 8 | 0.43 (0.21–0.91) | 4 | 0.73 (0.21–2.51) | 1 | 0.16 (0.02–1.22) | 3 | 0.42 (0.13–1.37) | ||
| P for trend | 0.0002 | 0.26 | 0.003 | 0.04 | ||||||
| C-statistic (95% CI) | 0.65 (0.52–0.77) | 0.65 (0.33–0.92) | 0.65 (0.33–0.93) | 0.66 (0.49–0.82) | ||||||
CVD: cardiovascular disease, including the first event of myocardial infarction, stroke or vascular death.
HR and the 95% CI after adjustment for age, sex and race-ethnicity based on Cox regression.
HR and the 95% CI after adjustment for age and sex based on Cox regression.
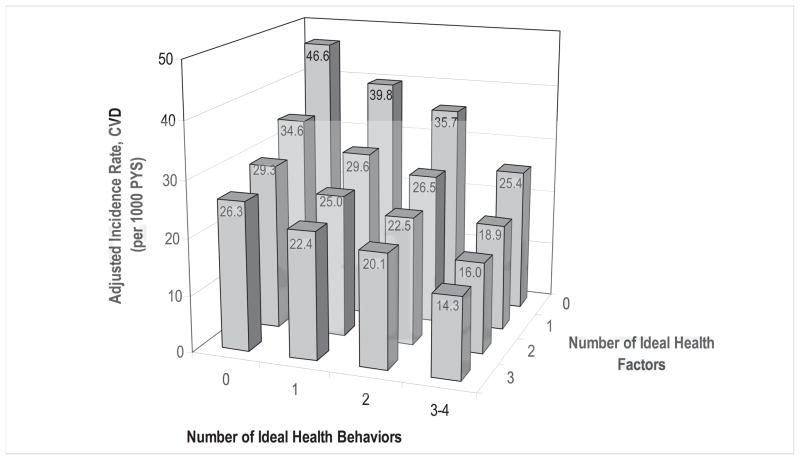
Figure 2 shows the adjusted incidence rates of CVD by the combinations of the numbers of ideal health behaviors and ideal health factors based on an additive model. The gradient for lower CVD incidence rates with a greater number of ideal health behaviors was observed across each of the subgroups by number of ideal health factors. Moreover, the rate was also lower with greater number of ideal health factors when controlling for the number of ideal health behaviors. Similarly, Cox regression analysis including both the number of ideal health behaviors and the number of ideal health factors in the model also showed that lower CVD risk is associated with increased number of ideal health behaviors (Wald Chi-square=15.84, df=3, p=0.001) and the number of ideal health factors (Wald Chi-square=22.79, df=3, p<0.0001), but there is no significant interaction between the two numbers (Wald Chi-square=6.74, df=9, p=0.66), suggesting that both ideal health behaviors and ideal health factors were independently associated with the lower CVD risk.
Figure 2.
Incidence rates of cardiovascular disease (CVD) by the numbers of ideal health behaviors (smoking, obesity, physical activity, and diet) and health factors (blood pressure, cholesterol, and glucose) in the total cohort. Incidence rates of cardiovascular disease by the numbers of ideal health behaviors and health factors were adjusted for age, sex and race-ethnicity, NOMAS (Northern Manhattan Study), 1993–2011.
Ideal CVH and Risk of MI and Stroke by Race/Ethnicity
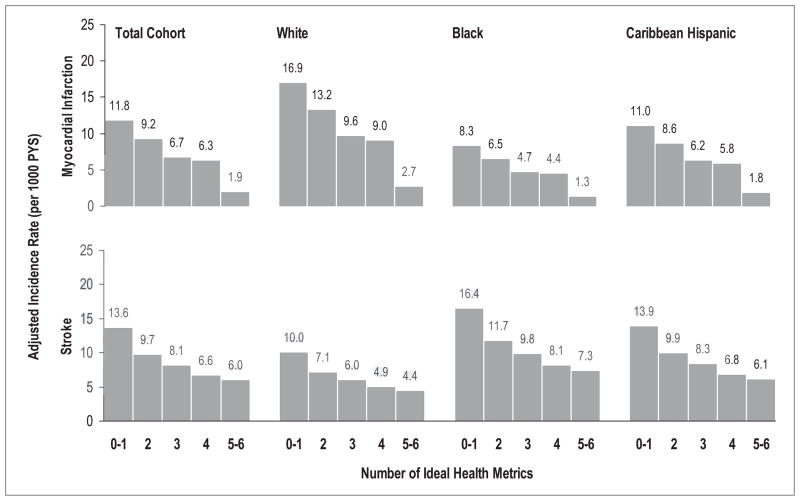
During the follow-up, 208 individuals had MI and 252 individuals had stroke as the first CVD event. For MI, the age and sex-adjusted incidence rate (crude rate) was 10.5 (11.9), 5.5 (5.6), and 7.6 (5.4) per 1000 person-years, respectively, in whites, blacks and Caribbean Hispanics. For stroke, the age and sex-adjusted incidence rate (crude rate) was 6.3 (7.1), 11.0 (11.1), and 9.6 (7.4) per 1000 person-years, respectively, in whites, blacks and Caribbean Hispanics. Figure 3 presents the age- and sex-adjusted incidence rates of MI and stroke according to the number of ideal CVH metrics in each race-ethnic group. Similar to the pattern of the relationship between the number of ideal CVH metrics and CVD risk, with an increasing number of ideal CVH metrics present, the adjusted incidence rate was lower for MI and stroke in whites, blacks, and in Caribbean Hispanics even though there were differences in the overall incidence rates across race-ethnic groups. Cox proportional hazard model also demonstrated a similar gradient relationship between the number of ideal CVH metrics and the HR for MI and stroke across the race-ethnic subgroups although no statistical significance was reached for MI in blacks and stroke in whites due to the small number of the events, with a discriminatory capability of C statistic ranging from 0.60 to 0.69 (Table 2). No significant interaction was detected between race-ethnicity and the number of ideal CVH metrics in the HRs for MI (Wald chi-square=5.70, df=8, p=0.68) and stroke (Wald chi-square=7.48, df=8, p=0.49).
Figure 3.
Incidence rates of myocardial infarction and stroke by the number of ideal health metrics in total cohort, whites, blacks, and Caribbean Hispanics. Incidence rates of myocardial infarction, stroke and vascular death by the number of ideal health metrics were adjusted for age, sex and race-ethnicity if applicable, NOMAS (Northern Manhattan Study), 1993–2011.
Ideal CVH and Vascular and Non-Vascular Death by Race/Ethnicity
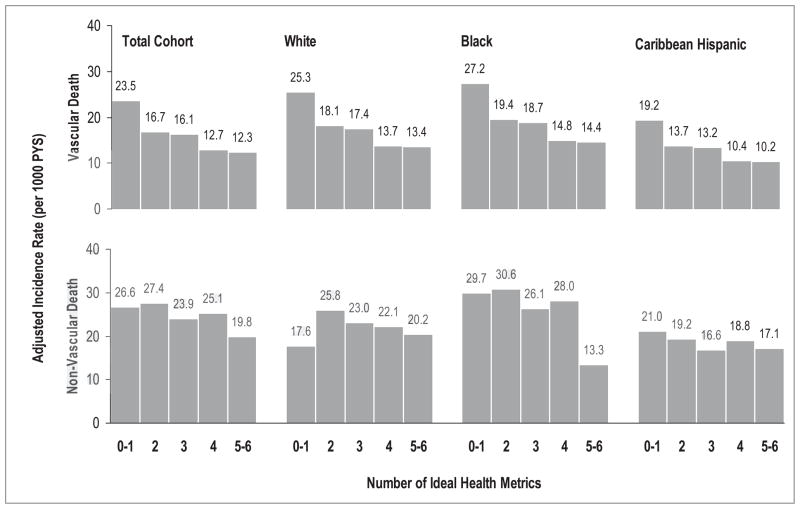
During the follow up, 1123 individuals had died, including 435 vascular and 688 non-vascular deaths. For vascular death, the age- and sex-adjusted (crude rate) mortality rate was 17.1 (20.1), 19.3 (19.5), and 14.0 (9.0) per 1000 person-years in whites, blacks, and Caribbean Hispanics, respectively. For non-vascular death, the age- and sex-adjusted (crude rate) mortality rate was 25.6 (29.8), 31.2 (31.1), and 21.0 (14.7) per 1000 person-years in whites, blacks, and Caribbean Hispanics, respectively. Figure 4 shows the age and sex-adjusted mortality rates of vascular and non-vascular deaths according to the number of ideal CVH metrics in each race-ethnic group. Compared to non-vascular death, vascular death showed a clearer gradient related to the number of ideal CVH metrics in the total cohort or specific ethnic group (Table 3).
Figure 4.
Mortality rates of vascular and non-vascular deaths by the number of ideal health metrics among in total cohort, whites, blacks, and Caribbean Hispanics. Mortality rates of vascular and non-vascular deaths by the number of ideal health metrics were adjusted for age, sex and race-ethnicity if applicable, NOMAS (Northern Manhattan Study), 1993–2011.
Table 3.
Hazard Ratios (HR) of Deaths by the Number of Ideal Cardiovascular Health Metrics
| Death | No. of Ideal Health Metrics | Total Cohort
|
White
|
Black
|
Caribbean Hispanic
|
||||
|---|---|---|---|---|---|---|---|---|---|
| No. case | HR (95% CI)* | No. case | HR (95% CI)† | No. case | HR (95% CI)† | No. case | HR (95% CI)† | ||
| All-Cause | 0–1 | 211 | Reference | 33 | Reference | 74 | Reference | 104 | Reference |
| 2 | 344 | 0.87 (0.73–1.03) | 87 | 1.03 (0.69–1.54) | 116 | 0.85 (0.64–1.15) | 141 | 0.84 (0.65–1.08) | |
| 3 | 342 | 0.78 (0.66–0.93) | 99 | 0.85 (0.57–1.27) | 120 | 0.75 (0.56–1.00) | 123 | 0.82 (0.63–1.06) | |
| 4 | 178 | 0.72 (0.59–0.89) | 70 | 0.78 (0.51–1.19) | 62 | 0.77 (0.55–1.09) | 46 | 0.65 (0.46–0.92) | |
| ≥5 | 48 | 0.59 (0.43–0.81) | 24 | 0.78 (0.46–1.33) | 10 | 0.45 (0.23–0.87) | 14 | 0.53 (0.30–0.93) | |
| P for trend | <0.0001 | 0.08 | 0.01 | 0.004 | |||||
| C-statistic (95% CI) | 0.70 (0.64–0.75) | 0.68 (0.56–0.80) | 0.68 (0.56–0.78) | 0.69 (0.59–0.78) | |||||
| Vascular | 0–1 | 95 | Reference | 19 | Reference | 33 | Reference | 43 | Reference |
| 2 | 127 | 0.70 (0.53–0.91) | 35 | 0.69 (0.39–1.21) | 42 | 0.65 (0.41–1.03) | 50 | 0.72 (0.48–1.08) | |
| 3 | 136 | 0.67 (0.51–0.87) | 36 | 0.49 (0.28–0.87) | 46 | 0.60 (0.38–0.95) | 54 | 0.88 (0.59–1.31) | |
| 4 | 59 | 0.51 (0.37–0.72) | 26 | 0.46 (0.25–0.84) | 21 | 0.55 (0.32–0.97) | 12 | 0.43 (0.23–0.82) | |
| ≥5 | 18 | 0.48 (0.29–0.80) | 10 | 0.54 (0.25–1.17) | 5 | 0.49 (0.19–1.25) | 3 | 0.30 (0.09–0.99) | |
| P for trend | <0.0001 | 0.02 | 0.03 | 0.01 | |||||
| C-statistic (95% CI) | 0.69 (0.60–0.78) | 0.68 (0.50–0.84) | 0.65 (0.48–0.81) | 0.69 (0.54–0.83) | |||||
| Non-Vascular | 0–1 | 116 | Reference | 14 | Reference | 41 | Reference | 61 | Reference |
| 2 | 217 | 1.01 (0.81–1.27) | 52 | 1.50 (0.83–2.71) | 74 | 1.02 (0.69–1.49) | 91 | 0.92 (0.67–1.28) | |
| 3 | 206 | 0.88 (0.70–1.10) | 63 | 1.34 (0.76–2.44) | 74 | 0.86 (0.59–1.27) | 69 | 0.78 (0.55–1.10) | |
| 4 | 119 | 0.90 (0.69–0.17) | 44 | 1.23 (0.67–2.26) | 41 | 0.95 (0.61–1.48) | 34 | 0.80 (0.52–1.22) | |
| ≥5 | 30 | 0.68 (0.45–1.02) | 14 | 1.12 (0.53–2.36) | 5 | 0.41 (0.16–1.04) | 11 | 0.67 (0.35–1.30) | |
| P for trend | 0.04 | 0.71 | 0.13 | 0.09 | |||||
| C-statistic (95% CI) | 0.68 (0.60–0.75) | 0.65 (0.49–0.79) | 0.66 (0.52–0.78) | 0.68 (0.55–0.79) | |||||
HR and the 95% CI after adjustment for age, sex and race-ethnicity based on Cox regression.
HR and the 95% CI after adjustment for age and sex based on Cox regression.
Discussion
In this multi-ethnic community-based prospective cohort of older individuals, the presence of a greater number of the ideal CVH metrics at baseline was associated with a markedly lower risk of CVD over a median follow up of 11 years. A similar pattern was also found for each outcome of stroke, MI, and vascular death in the separate analyses. This strong graded relationship was observed and the magnitude of the effects was quantitatively similar across whites, blacks, and Caribbean Hispanics. In addition, the lower CVD risk was associated with both higher numbers of ideal health behaviors (non-smoking, BMI < 25 kg/m2, adequate physical activity, healthy dietary quality) and health factors (untreated blood pressure <120/80 mmHg, untreated total cholesterol<200 mg/dL, untreated fasting glucose <100 mg/dL). Despite race-ethnic disparities in the prevalence of ideal CVH, our data provide evidence to support the uniform application of the AHA ideal CVH metrics for CVD risk assessment and health promotion for all Americans regardless of their race-ethnic backgrounds.
Our data indicated that there were substantial race-ethnic disparities in the prevalence of ideal health metrics and these disparities partially accounted for the differences in CVD risk. Figure 1 showed that the CVD incidence rates were similar between whites and blacks after adjustment for the number of ideal heath metrics. However, similar to the national data with lower CVD mortality in Hispanics compared with whites and blacks, our data showed that Hispanics had a lower risk for CVD even after adjustment for age, sex and the number of ideal health metrics, suggesting that other factors, including genetic and socio-cultural factors, may also contribute to this paradox. On the other hand, the observed prevalence of ideal diet in our study was equally low and agrees well with the national data (<0.5% ideal diet in both adults and children),11 suggesting that more longitudinal studies may be needed to evaluate the applicability of this metric across race-ethnic groups.
Prior studies have not evaluated the relationship between ideal CVH and specific event types or among Hispanics. In the ARIC study of middle-aged men and women a strong graded relationship was reported between an individual’s number of ideal cardiovascular health metrics and the future overall CVD risk both in whites and African Americans.13 The age, sex, and race-adjusted incidence rate of CVD was nearly 8-fold higher in the ARIC participants having 0 ideal CVH metric (32 per 1000 person-years) compared to those having 6 ideal CVH metrics (4 per 1000 person-years). No CVD events were observed among the individuals having 7 ideal CVH metrics after 20 years of follow-up in their relatively young cohort.
In contrast to the ARIC cohort, the NOMAS cohort, which has an older age distribution, had no participants with all 7 ideal CVH metrics and a much lower prevalence of 5–6 ideal CVH metrics (4.4% vs. 12.2%).13 Our findings clearly demonstrated a gradient in the adjusted hazard ratios across the number of ideal CVH metrics, and extended this graded association to Caribbean Hispanics, as well as to each separate CVD event. Although we studied an older cohort, we were able to document a 59% lower CVD risk for those having 5–6 ideal CVH metrics when compared with those having 0–1 ideal CVH metric. We were able to extend the results of ARIC beyond middle age adults and demonstrate a graded relationship between the number of ideal CVH metrics in an older, urban and multi-ethnic population. Interestingly, the greatest reduction in adjusted CVD incidence rate was found when comparing 0–1 to 2 ideal health metrics than for other increments of 1 health metric unit, suggesting that even having 2 ideal health metrics may lower CVD risk by ~27% (HR=0.73, Table 2). This sends an important message regarding initiating steps to ideal health even among those with the least ideal health. Our results have several important implications regarding the promotion of ideal cardiovascular health and the prevention of cardiovascular disease. Our data indicate that the prevalence of having more than 5 ideal CVH metrics is low. No one in our entire cohort had all 7 ideal CVH metrics, and a very small proportion had 5 or more ideal CVH metrics. More aggressive efforts are needed to begin at younger ages and shift more of the population toward ideal cardiovascular health. Our study, however, suggests that having a greater number of ideal cardiovascular health metrics could lead to substantial cardiovascular health benefits even in an older population. Our data showed that overall, the risk for vascular death was more than 30% lower for those having 2 or 3 ideal CVH metrics and 50% lower for those having 5 or more ideal CVH metrics when compared with those having 0–1 ideal CVH metrics. There are incremental benefits to increasing the number of ideal CVH metrics. Consumer campaigns emphasizing incremental changes in behavior will be beneficial and may allow for easier messaging for the public to attain achievable goals.
Our data provide strong evidence for the similar applicability of the AHA metrics in promoting cardiovascular health in whites, blacks, and Caribbean Hispanics. Although race-ethnic disparity in the prevalence of behaviors and health factors, incidence and mortality of CVD is well documented,8–10 our data shows a similar graded relationship between the number of ideal CVH metrics and the incidence rate of CVD across race-ethnic groups. Health promotion policies achieving a higher prevalence of ideal CVH metrics could lead to a lower risk for overall CVD, stroke and MI across all race and ethnic groups. Despite these similar quantitative effects of increased ideal CVH metrics across the race and ethnic groups, making the health and behavioral changes within black and Hispanic populations presents greater challenges due to the lower prevalence of ideal CVH, limited access to health care, and other cultural and socioeconomic issues.
Our data along with the findings from the ARIC study also strongly support the need for improvements in environments supporting health behaviors such as physical activity, smoking, obesity and diet that are just as important as policies controlling vascular factors such as blood pressure, cholesterol and blood glucose to lower CVD risk. Whereas there has been a decline in CVD mortality in the United States, the increased obesity and physical inactivity, as well as low consumption of healthy foods in US population, will likely lead to an increasing number of persons with cardiovascular disease.22, 23 Initiatives such as the Million Hearts program and follow-up to the United Nations Political declaration on Non-communicable disease that target tobacco control, obesity, and diet will be essential to the future reduction of cardiovascular diseases and stroke.24, 25
Strengths of our study include the community-based random sampling method, the inclusion of a tri-ethnic cohort with a sizeable number of Hispanics from the same community that allows for comparisons and helps minimize socioeconomic confounding, the availability of blood assessments and comprehensive data on health behaviors, and the excellent retention of the cohort with follow-up for as long as 16 years. Nevertheless, several limitations also deserve mention. First, the sample size was relatively small for whites and blacks but prominent for Caribbean Hispanics, limiting our ability to detect race-ethnic differences and generalize the findings to all Hispanics, given that Hispanics are a heterogeneous population. However, the observed gradients were consistent, steep, statistically significant and in agreement with other studies.13. Also, this consistency of risk gradients across ethnic groups was also seen in multiple regions of the world in the InterHEART study.26 Second, the crude scale used and the treatment of each individual metric as having an equal magnitude of effect could oversimplify the association. Third, this study did not include other cardiovascular events such as congestive heart failure, and the incidence rate of overall CVD could be underestimated.19 Fourth, cardiovascular health was defined based on the baseline assessments. Given over 10 years of follow-up, it is very likely that the levels of cardiovascular health factors may change over time, leading to the underestimation of true associations. In addition, the categories defined by the AHA for some metrics may be less than ideal. For example, some studies have showed that there is a continuous positive relationship between vascular risk and blood total cholesterol down to 160 mg/dL, without threshold.27, 28 The overall C statistics were below 0.7, suggesting that these weaknesses may lead to an underestimation of the true association between CVH metrics and CVD risk.23, 26 Thus, Stamler and others have demonstrated even larger protective effects.29, 30
In conclusion, our data documented a clear gradient relationship between ideal cardiovascular health and CVD risk across each race-ethnic subgroup and for each event type, including stroke, MI and vascular death. This evidence supports the application of the AHA ideal cardiovascular health metrics for CVD risk assessment and health promotion for all Americans.
Supplementary Material
Acknowledgments
The authors thank study participants for their collaboration and all staff of the Northern Manhattan Study for their dedication to the study especially Janet DeRosa.
Funding Sources: This work is supported by a grant from the National Institute of Neurological Disorders and Stroke (R37 NS 29993).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: Preliminary data for 2009. National Vital Statistics Report. 2011;59:1–67. [PubMed] [Google Scholar]
- 2.Capewell S, Ford ES, Croft JB, Critchley JA, Greenlund KJ, Labarthe DR. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bull World Health Organ. 2010;88:120–130. doi: 10.2471/BLT.08.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Young F, Capewell S, Ford ES, Critchley JA. Coronary mortality declines in the U.S. between 1980 and 2000 quantifying the contributions from primary and secondary prevention. Am J Prev Med. 2010;39:228–234. doi: 10.1016/j.amepre.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 8.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 9.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 10.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. The J Clin Endocrinol Metab. 2004;89:2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 12.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 15.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 16.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 17.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey. United States, 1985. Vital Health Stat. 1986;10:i–iv. 1–182. [PubMed] [Google Scholar]
- 18.Boden-Albala B, Elkind MS, White H, Szumski A, Paik MC, Sacco RL. Dietary total fat intake and ischemic stroke risk: the Northern Manhattan Study. Neuroepidemiology. 2009;32:296–301. doi: 10.1159/000204914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21:552–560. doi: 10.1016/s0197-2456(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D. Achieving cardiovascular health: a bleak outlook or tremendous potential? J Am Coll Cardiol. 2011;57:1697–1699. doi: 10.1016/j.jacc.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Smith SC, Holmes D, Shurin S, Brawley O, Cazap E, Glass R, Komajda M, Koroshetz W, Mayer-Davis E, Mbanya JC, Sledge G, Varmus H. Accelerating progress on non-communicable diseases. Lancet. 2011 Sep 16; doi: 10.1016/S0140-6736(11)61477-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Sacco RL, Frieden TR, Blakeman DE, Jauch EC, Mohl S. What the million hearts initiative means for stroke: a presidential advisory from the american heart association/american stroke association. Stroke. 2012;43:924–928. doi: 10.1161/STR.0b013e318248f00e. [DOI] [PubMed] [Google Scholar]
- 26.McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–589. doi: 10.1093/eurheartj/ehq448. [DOI] [PubMed] [Google Scholar]
- 27.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 28.Stamler J, Neaton JD. The Multiple Risk Factor Intervention Trial (MRFIT)--importance then and now. JAMA. 2008;300:1343–1345. doi: 10.1001/jama.300.11.1343. [DOI] [PubMed] [Google Scholar]
- 29.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 30.Daviglus ML, Liu K. Today’s agenda: we must focus on achieving favorable levels of all risk factors simultaneously. Arch Intern Med. 2004;164:2086–2087. doi: 10.1001/archinte.164.19.2086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.