Abstract
Rationale
Beta-adrenergic receptor (β-AR) stimulation produces sarcoplasmic reticulum (SR) Ca2+ overload and delayed after-depolarizations (DADs) in isolated ventricular myocytes. How DADs are synchronized to overcome the source-sink mismatch and produce focal arrhythmia in the intact heart remains unknown.
Objective
To determine if local β-AR stimulation produces spatio-temporal synchronization of DADs and to examine the effects of tissue geometry and cell-cell coupling on the induction of focal arrhythmia.
Methods and Results
Simultaneous optical mapping of transmembrane potential (Vm) and Ca2+ transients (CaT) was performed in normal rabbit hearts during subepicardial injections (50µL) of norepinephrine (NE) or control (normal Tyrodes). Local NE produced premature ventricular complexes (PVCs) from the injection site, which were dose-dependent (low-dose [30–60µM]: 0.45±0.62 vs. high-dose [125–250µM]: 1.33±1.46 PVCs/injection, p<0.0001) and were inhibited by propranolol. NE-induced PVCs exhibited abnormal Vm-Ca2+ delay at the initiation site, and were inhibited by either SERCA inhibition or reduced perfusate [Ca2+], indicating a Ca2+-mediated mechanism. NE-induced PVCs were more common at RV vs. LV sites (1.48±1.50 vs. 0.55±0.89, p<0.01) which was unchanged following chemical ablation of endocardial Purkinje fibers, suggesting source-sink interactions may contribute to the greater propensity to RV PVCs. Partial gap junction uncoupling with carbenoxolone (25µM) increased focal activity (2.18±1.43 vs. 1.33±1.46 PVCs/injection, p<0.05), further supporting source-sink balance as a critical mediator of Ca2+-induced PVCs.
Conclusions
These data provide the first experimental demonstration that localized β-AR stimulation produces spatio-temporal synchronization of SR Ca2+ overload and release in the intact heart and highlight the critical nature of source-sink balance in initiating focal arrhythmias.
Keywords: arrhythmia, mapping, norepinephrine, sarcoplasmic reticulum
Introduction
Sudden cardiac death (SCD) due to ventricular arrhythmias is a major cause of death in patients with heart failure (HF). Despite decades of research, anti-arrhythmic therapy has failed to reduce this risk.1 Beta-adrenergic receptor (β-AR) antagonists are a cornerstone of HF therapy2 and one of the few treatments to reduce SCD.3 However, the role of β-AR stimulation in arrhythmogenesis is poorly understood. In isolated failing ventricular myocytes, β-AR stimulation produces increased sarcoplasmic reticulum (SR) Ca2+ load, spontaneous SR Ca2+ release, delayed after-depolarizations (DADs) and triggered activity,4,5 providing a potential mechanistic link between β-AR stimulation and focal ventricular arrhythmia.6,7 However, DADs in isolated cells cannot be directly extrapolated to focal arrhythmias in the intact heart where strong electrotonic coupling to the surrounding myocardium acts as a ‘sink’ for local depolarizing current.8–10 Thus, a DAD occurring in a single cell (the ‘source’) cannot overcome the source-sink mismatch to produce propagating ectopic activity.11,12 In order for a DAD to produce focal activity in the intact heart, SR Ca2+ release must be synchronized across many cells to produce sufficient depolarizing current to overcome the source-sink mismatch. Indeed, mathematical models predict that in well-coupled tissue, synchronized SR Ca2+ release must occur in many thousands of myocytes to generate a single premature ventricular complex (PVC).12 The central questions as to how these cellular events are synchronized, both in space and time, and how many cells must be synchronized to produce focal arrhythmia in the intact heart remain unanswered.
We hypothesized that localized β-AR stimulation could produce spatio-temporal DAD synchronization in the intact heart. Experimental evidence suggests that hetero-geneous β-AR stimulation can occur in HF,13 as sympathetic nerve sprouting and remodeling,14 regional hyperinnervation,15,16 and differential release of the neurotransmitter norepinephrine (NE)17 have all been identified. Activation of β-AR by NE increases SR Ca2+ load and thus increases the likelihood of spontaneous SR Ca2+ release. Therefore, localized β-AR stimulation may produce spatial synchronization of SR Ca2+ overload among neighboring myocytes. Temporal synchronization of the NE-induced SR Ca2+ release is dictated by the timing of the preceding action potential (AP), Ca2+ transient (CaT) and recovery from refractoriness of ryanodine receptors (RyR).18,19 This combined spatio-temporal synchronization of SR Ca2+ overload and release is critical for producing a tissue-level DAD in the intact heart. If the DAD magnitude and local depolarizing current are large enough, the source-sink mismatch will be overcome and focal arrhythmia initiated.
We further hypothesized that, due to the critical nature of source-sink interactions in this scenario, tissue geometry and the degree of cell-cell coupling would contribute to focal arrhythmia propensity in response to localized β-AR stimulation. In order to test these hypotheses, simultaneous optical mapping of transmembrane potential (Vm) and CaT was performed during local subepicardial application of NE in normal rabbit hearts. The propensity of localized NE to initiate focal arrhythmia was quantified and the effects of tissue geometry and cell-cell coupling on the inducibility of PVCs were examined.
Methods
An expanded Methods section is available in the Online Data Supplement. All procedures involving animals were approved by the Animal Care and Use Committee of the University of California, Davis and adhered to the NIH Guide for the Care and Use of Laboratory Animals. Male New Zealand White rabbits (n=29) were anesthetized with an intravenous injection of pentobarbital sodium (50mg/kg). Hearts were excised, Langendorff-perfused at 37°C and loaded with RH237 and Rhod-2AM (Molecular Probes, Eugene, OR) for simultaneous fluorescent imaging of Vm and intracellular Ca2+. An electrocardiogram (ECG) was continuously recorded and pacing was from the basal left ventricle (LV). Blebbistatin (Tocris Bioscience, Ellisville, MO; 10–20µM) was used to eliminate motion artifact during optical recordings.20 Fluorescent signals were recorded using two CMOS cameras (MiCam Ultima-L, SciMedia) with a sampling rate of 1kHz and 100×100 pixels with a 35x35mm field of view.
Subepicardial injections were delivered via 30G needles with a 90° bend 1.5mm from the tip. Injections of normal Tyrodes (NT, 50µL: control) and NE (50µL, low-dose [30–60µM] or high-dose [125–250µM]) were delivered at different anatomical locations (LV base/LV apex/RV base/RV apex) in 15 hearts. In a subset of hearts (n=8), co-injection of NE and the fluorophore rhodamine-6G (R6G, 50µM, 528/547nm ex/em, Sigma, St. Louis, MO) was performed to visualize the epicardial area and transmural depth of tissue exposed to NE. In another subset (n=8 hearts), 25µM carbenoxolone (CBX) was added to produce partial gap junction (GJ) uncoupling. A further 14 hearts were used to study the effect of the β-AR antagonist propranolol (5–10µM, n=3), low perfusate [Ca2+] (0.33mM, n=3), the SR Ca2+-ATPase (SERCA2a) inhibitor cyclopiazonic acid (CPA, 30µM, n=2) and ablation of the RV endocardium with Lugol’s solution (n=3) on the occurrence of NE-induced PVCs. Local caffeine injections (10–40mM, 50µL, n=4) and global perfusion of isoproterenol (1µM, n=4) were also studied.
Data analysis was performed using two analysis programs (BV_Analyze, Brainvision, Japan; and Optiq, Cairn, UK). Activation time was determined as 50% rise time. For APs, repolarization time at 80% return to baseline was used to calculate action potential duration (APD80). For CaTs, duration was measured at 50% (CaTD50) and the time course of decay was quantified using the time constant (τ) of a single exponential fit of the decline (30–100%).21 Vm activation time was subtracted from Ca2+ activation time to produce maps of Vm-Ca2+ delay. Phase plots of the Vm/Ca2+ relationship were generated by plotting the normalized Vm values (x-axis) against the normalized Ca2+ values (y-axis) for the time course of a single AP, where counterclockwise chirality indicates normal Vm-Ca2+ coupling, and clockwise chirality indicates abnormal Vm-Ca2+ coupling.22 Conduction velocity (CV) was calculated as in Bayly et al.23 Continuous variables are presented as mean±SD. Comparisons between two groups of continuous data were made using a Student’s t-test, paired where appropriate, and categorical data using a Fisher’s exact test. Multiple comparisons were made using one- or two-way analysis of variance (ANOVA) with Bonferroni’s post-testing. P<0.05 was considered statistically significant.
Results
Local NE application induces focal arrhythmia
Baseline electrophysiological and Ca2+-handling parameters before injection and following complete washout of NE are detailed in the Online Data Supplement and in Supplemental Figure I. Local NE application resulted in PVCs in 15/15 hearts, whereas control injections (NT) produced PVCs in 4/13 hearts (p<0.001). Examples of focal arrhythmia induced by local NE application are shown in Figure 1A. PVCs are characterized by a broad QRS complex and ventriculo-atrial activation sequence, and arise from the injection site. Summary data are shown in Figure 1B. A range of NE doses were examined before grouping the data into high- and low-dose for statistical analysis (Supplemental Figure II). High-dose NE produced significantly more PVCs than NT (NE-high: 1.33±1.46 vs. NT: 0.17±0.43 PVCs/injection, p<0.0001, Figure 1B (i)) and a significantly higher proportion of NE applications resulted in at least one PVC (NE-high: 25/40 [63%] vs. NT: 8/48 [17%], p<0.001, Figure 1B (ii)). Low dose NE gave intermediate responses (NE-low: 0.45±0.62 vs. NE-high: 1.33±1.46 PVCs/injection, p<0.01, Figure 1B (i) and NE-low: 12/31 [39%] vs. NE-high: 25/40 [63%], p=0.058, Figure 1B (ii)).
Figure 1.
A) (i) ECG (black), atrial (red) and ventricular (blue) optical APs following subepicardial injection of 250µM norepinephrine (NE). Vm activation maps showing focal activation from the injection site. (ii) Alternate injection site. B) (i) PVCs/injection and (ii) proportion of injections resulting in at least one PVC for normal Tyrodes’ (NT, n=48 [13 hearts]), low dose NE (n=31 [10 hearts]) and high dose NE (n=40 [9 hearts]). C) The effect of 5µM and 10µM propranolol (PP) on responses to high dose NE (n=4). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Because NE stimulates α- as well as β-AR, the β-AR antagonist propranolol (PP 5–10µM, n=3) was added to the perfusate (Figure 1C). PP reduced PVCs/injection (NE-high: 1.00±0.95, +5µM PP: 0.32±0.48, +10µM PP: 0.06±0.25, p<0.001) and PVC occurrence (NE-high: 62%, +5µM PP: 32%, +10µM PP: 6%, p<0.05) in a dose-dependent manner, suggesting that PVCs were due to β- rather than α-AR stimulation. The propensity to NT-induced PVCs was unchanged with PP (25% vs. 21%, p=NS).
Local NE-induced PVCs are Ca2+-mediated
To investigate whether focal activity was due to local SR Ca2+ release, maps of Vm-Ca2+ delay were constructed. Spatial patterns of Vm-Ca2+ delay during NE-induced PVCs were compared to sinus rhythm, ventricular pacing and NT-induced PVCs, as shown in Figure 2. Mean epicardial Vm-Ca2+ delay was similar during sinus rhythm (7.04±0.94ms), ventricular pacing (7.02±0.95ms) and NT-induced PVCs (7.39±1.14ms, p=0.65). The presence of local NE did not shorten Vm-Ca2+ delay during sinus rhythm (Supplemental Figure IIIA) nor did Vm-Ca2+ delay exhibit restitution during shortening of coupling interval (Supplemental Figure IIIB). There were no significant differences in Vm rise times for sinus (14.1±4.1ms), pacing (18.4±4.0ms), NT-induced PVCs (17.0±2.6ms) or NE-induced PVCs (17.9±2.2ms).
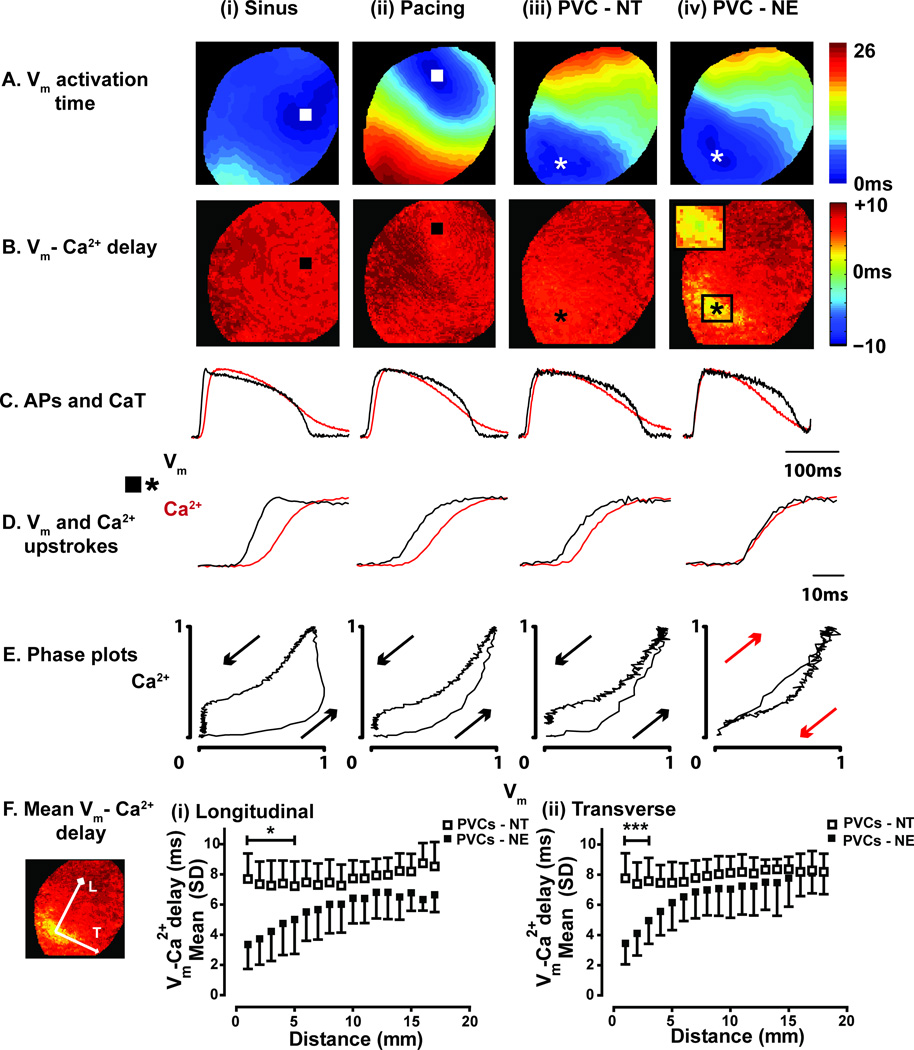
Figure 2.
A) Vm activation maps during (i) sinus rhythm, (ii) ventricular pacing, (iii) NT-induced PVC, (iv) NE-induced PVC. B) Maps of Vm-Ca2+ delay indicate homogeneous Vm-Ca2+ delay during sinus rhythm, pacing, and NT-induced PVCs. During NE-induced PVCs, Vm-Ca2+ delay is short at the site of earliest Vm activation. C) Optical APs (black) and CaTs (red) from the earliest activated sites. D) Vm and Ca2+ upstrokes from the earliest activated sites. E) Phase plots from the traces shown in C. Chirality is reversed during the NE-induced PVC. F) Mean Vm-Ca2+ delay as a function of distance from the earliest activation site along the (i) longitudinal and (ii) transverse axes of conduction. * p<0.05, ***p<0.001.
During normal excitation-contraction (EC) coupling, Ca2+ activation followed Vm with a relatively uniform delay across the epicardial surface (Figure 2B (i)-(iii)). However, during the majority of NE-induced PVCs, areas of abnormally short Vm-Ca2+ delay were evident at the site of earliest Vm activation (Figure 2B (iv)). APs and CaTs sampled from the site of earliest Vm activation (Figure 2C (iv)) demonstrate near-simultaneous Vm and Ca2+ upstrokes (Figure 2D (iv)) and reversed chirality of the Vm-Ca2+ phase plot (Figure 2E (iv)). The mean Vm-Ca2+ delay over distance from the earliest activation site for NT- and NE-induced PVCs are shown in Figure 2F and demonstrate the significantly shorter Vm-Ca2+ delay associated with NE-induced PVCs compared to NT-induced PVCs.
Simultaneous Vm/Ca2+ upstroke profiles are consistent with APs induced by SR Ca2+ release in isolated myocytes,24 in which caffeine (10mM) was used to rapidly activate RyR opening, and changes in Vm occurred instantaneously upon SR Ca2+ release. As subsarcolemmal Ca2+ instantaneously drives inward Na+/Ca2+ exchange (NCX) current, Vm/Ca2+ upstrokes are simultaneous.24 To test whether the same phenomenon occurs in the intact heart, local injections of caffeine were applied (Figure 3A-C). Local caffeine-induced PVCs displayed simultaneous Vm/Ca2+ upstrokes, exactly as observed during NE-induced PVCs. Corresponding negative control experiments were also performed, in which the probability of SR Ca2+ overload and release was reduced, either by lowering perfusate [Ca2+] (0.33mM) or by administration of the SERCA2a inhibitor CPA (30µM). As shown in Figure 3D, in the presence of low [Ca2+] NE-induced PVCs were reduced (0.83±0.70 vs. 0.20±0.46 PVCs/application, p<0.0001; 67 vs. 16%, p<0.001). Partial SERCA inhibition with CPA (approximately 50% prolongation of τ, data not shown) also reduced NE-induced PVCs (1.21±0.80 vs. 0.42±0.66 PVCs/application, p<0.001; 87 vs. 50%, p<0.05, Figure 3E). In contrast, the occurrence of NT-induced PVCs was not changed by either intervention.
Figure 3.
Left panel – positive control using local caffeine injections. Maps of A) Vm activation time and B) Vm-Ca2+ delay during (i) NE-induced PVC and (ii) caffeine-induced PVC (40mM). C) Vm and Ca2+ upstrokes from the earliest activated sites, showing near-simultaneous upstrokes in both cases. Right panel – negative control experiments with reduced SR Ca2+ load. D) PVC occurrence after reducing perfusate [Ca2+] to 0.33mM. E) PVC occurrence after 30µM CPA to partially inhibit SERCA2a activity. *p<0.05, ****p<0.0001.
Subcellular imaging has shown that spontaneous SR Ca2+ release events occur at characteristic times after the prior beat, depending on the recovery of SR Ca2+ content, SR Ca2+ load and RyR refractoriness,18,19 with a peak at 300–500ms.25 To assess whether this same temporal synchronization was evident for NE-induced PVCs, we measured the coupling interval for each PVC with respect to the previous beat (Supplemental Figure IIIC). The latencies of NE-induced PVCs cluster at coupling intervals of 300–400ms, consistent with a temporal synchronizing effect of RyR restitution. When PVCs were not elicited by NE, subthreshold Ca2+ elevation could be observed near the injection site (Supplemental Figure IV). This suggests that local NE application resulted in SR Ca2+ release from a group of cells, but in these cases was not large enough to initiate a PVC. Taken together, these data strongly implicate that focal activity during localized β-AR stimulation is mediated by local and relatively synchronous diastolic SR Ca2+ release.
RV sites are more prone to NE-induced PVCs
To determine whether differences in tissue geometry or electro�physiological characteristics may play a role in the genesis of focal arrhythmia, PVC inducibility was compared across different sites. There were no significant differences in PVC inducibility between apex and base (NE-high-apex: 1.29±1.54 vs. NE-high-base: 1.35±1.47 PVCs/injection, p=NS; NE-high-apex: 64% vs. NE-high-base: 62%, p=NS). In contrast, local NE in the RV produced more PVCs than in the LV (NE-high-RV:1.94±1.66 vs. LV:0.82±1.10 PVCs/injection, p<0.001, Figure 4A), and a higher proportion of NE injections resulted in at least one PVC (81% vs. 50%, p<0.05). Similar RV/LV trends were observed for low-dose NE. To determine if the differences in PVC inducibility between LV and RV were due to underlying electrophysiological heterogeneity, AP and CaT parameters were compared. There were no differences in CV, AP rise time, APD80, dispersion of APD80 or CaT dynamics between LV and RV (Table 1). To determine whether functional heterogeneity in the response to β-AR stimulation may exist between the ventricles, 1µM ISO was added globally. As shown in Table 1, there were no differences between LV and RV in the response to global β-AR stimulation with ISO.
Figure 4.
A) PVCs/injection in LV and RV. ***p<0.001 vs. LV. B) Vm activation maps (i) and R6G fluorescence (ii) following co-injection of NE and R6G. C) PVC probability at LV and RV sites as a function of NE tissue exposure. RV injection sites had a higher probability of PVC induction. r2: LV, 0.46, RV, 0.88; regression slopes: LV, 0.0021±0.0008, p<0.05 and RV, 0.0025±0.0005, p<0.05, p=NS, y-axis intercepts: LV, 0.03±0.19 vs. RV, 0.33±0.12, * p<0.05. D) Transmural histological sections of: (i) LV and (ii) RV injection sites with R6G fluorescence in green.
Table 1.
AP and CaT characteristics
| Mean±SD | LV | RV | P |
|---|---|---|---|
| Baseline | n=11 | n=11 | |
| APD80 (ms) | 185.3±18.6 | 183.3±17.7 | NS |
| APD80 dispersion (ms/mm2) | 0.23±0.21 | 0.22±0.17 | NS |
| AP rise time (ms) | 13.8±3.2 | 14.2±2.4 | NS |
| Conduction velocity (cm/s) | 58.5±9.7 | 56.8±12.3 | NS |
| CaT rise time (ms) | 20.4±1.5 | 21.3±2.4 | NS |
| CaT decay (τ, ms) | 65.5±9.8 | 66.3±7.9 | NS |
| Global ISO | n=4 | n=4 | |
| CaT rise time (ms) | 18.5±2.8 | 17.4±1.5 | NS |
| CaT decay (τ, ms) | 42.3±10.9 | 41.4±5.9 | NS |
AP= action potential, APD80=AP duration at 80% repolarization, CaT=calcium transient
Differences in tissue structure and local vasculature may result in non-uniform NE diffusion within tissue and may contribute to the regional differences in PVC propensity. In order to address this, the fluorophore R6G was added to NE to visualize the area of myocardium exposed. Figure 4B shows Vm activation time and R6G fluorescence following co-injection of NE and R6G. PVCs arise from the injection site and R6G fluorescence indicates the spatial extent of NE exposure. The area of epicardial tissue exposed to NE was correlated with PVC inducibility (Figure 4C). Both LV and RV injection sites displayed a similar increase in PVC probability with increasing NE exposure area (regression slopes: LV, 0.0021±0.0008, p<0.05 and RV, 0.0025±0.0005, p<0.05, LV vs. RV p=NS). However, for RV sites, PVC probability was higher across the range of exposure areas measured (y-axis intercepts: LV, 0.03±0.19 vs. RV, 0.33±0.12, p<0.05). Transmural histological sections at the injection site were then imaged to determine if the depth of NE exposure could account for the difference in PVC inducibility. Figure 4D shows LV and RV injection sites with similar epicardial areas of R6G fluorescence. In the LV, the R6G fluorescence extends to the midmyo�cardium, whereas in the RV, the R6G exposure is fully transmural. These differences in exposure may result in less source-sink mismatch in the RV compared to the LV (~2- vs. 3-dimensional), and therefore higher PVC probability.
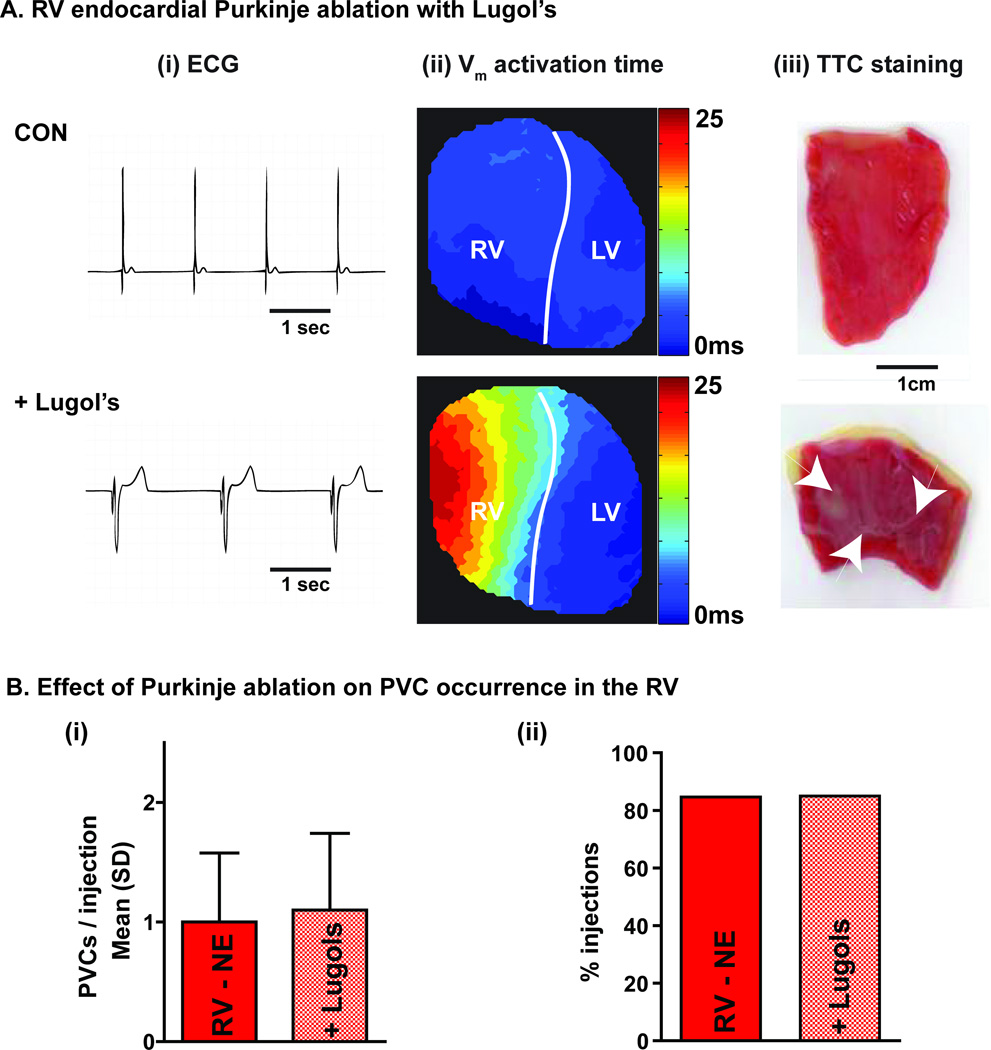
In order to investigate whether transmural NE exposure in the RV was activating endocardial Purkinje fibers to initiate focal activity, NE injections were repeated before and after RV endocardial chemical ablation with Lugol’s solution. As shown in Figure 5A, Lugol’s produced QRS prolongation and delayed RV activation during sinus rhythm, consistent with disruption of the Purkinje network, which was subsequently confirmed with triphenyl tetrazolium chloride (TTC) staining. The occurrence of NE-induced PVCs in the RV was not affected by chemical ablation of the endocardium (1.0±0.6 vs. 1.1±0.6 PVCs/injection, p=NS; 85 vs. 85%, p=NS, Figure 5B), suggesting that the Purkinje fibers are not playing a large role in initiating focal activity during local β-AR stimulation.
Figure 5.
A) RV endocardial ablation with Lugol’s solution. (i) ECG, (ii) Vm activation map and (iii) endocardial TTC staining before (CON) and after (+Lugol’s) RV endocardial Lugol’s. Purkinje ablation resulted in QRS prolongation and delayed RV epicardial activation. Tissue ablation was confirmed by the white appearance on TTC. B) Occurrence of PVCs was not affected by endocardial ablation.
Partial gap junction uncoupling promotes NE-induced PVCs
To further investigate the role of source-sink interactions, NE application was repeated in the presence of CBX to induce partial GJ uncoupling. 25µM CBX resulted in a ~25% decrease in CV in both the longitudinal (CVL) and transverse (CVT) directions (CVL 58.7±4.2 vs. 38.5±3.1cm/s, p<0.001; CVT 43.9±3.2 vs. 28.2±3.1cm/s, p<0.001) (Supplemental Figure V). The addition of CBX did not affect CaTD50 (140±9 vs.141±10ms, p=0.76) or Vm-Ca2+ delay during sinus rhythm (7.53±1.41 vs. 7.04±0.94ms, p=0.47) or ventricular pacing (7.12±1.58 vs. 7.02±0.95ms, p=0.89). With CBX, greater numbers of PVCs were induced by both low-dose NE (CBX: 1.48±1.33 vs. control: 0.45±0.62 PVCs/injection, p<0.01) and high-dose NE (CBX: 2.18±1.43 vs. control: 1.33±1.46 PVCs/injection, p<0.05) and a higher proportion of NE injections resulted in at least one PVC (NE-low: 76 vs. 39%, p<0.05; NE-high: 88 vs. 63%, p=0.06) (Figure 6).
Figure 6.
A) Number of PVCs/injection and B) proportion of injections resulting in at least one PVC for low (grey) and high dose NE (black) under control conditions (solid bars) and with CBX (hatched bars). *p<0.05 vs. control, **p<0.01 vs. control.
Source-sink interactions mediate the production of NE-induced PVCs
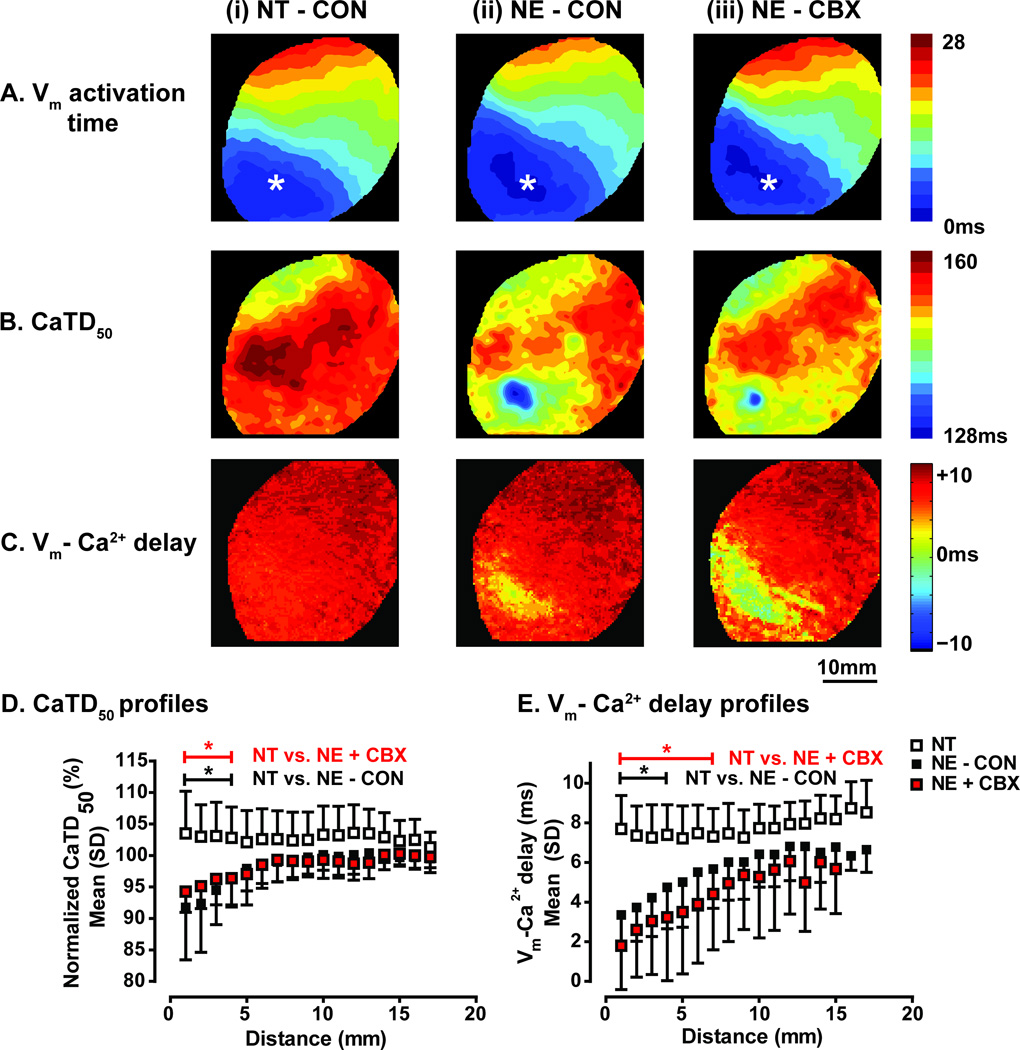
To determine the mechanism of increased propensity to focal arrhythmia during partial GJ uncoupling, the changes in Vm-Ca2+ dynamics were examined (Figure 7). β-AR activation produces more rapid SR Ca2+ uptake and thus faster CaT decline. Shortening of CaTD50 around the injection site was observed during NE- but not NT-induced PVCs (Figure 7B). This area of abbreviated CaTD50 can be used as a functional measure of the spatial extent of NE effects. However, due to electrotonic coupling, effects on Vm may not be seen across the same area. Thus, the area of abnormal Vm-Ca2+ delay was used as a functional estimate of the spatial extent over which Ca2+ release exerts an effect on Vm (Figure 7C). To quantify these parameters between hearts, normalized CaTD50 and Vm-Ca2+ delay during NT and NE-induced PVCs were plotted as a function of distance from the earliest Vm activation (Figure 7D and E, respectively [longitudinal direction only, transverse profiles shown in Supplemental Figure VI]). During NE-induced PVCs, CaTD50 was significantly shorter over the first 4mm, and this was unchanged with CBX (Figure 7D). Vm-Ca2+ delay was shorter over the first 4mm under control conditions, and this increased to 7mm with CBX (Figure 7E). Thus, after uncoupling, a larger effect on Vm is observed for the same NE effect on CaTD50. This is consistent with an increased propensity to Ca2+-mediated focal arrhythmia induced by NE during partial GJ uncoupling.
Figure 7.
A) Vm activation during (i) NT-induced PVC, (ii) NE-induced PVC and (iii) NE-induced PVC with CBX. B) Corresponding maps of CaTD50 and (C) Vm-Ca2+ delay. D) CaTD50 as a function of distance during NT-induced PVCs (n=11 [6 hearts]), NE-induced PVCs under control conditions (n=11 [7 hearts]) and NE-induced PVCs with CBX (n=10 [6 hearts]). During NE-induced PVCs, CaTD50 is significantly shorter (compared with NT-induced PVCs) for 4mm, and is not affected by CBX. E) Corresponding plot of Vm-Ca2+ delay. During NE-induced PVCs, Vm-Ca2+ delay is significantly shorter for 4mm in the longitudinal axis, increasing to 7mm with CBX. Similar results were observed in the transverse direction (Supplemental Figure VI). * p<0.05.
Discussion
This study tested the hypothesis that, in the intact heart, localized β-AR stimulation can provide spatio-temporal syn�chronization of SR Ca2+ release over many cells to overcome source-sink mismatch and produce focal arrhythmia. The results demonstrate that local NE application induces β-AR dependent, Ca2+-mediated focal arrhythmia in normal rabbit hearts. Quantification of Vm-Ca2+ dynamics established the mechanism of PVC induction. Abnormal Vm-Ca2+ delays were exclusively a feature of NE- and caffeine-induced PVCs, suggesting that focal arrhythmias induced by localized β-AR stimulation are mediated by SR Ca2+ overload and release. RV sites displayed a higher propensity to focal arrhythmia than LV. This difference could not be explained by electrophysiological differences or activation of Purkinje fibers and is thus likely due to the reduction in the source-sink mismatch in the thinner RV wall that more closely approximates a 2-dimensional geometry. Consistent with this finding, partial GJ uncoupling resulted in a reduction of the current sink and was associated with a higher propensity for focal arrhythmia in response to localized β-AR stimulation.
Short Vm-Ca2+ activation delay is indicative of Ca2+-mediated focal activity
During normal EC coupling, Vm-Ca2+ delay was 7–8ms, similar to that reported in rabbits22 and humans.26 In contrast, during NE-induced PVCs, significantly shorter Vm-Ca2+ delays (0–4ms) were observed at the initiation site. Such short Vm-Ca2+ delays were never observed during normal EC coupling, indicating that during NE-induced PVCs SR Ca2+ release likely started before the AP upstroke and opening of L-type Ca2+ channels. We tested the possibility that local NE application might accelerate Ca2+ release dynamics and abbreviate the Vm-Ca2+ delay during normal EC coupling. However, localized NE did not alter Vm-Ca2+ delay during sinus beats (Supplemental Figure IIIA). Vm-Ca2+ delay was also unaltered over a broad range of coupling intervals, ruling out EC coupling restitution kinetics as a cause of the abnormally short Vm-Ca2+ delay during NE-induced PVCs (Supplemental Figure IIIB). The observations that partial SERCA inhibition with CPA and low [Ca2+] perfusate reduced NE- but not NT-induced PVCs (Figure 3D-3E) as well as local intracellular Ca2+ rises during subthreshold NE application (Supplemental Figure IV) all support the conclusion that NE-induced PVCs are Ca2+-mediated. As short Vm-Ca2+ delays were only observed during NE and caffeine-induced PVCs, these data suggest that very short Vm-Ca2+ activation delays can be used as an indicator of Ca2+-mediated excitation.
In optical mapping studies of pharmacological QT prolongation22 and post-shock arrhythmias27 Ca2+ upstrokes preceding Vm upstrokes or slow diastolic Ca2+ elevation prior to Vm upstrokes have been reported. These models of Ca2+-mediated arrhythmia are characterized by ‘global’ SR Ca2+ overload and release, which results in gradual and spatially dyssynchronous Ca2+ elevation. In our experiments, we observed a different phenomenon, in that we saw rapid Ca2+ release in response to local NE. During NE- induced PVCs, Ca2+ and Vm upstrokes occurred nearly simultaneously at the site of earliest activation. This is highly analogous to rapid caffeine application in isolated cardiomyocytes, where synchronous SR Ca2+ release immediately activates NCX and depolarization occurs simultaneously with the Ca2+ rise.24 Indeed, local application of caffeine in the intact heart recapitulated these results (Figure 3A-C). Thus, although the simultaneous Vm/Ca2+ upstrokes reported here differ from those previously reported for Ca2+-mediated arrhythmia,22,27 this is likely attributable to the ‘local’ synchronous SR Ca2+ release produced in the current experiments as opposed to ‘global’ dyssynchronous Ca2+ release observed in other models.
Induction of a PVC requires temporal as well as spatial synchrony of SR Ca2+ release. We hypothesized that this temporal synchronization is dictated by the previous AP/CaT because of the time required for SR Ca2+ refilling and RyR restitution.18,19 Indeed, in Ca2+ overload conditions there is an increased amplitude, frequency and synchrony of Ca2+ waves.25 These factors increase the likelihood that neighboring Ca2+ overloaded myocytes will exhibit SR Ca2+ release in relative synchrony. We observed a clustering of NE-induced PVCs occurring 300–400ms after the previous beat (Supplemental Figure IIIC). In contrast, although the number of NT-induced PVCs was small, they appeared to show random coupling intervals. These data are consistent with a temporal synchronization of SR Ca2+ release in response to local NE through SR Ca2+ refilling and RyR restitution.
Regional heterogeneity in vulnerability to focal arrhythmia
Local β-AR stimulation was more likely to produce PVCs at RV than at LV sites. The finding of increased propensity to PVCs in the RV is congruent with clinical observations. Idiopathic VT/VF is often found to be triggered by PVCs arising from a focal RV origin, ablation of which has been shown to reduce subsequent VT/VF.28 Although PVC inducibility was related to area of epicardial NE exposure as assessed by co-injection of NE with fluorescent R6G (Figure 4C), differences in the area of NE exposure did not account for higher arrhythmia propensity in the RV. The mechanisms underlying the increased arrhythmogenic potential of the RV are not well understood. If differences in sympathetic drive exist, then this may play an important role. Differences in IK1 may influence the dominant frequency of VF in guinea-pig hearts.29 Although we did not detect significant interventricular electrophysiological or Ca2+ handling differences at baseline or during global β-AR stimulation (Table 1), it is possible that differences in specific channels, transporters (e.g. IK1, NCX, RyR), or β-ARs may contribute to this RV-LV difference. Varying degrees of interventricular heterogeneity in ion channel expression have been reported in different species,29,30 but optical mapping studies in intact rabbit hearts are congruent with our observations that no significant differences in overall electrophysiology exist between RV and LV at baseline.31
Another possible explanation for the RV/LV difference in PVC propensity may be that transmural NE exposure in the RV results in stimulation of the Purkinje network. As Purkinje fibers may be more prone to focal activity,27 activation with NE may result in greater PVC incidence in the RV. To test for this, we performed chemical ablation of the RV endocardial surface with Lugol’s solution and observed no significant difference in PVC propensity (Figure 5), suggesting that the higher incidence of PVCs in the RV is likely not attributable to Purkinje activation.
Tissue geometry and the source-sink balance
Another logical contributor to the observed RV/LV differences is inherent geometric source-sink balance. As illustrated in Figure 4D, for the same depth of NE exposure, the RV will experience 2-dimensional radial current sink around the circumference of the exposed area, whereas in the thicker-walled LV, the current sink is 3-dimensional. The importance of dimensionality of the surrounding current sink has been demonstrated in numerical simulations, which have estimated the number of cells with synchronized SR Ca2+ release required to produce a PVC.12 Xie et al calculated that in normal well-coupled 3D tissue, approximately 820,000 cells with synchronized SR Ca2+ release were required to produce a PVC, 100-fold more than were required in the equivalent 2D model.
In simulations, the total current source can be precisely controlled by changing the number of cells with synchronized SR Ca2+ release. In the present experiments, however, we can neither exactly measure nor systematically vary the total current source, but we can make estimates from functional measures of Vm-Ca2+ dynamics. One way to estimate the current source is to use the area over which local NE abbreviates CaTD (i.e. the area of tissue affected by NE). With strong electrotonic coupling, however, Vm is ‘clamped’ at the borders of this region, thus the alternative estimate, the area over which SR Ca2+ release drives depolarization (i.e. where Vm-Ca2+ delay is short) may be different. Using the dimensions of these functional measurements, along with anatomical surface and depth estimates from R6G imaging and applying them to a hemi-ellipse (surface radii, 0.5–5mm and depth of 1–2mm), the volume of current source estimated in the present study is 0.5–105mm3. Assuming a cell volume of 30pL32 and 30% extracellular space this equates to 12,200–2,400,000 cells. While this is a relatively crude estimate, the upper and lower limits are very similar to the estimates derived from the mathematical predictions (2D tissue ~8,000 cells and 3D tissue ~820,000 cells).12
The effect of partial GJ uncoupling
Partial GJ uncoupling with CBX resulted in a slowing of CV with preserved anisotropy ratio and APD, as has previously been reported in rabbit ventricular myocardium.33 Partial GJ uncoupling produced a two-fold increase in NE-induced PVCs (Figure 6). Partial uncoupling facilitates PVC propagation by ‘insulating’ the depolarized region (source) from the surrounding resting myocardium (sink),10 analogous to initiation of pacemaker activity in the sino-atrial node.34,35 In the case of local β-AR stimulation, a reduction in electrotonic current flow through GJ reduces the hyperpolarizing current from surrounding cells, which allows the current carried by NCX in response to SR Ca2+ release to have a larger effect on Vm. This is demonstrated by the significantly larger area of abnormally short Vm-Ca2+ delay observed following uncoupling (Figure 7E) despite a similar area of NE effect (Figure 7D). Given that the area of NE effect is not changed, the larger area of abnormal Vm-Ca2+ delay with CBX likely reflects poorer voltage clamp at the periphery, due to reduced coupling of the depolarizing source to the surrounding sink.
Local β-AR stimulation as a pathophysiological experimental paradigm
In the present study, we examined how localized β-AR stimulation produces spatio-temporal synchronization of SR Ca2+ overload and release across many cells to initiate focal arrhythmia. The data demonstrate that local NE induces Ca2+-mediated PVCs in intact hearts, providing a critical link from cellular studies of focal arrhythmia initiation via DADs to the tissue level. Intrasynaptic NE concentrations at vascular nerve terminals have been measured at up to 100µM, and an inverse relationship between intrasynaptic NE concentration and junctional space was observed.36 Because cardiac neuroeffector junctions are narrower, the NE concentrations used in the current study (30–250µM) may be similar to NE concentrations during physiological nerve activation, especially in HF when sympathetic drive is high. However, sympathetic stimulation does not typically result in PVCs in the normal rabbit or human heart and no exogenous NE application can fully recapitulate neuronal NE release.
Regionally heterogeneous sympathetic nerve sprouting, nerve remodeling, hyperinnervation13–15 and heterogeneous NE release17 all occur in HF. Focal arrhythmias are known to occur in animals and humans with HF due to dilated cardiomyopathy.6,7 Moreover, GJ uncoupling is a consistent feature of the electrophysiological phenotype in failing hearts.37, 38 Therefore, subepicardial NE injection is a useful model of the heterogeneous β-AR stimulation which occurs in HF and localized β-AR stimulation in the presence of GJ uncoupling represents a pathophysiologically relevant paradigm of focal arrhythmia induction. This is the first experimental study to determine a mechanistic link between altered local sympathetic stimulation and the generation of focal arrhythmia in the intact heart. Future studies should examine whether other features of HF remodeling also potentiate focal arrhythmias induced by local β-AR stimulation.
Study limitations
A small number of PVCs were induced by NT, likely due to activation of stretch-activated channels.39 As demonstrated in Figures 2 and 7, these were clearly distinguishable from NE-induced PVCs on the basis of Vm-Ca2+ delay. R6G was used to indicate the area of NE exposure and, as the two have similar molecular weight (319 vs. 479), we expect that the diffusion kinetics in tissue may be similar. However, R6G40 is more lipophilic41 and is not taken up by nerve terminals, thus does not demonstrate the time course of NE clearance from the tissue. There was variability in the area of tissue exposed to NE despite constant injection volume. The different patterns of R6G staining, both on the epicardium and transmurally, suggest this may be due to differences in local tissue architecture and microvasculature. Additional effects of β-AR stimulation (e.g. left-shift in Ca2+ current voltage-dependence, altered RyR properties), may further promote DADs and the development of focal arrhythmias, and these issues merit further study. In the current study, we did not determine the exact delay from NE injection to PVC appearance, partly because in dual Vm-Ca2+ imaging we could only record for several seconds. However, the first PVC typically occurred a few seconds after NE injection (as in Fig 1Ai and ii), consistent with the expectation that several beats would be required for β-AR activation to elevate SR Ca2+ load and release. As discussed above, focal epicardial application of NE only recapitulates some aspects of neuronal NE release, but does allow control and evaluation of exposed area. In future studies it would be valuable to assess the detailed time course of NE-induced PVCs and also to compare the responses to local injections to chemical or electrical stimulation of endogenous NE release.
Conclusions
Localized β-AR stimulation produced spatio-temporal synchronization of SR Ca2+ overload and release across many cells to produce focal activity in normal rabbit hearts. Source-sink interactions were found to be critically important in the generation of Ca2+-mediated focal arrhythmias. These data provide: (1) the first experimental demonstration of localized β-AR stimulation as a pathophysiologically relevant mechanism of focal arrhythmia initiation; (2) the first experimental evidence of a mechanistic link between sympathetic dysfunction and the generation of focal arrhythmias in the intact heart; (3) the first experimental estimation of the number of cells with synchronized SR Ca2+ release required to initiate focal arrhythmia and (4) a demonstration of the critical nature of the source-sink balance in the initiation of focal arrhythmias in the intact heart.
Supplementary Material
Novelty and significance.
What is known?
In isolated cardiac myocytes, β-adrenergic stimulation can cause spontaneous Ca2+ release from the sarcoplasmic reticulum, which may depolarize the membrane and lead to triggered action potentials.
In the intact heart, strong electrotonic coupling exists between cells, meaning that spontaneous Ca2+ release in a single cell cannot produce sufficient change in membrane potential to trigger propagating action potentials and arrhythmia (‘source-sink mismatch’).
For spontaneous Ca2+ release to trigger arrhythmias in the intact heart, it must be synchronized across many cells, but little is known about how many are required, or the mechanism of synchrony.
What new information does this article contribute?
Localized β-adrenergic stimulation by norepinephrine injection leads to Ca2+- mediated focal arrhythmia in the intact rabbit heart.
Local β-adrenergic stimulation synchronizes spontaneous Ca2+ release from the sarcoplasmic reticulum across thousands of cells, overcoming the source-sink mismatch.
Source-sink interactions are critically important in the generation of Ca2+-mediated focal arrhythmia.
This study addressed the important disconnect between our understanding of arrhythmia mechanisms in isolated cells and the intact heart. β-adrenergic receptor (β-AR) stimulation produces Ca2+ -mediated arrhythmias in cells, but electrotonic coupling in the intact heart means that spontaneous Ca2+ release in one cell is insufficient to significantly alter membrane potential (Vm). We examined how synchronization of spontaneous Ca2+ overload and release might occur in order for the source-sink mismatch to be overcome, and focal arrhythmia generated. Using dual optical mapping of Vm and Ca2+, we demonstrate that local norepinephrine application induces β-AR dependent, Ca2+-mediated focal arrhythmia in rabbit hearts. The source-sink balance was crucial to focal arrhythmia induction, which was potentiated when the source:sink ratio was increased, either by changes in tissue geometry or by reducing electrotonic conduction through gap junctions (both of which modify the sink). The data also allowed the first experimental estimation of the number of cells required to have synchronous Ca2+ release in order to initiate focal arrhythmia. These data provide the first demonstration of localized β-AR stimulation as a mechanism of focal arrhythmia initiation and evidence of a mechanistic link between sympathetic dysfunction and focal arrhythmias in the intact heart.
Acknowledgments
The authors thank Dr. Francis Burton for developing the analysis software package Optiq for the current project.
Sources of funding
British Heart Foundation: FS/10/64/28532 (R.C.M.), NIH P01:HL080101 (D.M.B), NIH P30:HL101280-01 (D.M.B. and C.M.R.), UC Davis Clinical and Translational Science Center (funded from NIH UL1:RR024146 to C.M.R.) and AHA 12SDG9010015 (C.M.R.).
Non-standard abbreviations and acronyms
- AP
Action potential
- APD
Action potential duration
- APD80
Action potential duration at 80% repolarization
- β-AR
Beta-adrenergic receptor
- BCL
Basic cycle length
- CaT
Calcium transient
- CaTD
Calcium transient duration
- CaTD50
Calcium transient duration at 50% amplitude
- CBX
Carbenoxolone
- CPA
Cyclopiazonic acid
- CV
Conduction velocity
- DAD
Delayed afterdepolarization
- DMSO
Dimethyl sulfoxide
- ECG
Electrocardiogram
- GJ
Gap junction
- HF
Heart failure
- LV
Left ventricle
- NE
Norepinephrine
- NT
Normal Tyrodes
- PP
Propranolol
- PVC
Premature ventricular complex
- R6G
Rhodamine-6G
- RyR
Ryanodine receptor
- RV
Right ventricle
- SCD
Sudden cardiac death
- SERCA
Sarcoplasmic reticulum Ca2+-ATPase
- SR
Sarcoplasmic reticulum
- TTC
Triphenyl tetrazolium chloride
- Vm
Transmembrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.The cardiac arrhythmia suppression trial. The New England Journal of Medicine. 1989;321:1754–1756. doi: 10.1056/NEJM198912213212510. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 3.Metoprolol cr/xl randomised intervention trial in congestive heart failure. Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, Pogwizd SM. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circulation Research. 2008;102:1389–1397. doi: 10.1161/CIRCRESAHA.107.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klopping C, Janse MJ. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovascular Research. 1994;28:1547–1554. doi: 10.1093/cvr/28.10.1547. [DOI] [PubMed] [Google Scholar]
- 6.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 7.Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation. 1992;86:1872–1887. doi: 10.1161/01.cir.86.6.1872. [DOI] [PubMed] [Google Scholar]
- 8.Joyner RW, Picone J, Veenstra R, Rawling D. Propagation through electrically coupled cells. Circulation Research. 1983;53:526–534. doi: 10.1161/01.res.53.4.526. [DOI] [PubMed] [Google Scholar]
- 9.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: A major mechanism of structural heart disease arrhythmias. PACE. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 10.Rohr S, Kucera JP, Fast VG, Kléber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- 11.Houser SR. When does spontaneous sarcoplasmic reticulum Ca2+ release cause a triggered arrythmia? Cellular versus tissue requirements. Circulation Research. 2000;87:725–727. doi: 10.1161/01.res.87.9.725. [DOI] [PubMed] [Google Scholar]
- 12.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 14.Nori SL, Gaudino M, Alessandrini F, Bronzetti E, Santarelli P. Immunohistochemical evidence for sympathetic denervation and reinnervation after necrotic injury in rat myocardium. Cell Mol Biol. 1995;41:799–807. [PubMed] [Google Scholar]
- 15.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 16.Barber MJ, Mueller TM, Henry DP, Felten SY, Zipes DP. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation. 1983;67:787–796. doi: 10.1161/01.cir.67.4.787. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol HCP. 2004;286:H2229–H2236. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- 18.Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- 19.Bers DM, Bassani RA, Bassani JW, Baudet S, Hryshko LV. Paradoxical twitch potentiation after rest in cardiac muscle: Increased fractional release of SR calcium. J Mol Cell Cardiol. 1993;25:1047–1057. doi: 10.1006/jmcc.1993.1117. [DOI] [PubMed] [Google Scholar]
- 20.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 21.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circulation Research. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 22.Choi B-R, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and torsade de pointes in rabbit hearts with type 2 long qt syndrome. J Physiol (Lond) 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE trans BME. 1998;45:563–571. doi: 10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 24.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circulation Research. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 25.Wasserstrom JA, Shiferaw Y, Chen W, Ramakrishna S, Patel H, Kelly JE, O’Toole MJ, Pappas A, Chirayil N, Bassi N, Akintilo L, Wu M, Arora R, Aistrup GL. Variability in timimg of spontaneous calclium release in the intact rat heart is determined by the time course of sarcoplasmic reticulum calclium load. Circulation Research. 2010;107:1117–1126. doi: 10.1161/CIRCRESAHA.110.229294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation. 2011;123:1881–1890. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: Role of purkinje fibers and triggered activity. Circulation Research. 2010;106:399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maury P. Why is the ventricular outflow tract so arrhythmogenic (or is it really?) BMJ. 2011;97:1631–1633. doi: 10.1136/heartjnl-2011-300465. [DOI] [PubMed] [Google Scholar]
- 29.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: A determinant of rotor dynamics in ventricular fibrillation. Circulation Research. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 30.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 31.Caldwell JC, Burton FL, Smith GL, Cobbe SM. Heterogeneity of ventricular fibrillation dominant frequency during global ischaemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2007;18:854–861. doi: 10.1111/j.1540-8167.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 32.Satoh H, Delbridge LM, Blatter LA, Bers DM. Surface:Volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements. Biophys J. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JPP, Wilms-Schopman FJG, Wiegerinck RF, Bourier J, Belterman CNW, Coronel R, Verheijck EE. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc Res. 2003;60:288–297. doi: 10.1016/j.cardiores.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Joyner RW, van Capelle FJ. Propagation through electrically coupled cells. How a small sa node drives a large atrium. Biophys J. 1986;50:1157–1164. doi: 10.1016/S0006-3495(86)83559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 36.Bevan JA, Su C. Variation of intra- and perisynaptic adrenergic transmitter concentrations with width of synaptic cleft in vascular tissue. J Pharm Exp Ther. 1974;190:30–38. [PubMed] [Google Scholar]
- 37.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 38.Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Kohl P, Bollensdorff C, Garny A. Effects of mechanosensitive ion channels on ventricular electrophysiology: Experimental and theoretical models. Exp Physiol. 2006;91:307–321. doi: 10.1113/expphysiol.2005.031062. [DOI] [PubMed] [Google Scholar]
- 40.Steele TW, Huang CL, Kumar S, Widjaja E, Chiang Boey FY, Loo JS, Venkatraman SS. High-throughput screening of plga thin films utilizing hydrophobic fluorescent dyes for hydrophobic drug compounds. J Pharm Sci. 2011;100:4317–4329. doi: 10.1002/jps.22625. [DOI] [PubMed] [Google Scholar]
- 41.Gulyaeva N, Zaslavsky A, Lechner P, Chlenov M, Chait A, Zaslavsky B. Relative hydrophobicity and lipophilicity of beta-blockers and related compounds as measured by aqueous two-phase partitioning, octanol-buffer partitioning, and hplc. Eur J Pharm Sci. 2002;17:81–93. doi: 10.1016/s0928-0987(02)00146-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.