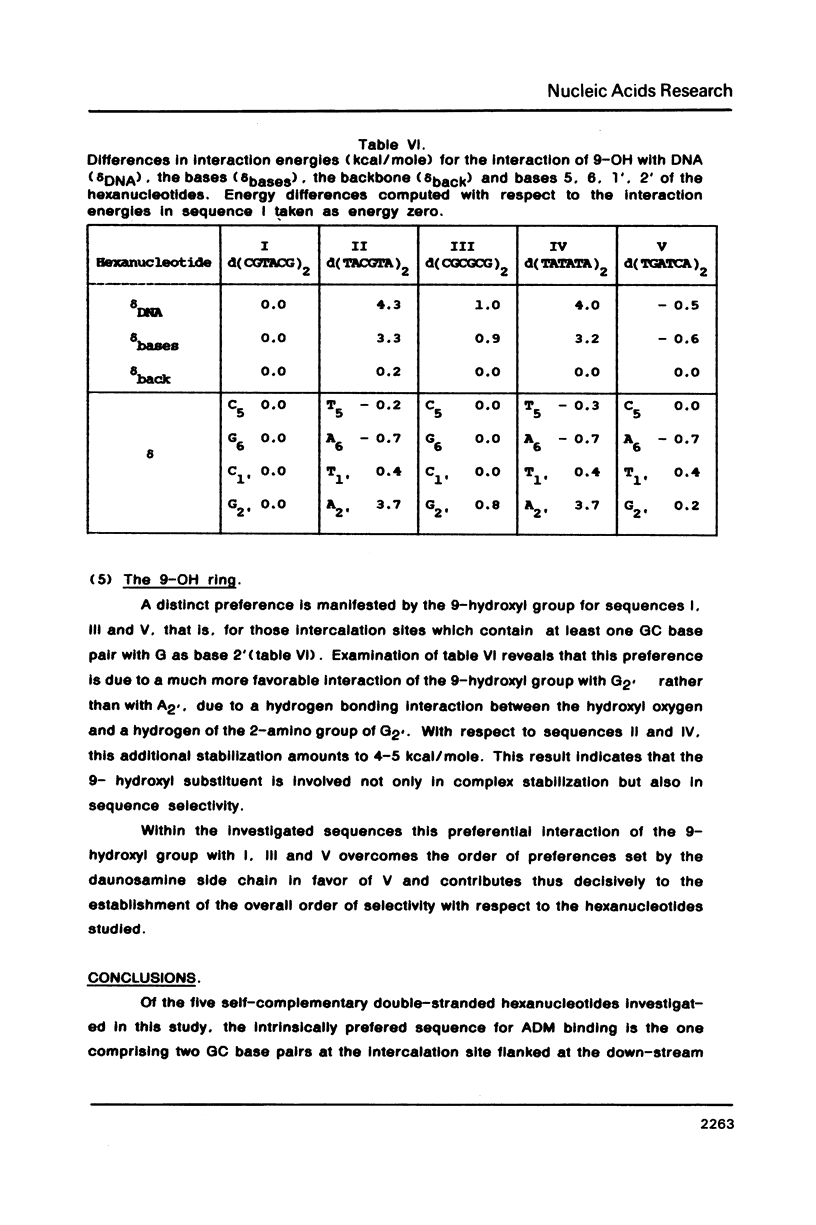
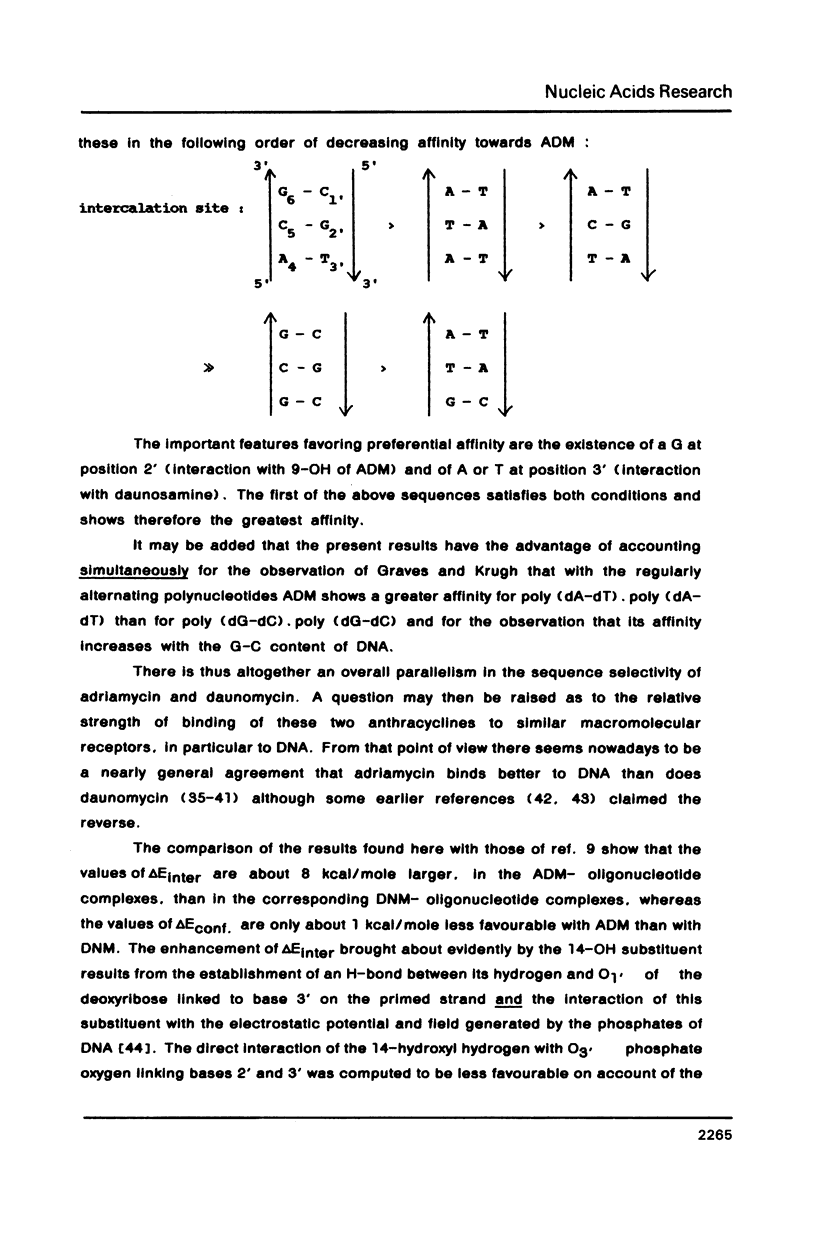
Abstract
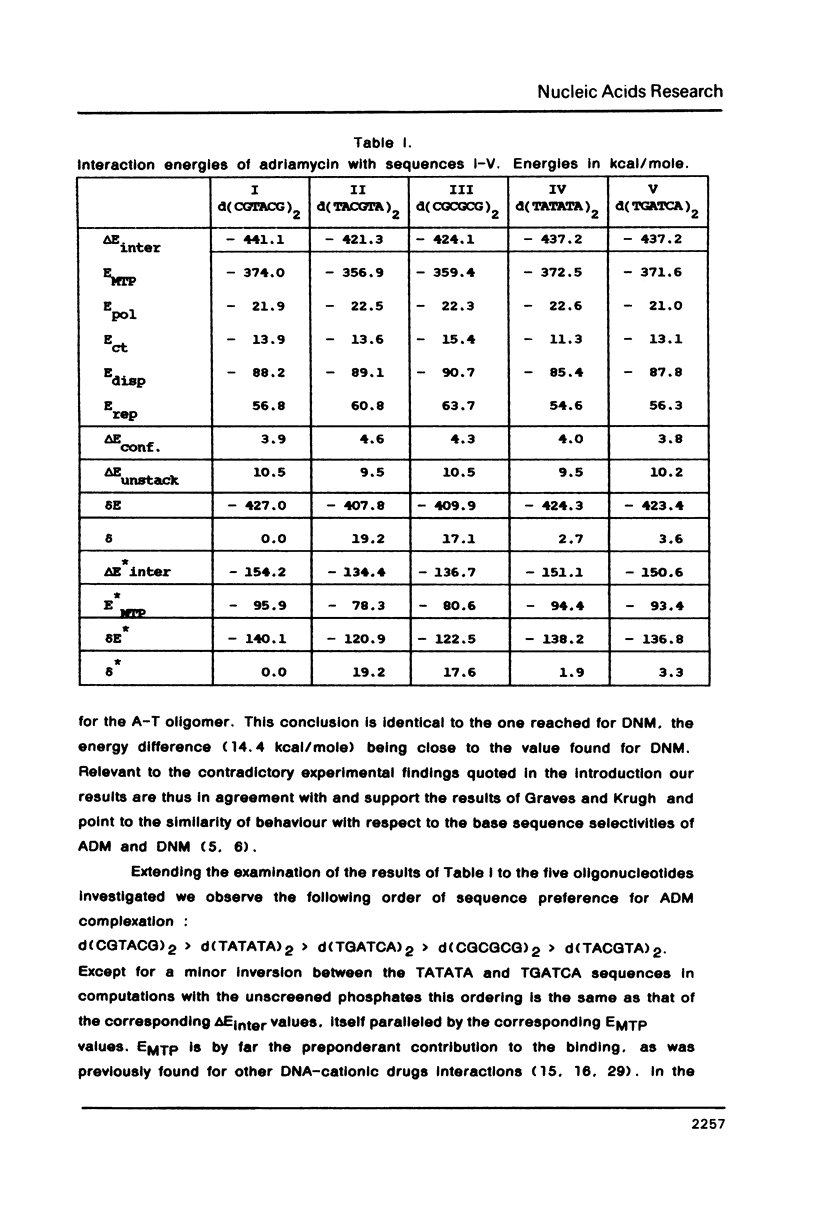
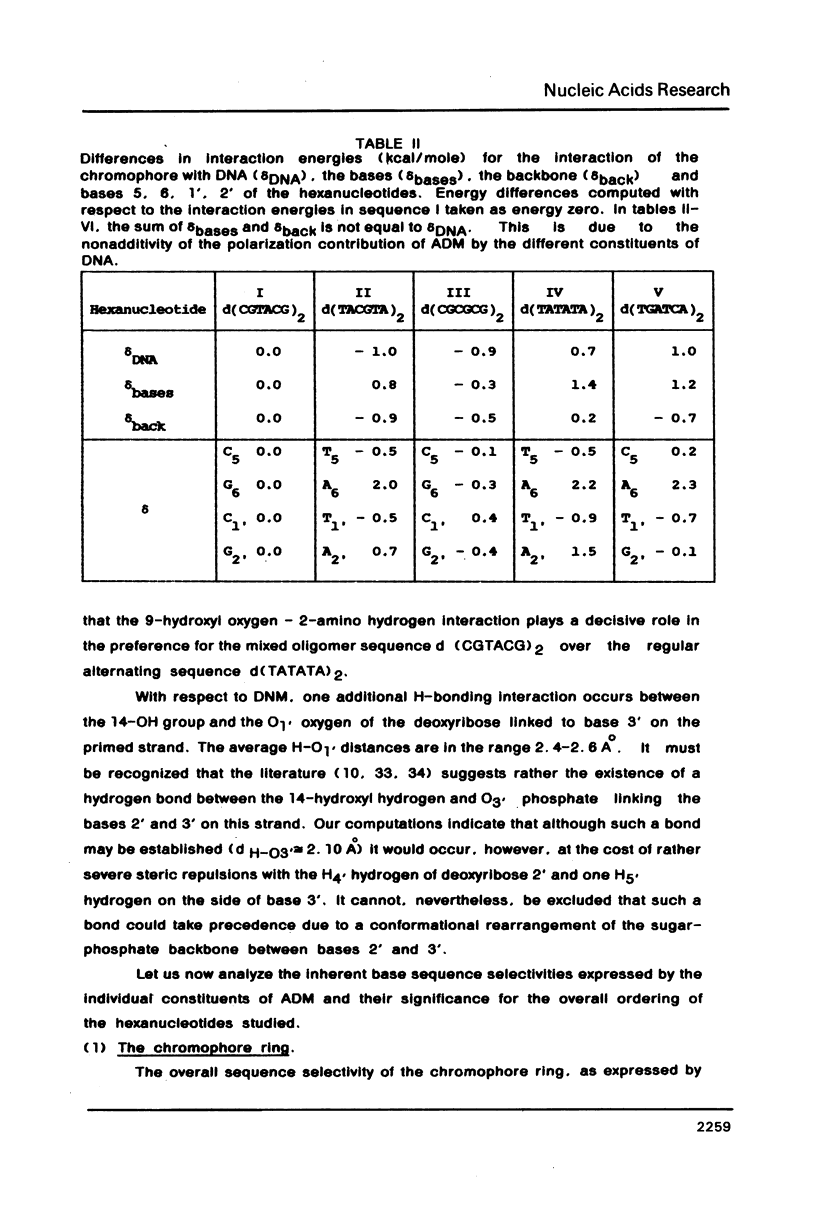
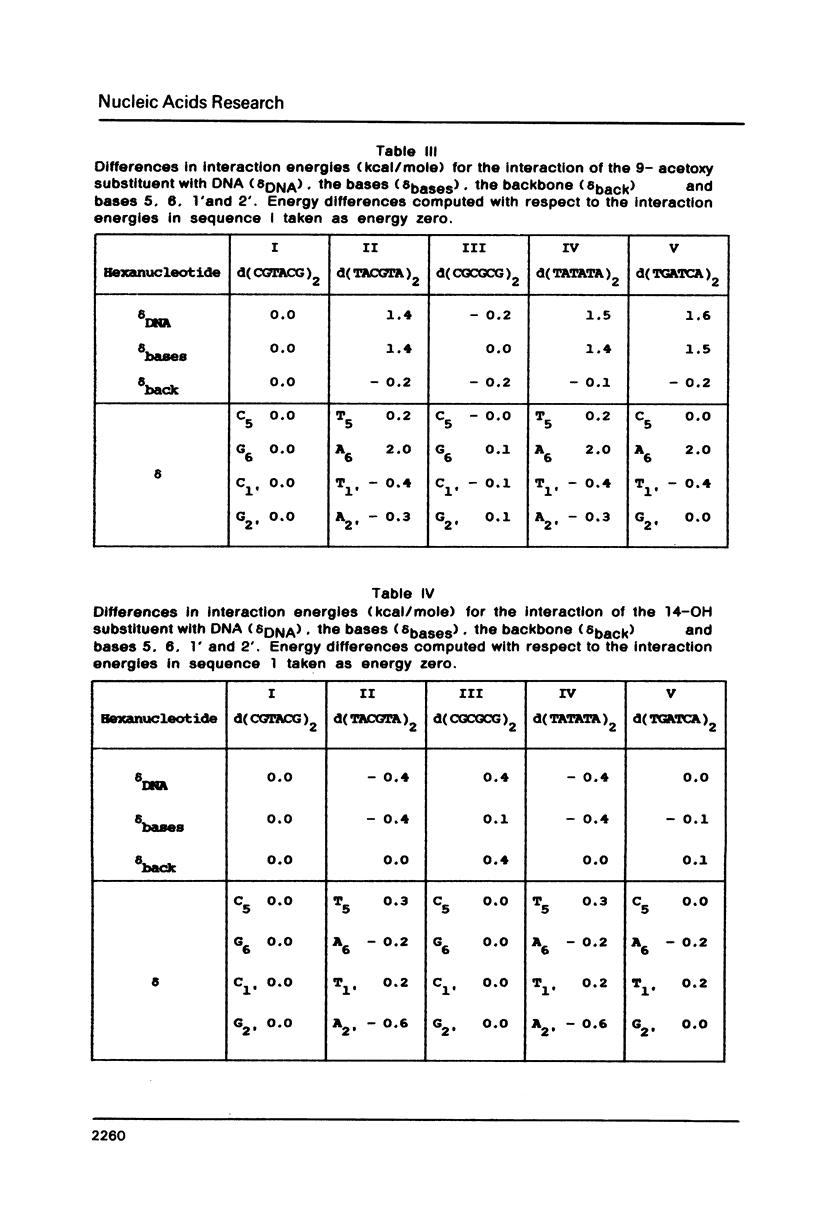
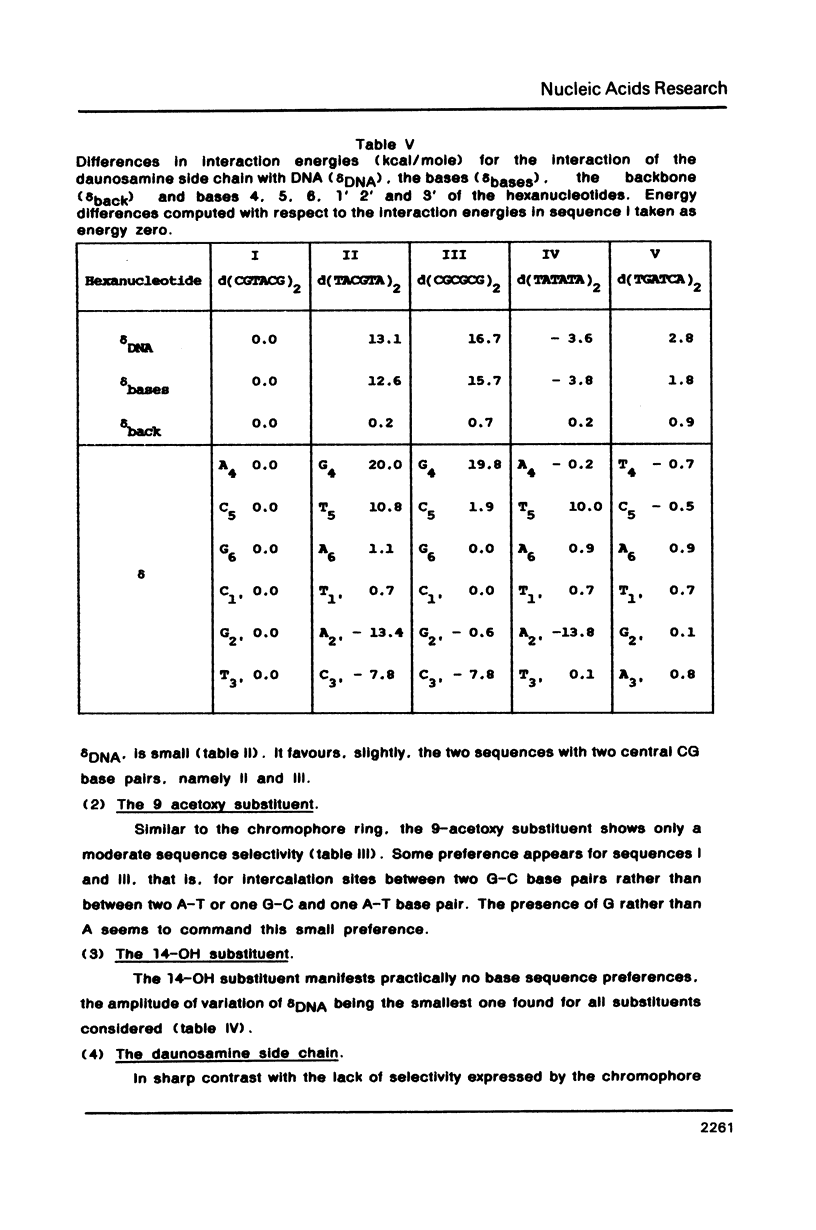
Theoretical computations are performed on the structural and energetical factors involved in the sequence selective binding of adriamycin (ADM) to five self-complementary double-stranded hexanucleotides. Among the two regularly alternating hexanucleotides d (TATATA)2 and d (CGCGCG)2, a stronger binding is predicted for the former. The strongest complex is computed, however, for the mixed hexanucleotide d (CGTACG)2, containing the intercalation site between two CG base pairs and an adjacent TA base pair. The overall sequence preference is the result of an intricate interplay of sequence preferences of the constituents in particular of daunosamine and the 9-OH substituent. Altogether, the selective base pair recognition by adriamycin cannot be defined in terms of the two base pairs implicated in the intercalation site alone but must be expressed in terms of a triplet of base pairs.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Marco A., Casazza A. M., Dasdia T., Necco A., Pratesi G., Rivolta P., Velcich A., Zaccara A., Zunino F. Changes of activity of daunorubicin, adriamycin and stereoisomers following the introduction or removal of hydroxyl groups in the amino sugar moiety. Chem Biol Interact. 1977 Dec;19(3):291–302. doi: 10.1016/0009-2797(77)90052-7. [DOI] [PubMed] [Google Scholar]
- Double J. C., Brown J. R. The interaction of aminoalkylaminoanthraquinones with deoxyribonucleic acid. J Pharm Pharmacol. 1975 Jul;27(7):502–507. doi: 10.1111/j.2042-7158.1975.tb09492.x. [DOI] [PubMed] [Google Scholar]
- DuVernay V. H., Jr, Pachter J. A., Crooke S. T. Deoxyribonucleic acid binding studies on several new anthracycline antitumor antibiotics. Sequence preference and structure--activity relationships of marcellomycin and its analogues as compared to adriamycin. Biochemistry. 1979 Sep 4;18(18):4024–4030. doi: 10.1021/bi00585a028. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Grier D., Fingerle R. E., Reimer R., Levy R., Pearce S. W., Wilson W. D. Interaction specificity of the anthracyclines with deoxyribonucleic acid. Biochemistry. 1976 May 18;15(10):2062–2070. doi: 10.1021/bi00655a006. [DOI] [PubMed] [Google Scholar]
- Graves D. E., Krugh T. R. Adriamycin and daunorubicin bind in a cooperative manner to deoxyribonucleic acid. Biochemistry. 1983 Aug 2;22(16):3941–3947. doi: 10.1021/bi00285a033. [DOI] [PubMed] [Google Scholar]
- Graves D. E., Krugh T. R. Adriamycin and daunorubicin bind in a cooperative manner to deoxyribonucleic acid. Biochemistry. 1983 Aug 2;22(16):3941–3947. doi: 10.1021/bi00285a033. [DOI] [PubMed] [Google Scholar]
- Gresh N., Pullman B. A theoretical study of the nonintercalative binding of berenil and stilbamidine to double-stranded (dA-dT)n oligomers. Mol Pharmacol. 1984 May;25(3):452–458. [PubMed] [Google Scholar]
- Gresh N. Theoretical study of the binding of aliphatic diamines to the minor groove of a B-DNA (dA-dT)11 oligomer. Biopolymers. 1985 Aug;24(8):1527–1542. doi: 10.1002/bip.360240809. [DOI] [PubMed] [Google Scholar]
- Manfait M., Alix A. J., Jeannesson P., Jardillier J. C., Theophanides T. Interaction of adriamycin with DNA as studied by resonance Raman spectroscopy. Nucleic Acids Res. 1982 Jun 25;10(12):3803–3816. doi: 10.1093/nar/10.12.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle S., Taylor G. Nucleic acid binding drugs. Part IV. The crystal structure of the anti-cancer agent daunomycin. Biochim Biophys Acta. 1977 Dec 14;479(4):450–459. doi: 10.1016/0005-2787(77)90038-7. [DOI] [PubMed] [Google Scholar]
- Newlin D. D., Miller K. J., Pilch D. F. Interactions of molecules with nucleic acids. VII. Intercalation and T.A specificity of daunomycin in DNA. Biopolymers. 1984 Jan;23(1):139–158. doi: 10.1002/bip.360230111. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L., Rein R. Energetic and structural aspects of ethidium cation intercalation into DNA minihelices. Biopolymers. 1979 Nov;18(11):2821–2847. doi: 10.1002/bip.1979.360181112. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L., Rein R. Energetics of intercalation specificity. I. Backbone unwinding. Biopolymers. 1979 May;18(5):1277–1291. doi: 10.1002/bip.1979.360180517. [DOI] [PubMed] [Google Scholar]
- Pack G. R., Loew G. Origins of the specificity in the intercalation of ethidium into nucleic acids. A theoretical analysis. Biochim Biophys Acta. 1978 Jun 22;519(1):163–172. doi: 10.1016/0005-2787(78)90070-9. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Rice J. A. Hydrogen bonding, overlap geometry, and sequence specificity in anthracycline antitumor antibiotic.DNA complexes in solution. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3333–3337. doi: 10.1073/pnas.78.6.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman L. F., Simpkins H. The differential effects produced by daunomycin and adriamycin on RNA, polynucleotides, single stranded, supercoiled DNA, and nucleosomes. Biochem Biophys Res Commun. 1985 Sep 16;131(2):1033–1040. doi: 10.1016/0006-291x(85)91343-9. [DOI] [PubMed] [Google Scholar]
- Pigram W. J., Fuller W., Hamilton L. D. Stereochemistry of intercalation: interaction of daunomycin with DNA. Nat New Biol. 1972 Jan 5;235(53):17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- Pullman T. M. The effectiveness of pit and fissure sealants. J Mich Dent Assoc. 1983 Feb;65(2):91–93. [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K. C., Yip K. F. Effect of deoxyribonuclease on adriamycin-polynucleotide complexes. Cancer Res. 1976 Sep;36(9 PT1):3367–3373. [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Woo N. H., Seeman N. C., Rich A. Crystal structure of putrescine diphosphate: a model system for amine-nucleic acid interactions. Biopolymers. 1979 Mar;18(3):539–552. doi: 10.1002/bip.1979.360180306. [DOI] [PubMed] [Google Scholar]
- Yonekawa H., Gotoh O., Motohashi J., Hayashi J. I., Tagashira Y. Positioning of the A . T-rich regions in rat mitochondrial DNA by electron microscopy and analysis of the hysteresis of denaturation. Biochim Biophys Acta. 1978 Dec 21;521(2):510–519. doi: 10.1016/0005-2787(78)90293-9. [DOI] [PubMed] [Google Scholar]
- Zakrzewska K., Lavery R., Pullman B. Theoretical studies of the selective binding to DNA of two non-intercalating ligands: netropsin and SN 18071. Nucleic Acids Res. 1983 Dec 20;11(24):8825–8839. doi: 10.1093/nar/11.24.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehl U., Förster W. Prostaglandin I2 (PG I2) fails to inhibit ADP induced platelet aggregation in vivo in rats. Prostaglandins Leukot Med. 1984 Oct;16(1):45–56. doi: 10.1016/0262-1746(84)90085-4. [DOI] [PubMed] [Google Scholar]
- Zunino F., Di Marco A., Zaccara A., Gambetta R. A. The interaction of daunorubicin and doxorubicin with DNA and chromatin. Biochim Biophys Acta. 1980 Apr 30;607(2):206–214. doi: 10.1016/0005-2787(80)90073-8. [DOI] [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Di Marco A., Zaccara A. Interaction of daunomycin and its derivatives with DNA. Biochim Biophys Acta. 1972 Sep 14;277(3):489–498. doi: 10.1016/0005-2787(72)90092-5. [DOI] [PubMed] [Google Scholar]



