Abstract
The T-type Ca2+ channel (TTCC) plays important roles in cellular excitability and Ca2+ regulation. In the heart, TTCC is found in the sinoatrial nodal (SAN) and conduction cells. Cav3.1 encodes one of the three types of TTCCs. To date, there is no report regarding the regulation of Cav3.1 by β-adrenergic agonists, which is the topic of this study. Ventricular myocytes (VMs) from Cav3.1 double transgenic (TG) mice and SAN cells from wild type, Cav3.1 knockout, or Cav3.2 knockout mice were used to study β-adrenergic regulation of overexpressed or native Cav3.1-mediated T-type Ca2+ current (ICa-T(3.1)). ICa-T(3.1) was not found in control VMs but was robust in all examined TG-VMs. A β-adrenergic agonist (isoproterenol, ISO) and a cyclic AMP analog (dibutyryl-cAMP) significantly increased ICa-T(3.1) as well as ICa-L in TG-VMs at both physiological and room temperatures. The ISO effect on ICa-L and ICa-T in TG myocytes was blocked by H89, a PKA inhibitor. ICa-T was detected in control wildtype SAN cells but not in Cav3.1 knockout SAN cells, indicating the identity of ICa-T in normal SAN cells is mediated by Cav3.1. Real-time PCR confirmed the presence of Cav3.1 mRNA but not mRNAs of Cav3.2 and Cav3.3 in the SAN. ICa-T in SAN cells from wild type or Cav3.2 knockout mice was significantly increased by ISO, suggesting native Cav3.1 channels can be upregulated by the β-adrenergic (β-AR) system. In conclusion, β-adrenergic stimulation increases ICa-T(3.1) in cardiomyocytes, which is mediated by the cAMP/PKA pathway. The upregulation of ICa-T(3.1) by the β-adrenergic system could play important roles in cellular functions involving Cav3.1.
Introduction
T-type Ca2+ channels (TTCCs or Cav3) belong to one of the families of voltage-dependent Ca2+ channels. These channels are activated and inactivated at low membrane potentials (the threshold is about −60 mV) with rapid time-dependent decay (transient) and tiny single channel currents and thus termed T-type. They are encoded by three genes, Cav3.1 (α1G), Cav3.2 (α1H) and Cav3.3 (α1I) [1], [2], [3], [4], [5]. The identification of the genes encoding TTCCs [2], [3], [5] allows the examination of the properties, distribution and function of each subtype of TTCCs and offers the potential to design isoform-specific TTCC antagonists to treat related channelopathies.
TTCCs are present in a wide variety of tissues including the heart, brain, skeletal muscle, testis and spermatids, indicating multiple functions of these channels such as cardiac rhythm generation, neuronal excitability, hormone secretion, neurotransmitter release, vascular tone regulation, muscle contraction, gene expression, cell metabolism, differentiation, and proliferation [2], [3], [5], [6]. Therefore, abnormal expression and function of TTCCs are associated with many diseases including cardiac hypertrophy and arrhythmia, hypertension, epilepsy, autism, and cancer [6].
TTCCs are expressed in the whole heart during the embryonic stage but their expression in the ventricle decreases rapidly after birth [7]. Cav3.1 and Cav3.2 expression is retained in the sinoatrial node (SAN), atrioventricular node (AVN) and Purkinje fibers of the adult heart, indicating a role in cardiac automaticity and conduction [7]. Mice deficient of Cav3.2 showed normal sinoatrial rhythm [8], but mice lacking Cav3.1 had prolonged SAN recovery time, slowed pacemaker activity of SAN cells and heart rate, and delayed atrioventricular conduction. These results indicate Cav3.1, rather than Cav3.2, is the major TTCC participant in cardiac rhythm generation in the mouse heart [9].
Since β-adrenergic system is critical for heart rate regulation and Cav3.1 is involved in cardiac rhythm generation, it is important to examine the regulation of the TTCC by the β-adrenergic/PKA system. The regulation of TTCCs by cAMP-dependent protein kinase A (PKA) has been controversial probably due to the differences in experimental conditions, cell types and the existence of specific isoforms [10]. In general it is believed that PKA has little effects on TTCCs [11], [12], [13]. Phosphorylation of Cav3.2 by PKA has been shown to permit the inhibitory effect of Gβγ dimmers [14]. In contrast, T-type Ca2+ current (ICa-T, probably through Cav3.2 because it was sensitive to low concentration of Ni2+) in frog atrial myocytes was reported to be increased by isoproterenol via a cAMP/PKA independent mechanism [15]. The same group showed that cAMP/PKA downstream to β-adrenergic receptor might phosphorylate a protein to enhance high-voltage prepulse-induced facilitation of TTCCs [16]. In addition, Lenglet et al. also reported that Cav3.2 TTCC activity recorded in rat glomerulosa cells was augmented by PKA after the stimulation of 5HT7 receptors [17]. To date, there is no report of the regulation of Cav3.1 by the β-adrenergic receptor/cAMP/PKA cascade in cardiac or other native mammalian cells.
In this study, we sought to determine whether Cav3.1 is regulated by β-adrenergic receptor/PKA signaling pathway using ventricular myocytes from Cav3.1 transgenic mice and sinoatrial node cells from wildtype or Cav3.2 knockout mice. We have found that the activity of both overexpressed and native Cav3.1 channel is enhanced by a β-adrenergic agonist, isoproterenol. This effect was mediated by the adenylyl cyclase/cAMP/PKA system because cAMP recapitulated the effect of isoproterenol while a PKA inhibitor (H89) abolished the effect of ISO on ICa-T(3.1). The upregulation of Cav3.1 by PKA may contribute to the regulation of the heart rate by the β-adrenergic system.
Materials and Methods
Ethical Approval
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Use and Care Committee at Temple University.
Animal Models
A mouse model with cardiac-specific (α-MHC controlled) and conditional (tet-off, controlled by doxycycline, DOX) overexpression of mouse Cav3.1 (Genebank: NM_009783) was established with the bitransgenic system developed by Sanbe A et al [18]. Cav3.1 transgenic (TG) mice were used for isolating ventricular myocytes (VMs). Cav3.1 knockout and Cav3.2 knockout mouse colonies were obtained from the Molkentin’s group [19] for isolating the SAN cells.
Ventricular Myocyte and Sinoatrial Node Cell Isolation
Mouse ventricular myocytes (VMs) were isolated using a constant-pressure Langendorff apparatus as described [20]. Animals were anesthetized with sodium pentobarbital (120 mg/kg body weight). The heart was excised and digested via retrograde perfusion of the heart with normal Tyrode solution containing type II collagenase (290 U/mL) and (in mM): CaCl2 0.02, glucose 10, HEPES 5, KCl 5.4, MgCl2 1.2, NaCl 150, sodium pyruvate 2 (pH 7.4 with NaOH). After 8–10 min, the ventricles were minced and isolated ventricular myocytes were dissociated by gentle aspiration of minced tissue. Isolated VMs then were filtered with a 200 µm-diameter mesh. VMs were maintained in normal Tyrode solution containing 0.5% bovine serum albumin and the extracellular Ca2+ was titrated up to 1 mM. Myocytes were used within 8 hours after isolation.
SAN cells were isolated as described previously [21], [22]. Animals were anesthetized with sodium pentobarbital (120 mg/kg BW, intraperitoneal injection) and heparinized intravenously. SAN cells were isolated with a “chunk” technique as follow: After the heart was excised, it was placed into Tyrode’s solution (35°C) containing (in mM) 140 NaCl, 5.0 HEPES, 5.5 Glucose, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2 (pH = 7.4). The SAN region was the one with spontaneous activity and where the contraction of the heart initiates. The SAN tissue was dissected out with a dissecting scope according to the landmarks of the heart (delimited by the orifice of superior vena cava, crista terminalis and atrial septum) as described [21], [22]. The SAN tissue was cut into smaller pieces, which were transferred and rinsed in a “low Ca2+” digestion containing (mM) 140 NaCl, 5.0 Hepes, 5.5 Glucose, 5.4 KCl, 0.2 CaCl2, 0.5 MgCl2, 1.2 KH2PO4, 50 Taurine, and 1 mg/mL BSA (pH = 6.9). Then SAN tissue pieces were digested in 5 mL of “low Ca2+” solution containing collagenase type I (Worthington, 225 U/mL), elastase (Worthington, 1.8 U/mL), and protease type XIV (0.8 U/mL, Sigma) for 30 min at 37°C. After digestion, the tissue was washed with 10 mL of Kraft-Bruhe medium containing (mM) 100 potassium glutamate, 5.0 Hepes, 20 Glucose, 25 KCl, 10 potassium aspartate, 2.0 MgSO4, 10 KH2PO4, 20 taurine, 5 creatine, 0.5 EGTA, and 1 mg/mL BSA (pH = 7.2) for 3 times and then the cells were dissociated with a transfer pipette by pipetting up and down the tissue chunks. Dissociated cells were put at room temperature for 5 min. The cells were stored at 4°C and studied within 5 hours.
Electrophysiology
Ca2+ currents (ICa) were measured using whole-cell voltage-clamp techniques with an Axopatch 2B voltage-clamp amplifier and pClamp8 software and 1–3 MΩ pipettes. Ca2+ currents were measured under discontinuous voltage-clamp mode. The real clamping voltage and Ca2+ currents were measured simultaneously. To achieve good voltage control, the gain was set between 8 to 50. To ensure the quality of our data, only data with the loss of voltage control <10 mV were included for our report. VMs were placed in a heated chamber (35±2°C or room temperature) on the stage of an inverted microscope (Nikon Diaphot, Japan) and initially perfused with normal Tyrode solution. The pipette contained a Na+-free and K+-free solution consisting of (in mM): Cs-aspartic acid 130, EGTA 10, MgCl2 1, NMDG 10, HEPES 10, TEA-Cl 20, Tris-ATP 5, Tris-GTP 0.25, pH 7.2. Once a gigaohm seal was obtained, the patched membrane was ruptured to allow 10 minutes of dialysis of the myocyte. The perfusate was switched to a Na+-free, K+-free solution containing (in mM): CaCl2 2, 4-aminopyridine (4-AP) 2, CsCl 5.4, Glucose 10, HEPES 5, MgCl2 1.2, NMDG 150, pH 7.4. Myocyte capacitance was obtained with the membrane test function in Clampex 8.0, which hyperpolarizes the cell for −5 mV from the holding potential to measure cell capacitance. The total calcium current (ICa)-voltage relationship was determined by measuring ICa from a holding potential of −90 mV using square wave pulses in a 10 mV- increment. ICa-L was measured at the same test voltages from a holding potential of −50 mV. To minimize run-down of Ca2+ currents, these two Ca2+ currents measured at the same test potential were separated by a 1000 ms holding potential of −50 mV ( Figure 1B ) and 20 s was set between sweeps. ICa-T at each test membrane potential was determined by subtracting raw ICa-L from raw total ICa at this test potential, which is a traditional way for measuring ICa-T [1]. Mibefradil (1 µM) or BayK 8644 (1 µM) was applied via perfusate to the cell while the Ca2+ currents measured at the test potential −40 mV from the Vh of −90 mV and at 10 mV from the Vh of −50 mV to 10 mV were continuously monitored. BayK 8644, a DHP agonist, was selected because it could readily and affirmatively determine if BayK 8644 had any stimulatory effect on ICa-T even in the presence of a rundown of ICa-T.
Figure 1. ICa-T(3.1) was expressed only in Cav3.1 TG ventricular myocytes and sensitive to mibefradil but not to dihydropyridines.
A, A schematic of the bitransgenic inducible expression system for cardiac-specific Cav3.1 overexpression. tTA is the tetracycline-controlled transactivator system. tetR, tet-repressor cDNA fused to VP16 (activator domain); tet-O, tet-operon. B, Raw currents of total ICa, ICa-L and ICa-T in a control and a TG VMs. No ICa-T was present in this control cell but robust ICa-T found in the TG VM. C & D, Averaged I–V curves of total ICa, ICa-L and ICa-T in control (n = 12) and TG (n = 13) ventricular myocytes. No ICa-T was detected in control VMs but great ICa-T found in TG VMs.
To determine ISO effect on the current-voltage (I–V) relationships of ICa-L and ICa-T, I–V relationships of total ICa and ICa-L were recorded at baseline. Subsequently, 1 µM isoproterenol (ISO, Sigma) were applied through perfusate while the currents elicited from the Vh = −90 mV to −40 mV (mostly ICa-T) and from the Vh = −50 mV to 10 mV (mostly ICa-L) were monitored until the maximum effect of ISO was observed. Then, the I–V relationships of total ICa, ICa-L and ICa-T were determined again as described above. To determine if direct activation of PKA was able to regulate ICa-T, a nonhydrolyzable cAMP analog (dibutyryl-cAMP, Sigma, 10 µM) was dialyzed into the cell for 10 minutes and the ICa-L and ICa-T were determined afterwards [23]. To determine if ISO effects on ICa-L and ICa-T is mediated by PKA, a PKA inhibitor (H89, Sigma, 5 µM), was included in the pipette filling solution and patched VMs were dialyzed for 10 minutes. Thereafter, the I–V relationships of total ICa, ICa-L and ICa-T were measured before and after the application of ISO.
To investigate the effects of ISO and db-cAMP on voltage-dependent inactivation of ICa-L and ICa-T(3.1), double-pulse protocols were applied with or without these drugs. For ICa-T(3.1), the first pulse was the prepulse to different test voltages (−110 mV to −10 mV in a10 mV-increment) from the holding potential −90 mV. The second pulse was separated by a 5 ms repolarizing period to −90 mV from the prepulses and depolarized to −40 mV, a voltage almost only ICa-T and minimal ICa-L contamination could be recorded. For ICa-L, the prepulses were evoked from the holding potential of −50 mV to different membrane potentials and the second pulse was evoked from a short return to −50 mV for 5 ms to 0 mV (for 400 ms).
Since in small SAN cells, rundown of ICa-L and ICa-T was faster (often ICa-T was decreased by >30% within 10 minutes), it became a significant issue to study ISO effects if a long recording time was needed. Full I–V curves before and after ISO could not obtained. Therefore, to study ISO effects on ICa-T in SAN cells, we only studied ISO effect at a single voltage (from the holding potential of −90 mV to −40 mV) in the presence of nifedipine (an ICa-L blocker, 10 µM). The use of nifedipine ensured only ICa-T was recorded at this voltage even ISO could shift the activation of ICa-L to more negative voltage and thus avoided the use of double pulses to minimize recording time to minimize ICa-T rundown.
The conductance of L- and T-type Ca2+ channels (GCa) was calculated by dividing the current by the driving force (Vt-ECa’, where ECa’ stands for the apparent reversal potential of Ca2+). The activation-voltage (G-V) relationships were plotted using normalized L- or T-type Ca2+ channel conductance (GCa/GCa,max) as the y-axis and test potentials as the x-axis. The G-V curve is fitted with a Boltzmann function G/Gmax = 1/[1+exp[−(Vt−V0.5, d∞)/k]], where Vt is the test voltage and V0.5, d∞is the voltage at which half of the Gmax can be elicited, k is the slope factor. The voltage-dependent inactivation curve (f∞) was fitted with a Boltzmann function G/Gmax = 1/[1+exp[−(Vt−V0.5, f∞)/k]], where Vt is the test voltage and V0.5, f∞is the voltage at which half of the channels were inactivated, k is the slope factor.
Real-time PCR
SAN tissue was pooled from 4–8 mice for wild type (n = 24) and transgenic (n = 19) animals. Total mRNA was extracted from snap-frozen SAN tissue, ventricular tissue, or brain (as a positive control for T-type Ca2+ channel expression) using Trizol reagent and quantitated by a UV spectrometer. Real-time PCR was done with the SYBR Green Real Time PCR kit (Applied Biosystems, Carlsbad, CA) according to the instruction of the kit and an Eppendorff Mastercycler RT-PCR machine. GAPDH was used as the internal control. The ΔΔCt-method was used to determine the abundance of Cav3 mRNAs relative to GAPDH. The primers were (5′ to 3′): Cav3.1: forward: TGTGGAAATGGTGGTGAAGA and reverse: ACTGCGGAGAAGCTGACATT; Cav3.2: forward: GCTGTTTGGGAGGCTAGAAT and reverse: CGAAGGTGACGAAGTAGACG; Cav3.3: forward: TGGGCATTTTTGGCAAGAA and reverse: CAGTGCGGATGGCTGACA; GAPDH: forward: TGCACCACCAACTGCTTAG and reverse: GATGCAGGGATGATGTTC.
Data Analysis
Obtained data were analyzed offline with Clampfit 8 (Molecular Device, CA) as described previously [23], managed with Microsoft Excel and presented with GraphPad Prizm 5.0 (La Jolla, CA, USA). In short, ICa-T was obtained by subtracting the raw ICa-L from the raw total ICa as described previously [24]. The current-voltage (I–V) relationships were constructed by plotting the peak amplitudes of total ICa, ICa-L and derived ICa-T against the test voltages.
Statistics
Data in the text are reported as mean±SEM. When appropriate, paired and unpaired T-test, ANOVA or ANOVA for repeated measures were used to detect significance with SAS 9.0 (SAS Institute Inc.). P values of ≤0.05 were considered significant. In this paper, n is the number of cells examined from at least 3 animals.
Results
Cav3.1 Mediated T-type Ca2+ Current (ICa-T(3.1)) Was Observed Only in VMs from Cav3.1 Double Transgenic Mice
To examine whether Cav3.1 was expressed in our transgenic system, ICa-T was measured in VMs from transgenic (TG) and control hearts. Typical examples of total Ca2+ current (ICa), L-type Ca2+ current (ICa-L) and T-type Ca2+ current (ICa-T) in control and TG VMs are shown in Figure 1B . The current-voltage relationships (I–V curves) of total ICa, ICa-L and ICa-T in both control (n = 12) and TG myocytes (n = 5) are shown in Figure 1C and D . There was no detectable ICa-T observed in all 12 tested control cells ( Figure 1B and C ) but a great density of ICa-T (maximum ICa-T amplitude: −12.1±1.3pA/pF) was found in Cav3.1 TG VMs ( Figure 1B and D ). ICa-T had a threshold of ∼−60 mV and peaked at ∼−40 mV. The time-dependent decay rates (kinetics) of the ICa-T were significantly faster than those of the ICa-L recorded in the same cell ( Figure 1B ), as reported previously [1].
To confirm the identity of putative ICa-T, mibefradil (1 µM) was used to determine its sensitivity to this TTCC antagonist. Mibefradil suppressed ICa-T at −40 mV by 66.8±8.7% ( Figure 2A and C ) but had minimal effect on ICa-L at 10 mV (decreased by 11.1±2.8%; Figure 2B and D ). To further confirm that ICa-T could be effectively separated by holding the cell at different membrane potentials with minimal contamination of ICa-L, BayK 8644, a dihydropyridine agonist specific for ICa-L, was used to test dihydropyridine effect on the two components of ICa. As suggested in Figure 1C and D , ICa recorded at −40 mV from Vh of −90 mV was almost completely consisting of ICa-T while ICa recorded at 10 mV from Vh of −50 mV should be only ICa-L. As predicted, ICa at −40 mV from the Vh of −90 mV was not changed by BayK ( Figure 2E, F and H ) while ICa at 10 mV from the Vh of −50 mV was significantly increased 49.7±0.8% by BayK ( Figure 2E, G and H ). When the I–V curves of presumable ICa-L and ICa-T, separated by the strategy of holding the cell at different membrane potentials, were examined, BayK evidently increased the presumable ICa-L but not the presumable ICa-T ( Figure 2I, J and K ). These results suggest that the separation of ICa-L and ICa-T by holding the cell at different membrane potentials is effective, and that ICa-T is not sensitive to dihydropyridines but sensitive to mibefradil.
Figure 2. ICa-T(3.1) in Cav3.1 TG ventricular myocytes was sensitive to mibefradil but not to dihydropyridines.
A & B, Time courses of amplitude changes of presumable ICa-L and ICa-T in response to mibefradil. C & D, Relative changes of ICa-T (C) and ICa-L (D) in response to mibefradil. E, Raw currents recorded at −40 mV from Vh = −90 mV and at 10 mV from Vh = −50 mV before and after the application of BayK 8644. F & G, Time courses of amplitude changes of presumable ICa-L and ICa-T in response to BayK. H, Normalized increases in presumable ICa-L and ICa-T by BayK. I, J and K, I–V curves of total ICa, ICa-L and ICa-T before and after BayK.
ISO Significantly Increased ICa-T(3.1) in TG VMs
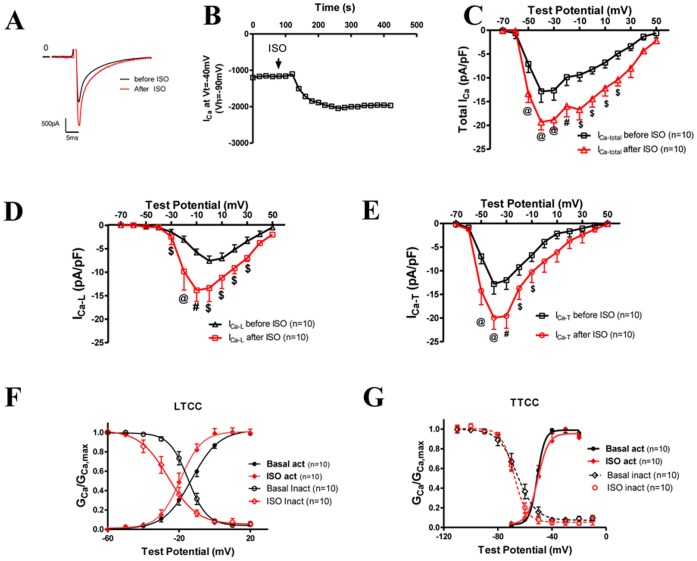
To study whether ICa-T(3.1) can be regulated by the β-adrenergic system in cardiac myocytes, isoproterenol, a β-adrenergic agonist, was applied to TG VMs. Since ICa at −40 mV from the Vh of −90 mV was almost completely consisting of the T-type Ca2+ current, ISO effect on ICa-T(3.1) was monitored by recording ICa at −40 mV. Figure 3A showed ICa at −40 mV before and after ISO and Figure 3B showed the time course of the ISO effect on a myocyte, suggesting a significant upregulation of ICa-T(3.1). I–V curves of total ICa ( Figure 3C ), ICa-L ( Figure 3D ), and ICa-T ( Figure 3E ) before and after ISO showed that β-adrenergic stimulation (ISO) significantly increased both ICa-L and ICa-T at most test voltages. The maximal ICa-L was increased by 81.6% (from −7.6±1.2pA/pF to −13.8±1.3pA/pF) and the maximal ICa-T(3.1) was increased by 55.5% (from −12.8±1.9pA/pF to −19.9±2.4pA/pF). It is well known that β-adrenergic stimulation shifts the activation of ICa-L to more negative voltages and accelerates the decay of ICa-L via a Ca2+-dependent inactivation mechanism [24]. In this study, we found that ISO shifted the peak ICa voltage for ICa-L but not for ICa-T(3.1) ( Figure 3D and E ). It seems that there is no voltage dependent effect of ISO because ISO did not change the voltage-dependence of ICa-T(3.1) activation and inactivation although it shifted the activation and inactivation of ICa-L to more negative voltages ( Figure 3F and 3G ).
Figure 3. ISO significantly increased ICa-T(3.1) and ICa-L.
A, Raw current recordings at −40 mV from the Vh of −90 mV before and after ISO. B, Time course of the change of the amplitude of ICa measured at the test potential of −40 mV from the Vh = −90 mV, which was almost completely consisting of ICa-T(3.1). C-E, Averaged I–V curves of total ICa (C), ICa-L (D), and ICa-T (E) before and after ISO application. ISO increased total ICa, ICa-T and ICa-L at most membrane potentials. ISO shifted the voltage at which ICa-L peaked from 0 mV to −10 mV but did not change the voltage (−40 mV) at which ICa-T(3.1) peaked. F and G: the voltage-dependent activation and inactivation curves of ICa-L and ICa-T before and after the application of ISO. @: p<0.001; #: p<0.01; $: p<0.05; TG vs. control at the same test potential. Statistics were done with two-way ANOVA and post-hoc t-test.
Nonhydrolyzable Dibutyryl-cAMP (db-cAMP) Significantly Increased ICa-T(3.1) at Both Physiological and Room Temperatures
The stimulatory effect of β-adrenergic agonists on calcium channels in cardiac myocytes can be mediated by mulitple mechanisms [24]: 1, PKA activation through the β-adrenergic receptor/Gαs/adenyl cyclase/cAMP/PKA pathway; 2, direct modulation by Gαs or Gβγ after the binding of adrenergic agonists to the adrenergic receptor and subsequent dissociation of Gαs from Gβγ dimer; 3. The newly found cAMP sensor, EPAC (exchange protein directly activated by cAMP), regulates ICa-T. We tested if direct activation of PKA by a cAMP analog (db-cAMP) was able to stimulate ICa-T(3.1). Db-cAMP significantly increased ICa-L by 154.9% (with db-cAMP −15.5±3.3pA/pF versus without db-cAMP −6.1±0.8pA/pF, Figure 4B ) and increased ICa-T(3.1) by 102.8% (with db-cAMP 22.7±2.6pA/pF vs. without db-cAMP −11.2±0.9pA/pF, Figure 4C ). The voltage-dependence of ICa-T activation and inactivation was not altered by db-cAMP ( Figure 4F ) although db-cAMP shifted the activation curve and inactivation curve to the left ( Figure 4E ). These findings clearly show that the ISO effects of ICa-T(3.1) are recapitulated by cAMP, indicating that the upregulation of ICa-T(3.1) by ISO is mediated by the cAMP dependent PKA signaling pathway.
Figure 4. A nonhydrolyzable cAMP analog, db-cAMP, significantly increases ICa-T and ICa-L.
A, Example currents of total ICa, ICa-L and ICa-T without (top) or with db-cAMP (lower) in the pipette recorded in TG VMs. Currents were normalized to cell capacitance for comparison and the scale bars are the same for both without or with db-cAMP recordings. B–D, Averaged I–V curves of total ICa-total (B), ICa-L (C) and ICa-T (D) with or without db-cAMP in the pipette. There are significantly increases in both ICa-L and ICa-T(3.1). Db-cAMP shifted the peak voltage of ICa-L to more negative voltages but did not change that of ICa-T(3.1) in TG VMs. E and F: the voltage-dependent activation and inactivation curves of ICa-L and ICa-T with or without db-cAMP. @: p<0.001; #: p<0.01; $: p<0.05; TG vs. control at the same test potential. Statistics were done with two-way ANOVA and post-hoc t-test.
The Effect of ISO on ICa-T (3.1) can be Blocked by a PKA Inhibitor, H-89
Albeit our results with db-cAMP imply that the stimulatory effect of ISO might be mediated by PKA, recently another cAMP sensor, an exchange protein directly activated by cAMP (EPAC), has been found in cells from various tissues including the heart [25]. To rule out the involvement of EPAC and confirm the role of PKA in the stimulatory effects of ISO, a PKA-specific inhibitor, H89, was included in the pipette filling solution. In the presence of H89, ISO did not increase the current recording at the Vt = −40 mV from Vh = −90 mV (mainly ICa-T(3.1)) before and after the application of ISO (raw currents in Figure 5A and time course in Figure 5B ). I–V curves of ICa-L and ICa-T were not changed by ISO with H89 in the pipette filling solution ( Figure 5C & D ). These results support the idea that PKA activated by β-adrenergic agonists causes the upregulation of ICa-T(3.1).
Figure 5. The effect of ISO on ICa-T and ICa-L can be blocked by a PKA Inhibitor, H89.
A, Raw currents evoked from the holding potential of −90 mV to −40 mV before and 4 minutes after the application of ISO in a TG myocyte dialyzed with pipette H89 (10 µM). B, The time course of the change of the amplitude of ICa-T(3.1) (recorded at Vt = −40 mV from the Vh = −90 mV). H89 blocked the effect of ISO on ICa-T(3.1). C & D, Averaged I–V curves of ICa-L and ICa-T in TG VMs dialyzed with H89 before and after the application of ISO.
ICa-T(3.1) is the T-type Ca2+ Current in Mouse SAN Cells and is Upregulated by Isoproterenol
At last, we examined if native ICa-T in SAN cells is regulated by the β-adrenergic system. Total ICa, ICa-T and ICa-L were recorded from wild type and Cav3.1 KO SAN cells. In wild type SAN cells, ICa-T was clearly recorded with a maximum current of −2.08±0.64pA/pF ( Figure 6 A–C). In contrast, in the SAN cells from Cav3.1 knockout mice, T-type Ca2+ current is completely absent, indicating that Cav3.1 is the major mediator of ICa-T in mouse SAN cells ( Figure 6 D–F) as previous study suggests [9]. Real-time PCR further confirmed that the major TTCC in the SAN is Cav3.1. Cav3.2 mRNA abundance was very low and Cav3.3 mRNA was not detectable in SAN tissue although all three types of TTCCs were detected in the brain ( Figure 6 G). ICa (presumably ICa-T) measured at −40 mV in wild-type SAN cells bathed in 10 µM nifedipine (to block ICa-L) was increased by 115.7±21.1% with 1 µM ISO ( Figure 6 H and I). To further make sure that ICa-T in SAN cells was Cav3.1-mediated and could be stimulated by 1 µM ISO, ICa-T was recorded in Cav3.2 knockout SAN cells and ISO did increase ICa-T in these cells by 161.0±20.5% ( Figure 6 J and K).
Figure 6. ICa-T in mouse SAN cells is mediated by Cav3.1 and can be increased by ISO.
I–V curves of total ICa, ICa-L and ICa-T in wild-type control (A, B and C) and in Cav3.1 knockout (D, E and F) SAN cells were shown in A-F, showing ICa-T is present in WT cells but not Cav3.1 KO cells. G, mRNA abundance of Cav3.1, Cav3.2, Cav3.3 in wildtype brain, wildtype ventricle, wildtype SAN, Cav3.1 TG brain, Cav3.1 TG ventricle, and Cav3.1 TG SAN, measured by real-time PCR. H and J, time courses of the change of ICa-T (recorded at −40 mV in the presence of nifedipine) amplitudes by ISO+nifedipine in control wildtype (H) and Cav3.2 KO (J) SAN cells. I and K, normalized increases of ICa-T (recorded at −40 mV in the presence of nifedipine) by ISO+nifedipine in control wildtype (I) and Cav3.2 KO (K) SAN cells.
Discussion
What are the New Findings in this Study?
First, we found that β-adrenergic stimulation increased ICa-T(3.1) in cardiac myocytes expressing exogenous or endogenous Cav3.1 channels. To our knowledge, this is the first detailed study of β-adrenergic regulation of one specific subtype of TTCCs in cardiac myocytes thus far. Second, the cAMP/PKA pathway mediates the activity enhancing effect of β-adrenergic stimulation.
The Regulation of TTCCs by the β-adrenergic/PKA Pathway
TTCCs are distributed in various cell types serving a wide range of functional roles [3], [26]. Abnormal expression of T-type Ca2+ channels is involved in pathological conditions including epilepsy [27], neurogenic pain [28], cancer [29], [30], and cardiac hypertrophy [31]. Therefore, the regulation of TTCCs has been studied since their discoveries [10]. However, early studies often show conflicting results possibly due to unknown molecular identities and TTCC heterogeneity in cells [10]. Interestingly, it has been shown that the same neurotransmitter, hormone, or protein kinase exerts different effects on subtypes of TTCCs or on the same TTCC subtype in different tissues [10]. In addition, in our study, we have observed a run-down phenomenon of ICa-T at baseline or during β-adrenergic stimulation in some myocytes, which could mask the stimulatory effects of β-adrenergic.
Specifically for β-adrenergic/PKA regulation of TTCCs, it remains controversial [32]. Most previous studies showed little effect of PKA on TTCCs [11], [12], [13]. Phosphorylation of Cav3.2 by PKA permits the inhibition of ICa-T by Gβγ dimmers [14]. On the other hand, T-type Ca2+ current has been reported be increase by ISO in frog atrial cells through a cAMP/PKA-independent mechanism [15] while in rat glomerulosa cells by 5-HT via a PKA-dependent mechanism [17]. Cav3.2 channel activity can be increased by db-cAMP in heterologous systems as well [32], [33]. To date, there has been no report about Cav3.1 regulation by β-adrenergic system, which is not present in heterologous systems. It has been shown that in the mouse heart, Cav3.1 is the primary TTCC found in SAN cells as our study also suggests [9]. Here, for the first time, we have shown that in cardiac myocytes only expressing Cav3.1 (TG myocytes or Cav3.2 knockout SAN cells), ICa-T(3.1) was significantly increased by ISO. Db-cAMP reproduces the effect of ISO on ICa-L(3.1) at both room and physiological temperatures while H89 (a PKA inhibitor) blocked ISO effect on ICa-L and ICa-T in TG myocytes, confirming that the stimulatory effect of ISO on ICa-L and ICa-T is mediated by PKA.
Potential Mechanisms for PKA-dependent Upregulation of ICa-T(3.1)
In the current study, we did not determine how ICa-T(3.1) is regulated by PKA activated by ISO. PKA might phosphorylate Cav3.1 directly as it does on L-type (Cav1) Ca2+ channels. Since it is generally believed that Cav3 channels do not have accessory subunits, the PKA site(s) could be on the Cav3.1α subunit. It is possible that PKA-dependent phosphorylation of Cav3.1 augments the open probability of the channel, as it does to the L-type Ca2+ channel. A second possibility is that PKA might phosphorylate another molecule to indirectly augment the open probability of the channel. Further studies will be needed to define the mechanism.
The effect of β-adrenergic stimulation on ICa-T could be reversed by dephosphorylation of Cav3.1α or a related protein if PKA phosphorylation is the mechanism for the upregulation of Cav3.1 activity. Multiple protein serine/threonine phosphatases including PP1, PP2A, and PP2B, are expressed in cardiac myocytes. Which phosphatase is involved in Cav3.1 by ISO warrants further investigation.
Relevance of β-adrenergic Mediated Upregulation of ICa-T(3.1) in the Heart
In the heart, T-type calcium channels contribute to the heart rate generation [34], and it is possible that β-adrenergic/PKA regulation of Cav3.1 may participate in the positive chronotropic effects of β-adrenergic agonists. ICa-T is also reported to be reemerged and a participator in excitation-contraction coupling in stressed hearts; it is likely that β-adrenergic mediated upregulation of ICa-T might also contribute to cardiac contraction in stressed hearts although our previous studies have shown that overexpressed Cav3.1 is not effective to load the SR and trigger SR Ca2+ release [35]. Furthermore, if there is abnormally high ICa-T (e.g., overstimulation of the Cav3 channels by the sympathetic nervous system) in the cardiac pacemaking tissues and the conduction system, there could be tachycardia or atrial fibrillation or ectopic ventricular contraction. We have observed ectopic ventricular contraction in some Cav3.1 TG mice. In line with this, blocking Cav3 channels is able to reduce arrhythmic events and sudden cardiac death in a mouse heart failure model [36].
Conclusion
In cardiac myocytes, Cav3.1 current is increased by β-adrenergic agonists. This effect is mediated by protein kinase A. The regulation of Cav3.1 by β-adrenergic/PKA signaling pathway could play a role in heart rate regulation, arrhythmias and regulating other cellular functions involving Cav3.1.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National Institutes of Health (R01-HL088243 to X.C.) and American Heart Association (SDG Grant 0730347N to X.C. and Predoctoral Fellowship 09PRE2260943 to Y.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akaike N. T-type calcium channel in mammalian CNS neurones. Comp Biochem Physiol C. 1991;98:31–40. [PubMed] [Google Scholar]
- 2.Nilius B, Talavera K, Verkhratsky A. T-type calcium channels: the never ending story. Cell Calcium. 2006;40:81–88. doi: 10.1016/j.ceca.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Reyes E, Lory P. Molecular biology of T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:605–609. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- 6.Shuba YM, Perez-Reyes E, Lory P, Noebels J. T-type calcium channels: from discovery to channelopathies, 25 years of research. Channels (Austin) 2008;2:299–302. doi: 10.4161/chan.2.4.6577. [DOI] [PubMed] [Google Scholar]
- 7.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, et al. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 9.Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- 10.Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985;86:1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher R, Johnston D. Differential modulation of single voltage-gated calcium channels by cholinergic and adrenergic agonists in adult hippocampal neurons. J Neurophysiol. 1990;64:1291–1302. doi: 10.1152/jn.1990.64.4.1291. [DOI] [PubMed] [Google Scholar]
- 13.Benham CD, Tsien RW. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C, Depuy SD, Yao J, McIntire WE, Barrett PQ. Protein kinase A activity controls the regulation of T-type CaV3.2 channels by Gbetagamma dimers. J Biol Chem. 2009;284:7465–7473. doi: 10.1074/jbc.M808049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez JL, Vassort G. Properties of the low threshold Ca current in single frog atrial cardiomyocytes. A comparison with the high threshold Ca current. J Gen Physiol. 1992;100:519–545. doi: 10.1085/jgp.100.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez JL, Rubio LS, Vassort G. Facilitation of T-type calcium current in bullfrog atrial cells: voltage-dependent relief of a G protein inhibitory tone. J Physiol 491 (Pt. 1996;2):321–334. doi: 10.1113/jphysiol.1996.sp021218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenglet S, Louiset E, Delarue C, Vaudry H, Contesse V. Involvement of T-type calcium channels in the mechanism of action of 5-HT in rat glomerulosa cells: a novel signaling pathway for the 5-HT7 receptor. Endocr Res. 2002;28:651–655. doi: 10.1081/erc-120016981. [DOI] [PubMed] [Google Scholar]
- 18.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, et al. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama H, Bodi I, Correll RN, Chen X, Lorenz J, et al. alpha1G-dependent T-type Ca2+ current antagonizes cardiac hypertrophy through a NOS3-dependent mechanism in mice. J Clin Invest. 2009;119:3787–3796. doi: 10.1172/JCI39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52:51–64. doi: 10.1016/s0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 24.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 25.Metrich M, Morel E, Berthouze M, Pereira L, Charron P, et al. Functional characterization of the cAMP-binding proteins Epac in cardiac myocytes. Pharmacol Rep. 2009;61:146–153. doi: 10.1016/s1734-1140(09)70017-9. [DOI] [PubMed] [Google Scholar]
- 26.Lory P, Bidaud I, Chemin J. T-type calcium channels in differentiation and proliferation. Cell Calcium. 2006;40:135–146. doi: 10.1016/j.ceca.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Park D, Choi S, Lee S, Sun M, et al. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- 29.Adams PJ, Snutch TP. Calcium channelopathies: voltage-gated calcium channels. Subcell Biochem. 2007;45:215–251. doi: 10.1007/978-1-4020-6191-2_8. [DOI] [PubMed] [Google Scholar]
- 30.Taylor JT, Zeng XB, Pottle JE, Lee K, Wang AR, et al. Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol. 2008;14:4984–4991. doi: 10.3748/wjg.14.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 32.Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, et al. Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem. 2007;282:32710–32718. doi: 10.1074/jbc.M702746200. [DOI] [PubMed] [Google Scholar]
- 33.Kim JA, Park JY, Kang HW, Huh SU, Jeong SW, et al. Augmentation of Cav3.2 T-type calcium channel activity by cAMP-dependent protein kinase A. J Pharmacol Exp Ther. 2006;318:230–237. doi: 10.1124/jpet.106.101402. [DOI] [PubMed] [Google Scholar]
- 34.Huc S, Monteil A, Bidaud I, Barbara G, Chemin J, et al. Regulation of T-type calcium channels: signalling pathways and functional implications. Biochim Biophys Acta. 2009;1793:947–952. doi: 10.1016/j.bbamcr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Jaleel N, Nakayama H, Chen X, Kubo H, MacDonnell S, et al. Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circ Res. 2008;103:1109–1119. doi: 10.1161/CIRCRESAHA.108.185611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita H, Kuwahara K, Takano M, Arai Y, Kuwabara Y, et al. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation. 2009;120:743–752. doi: 10.1161/CIRCULATIONAHA.109.857011. [DOI] [PubMed] [Google Scholar]