Abstract
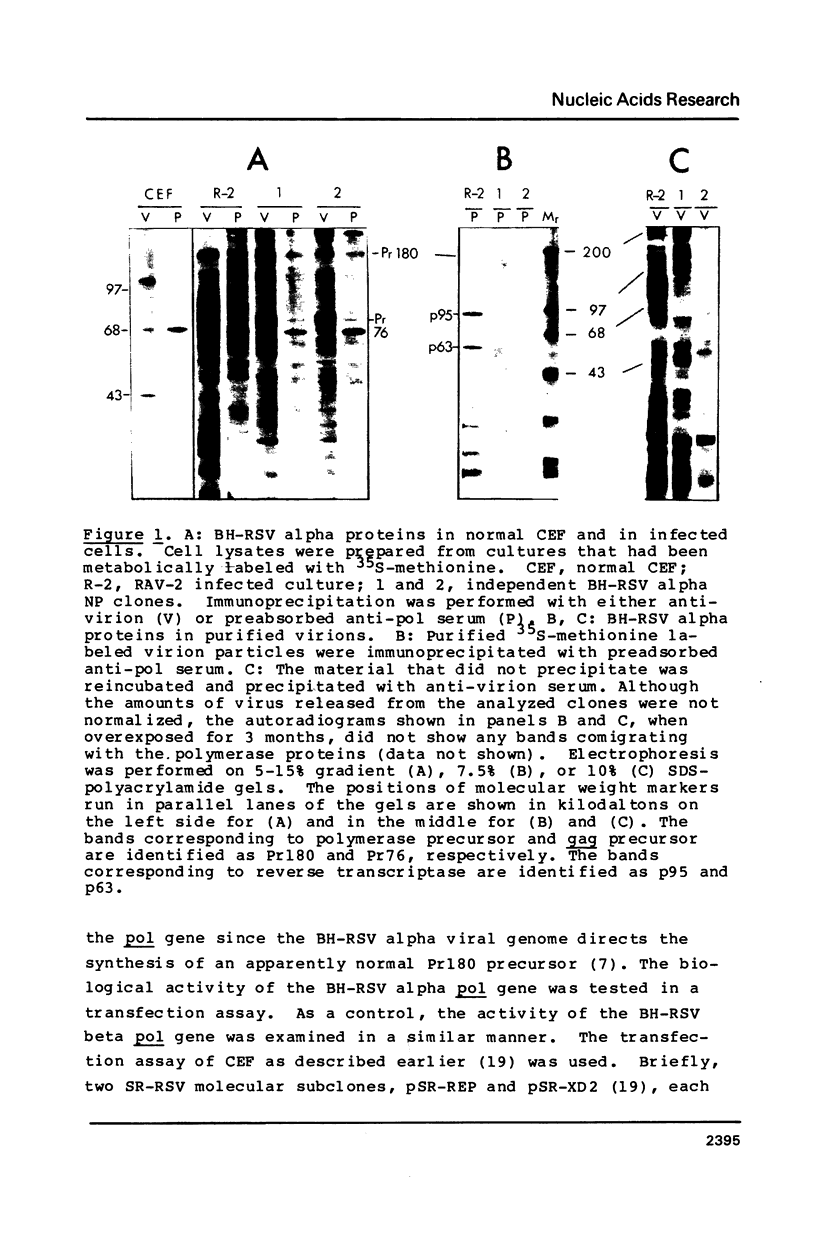
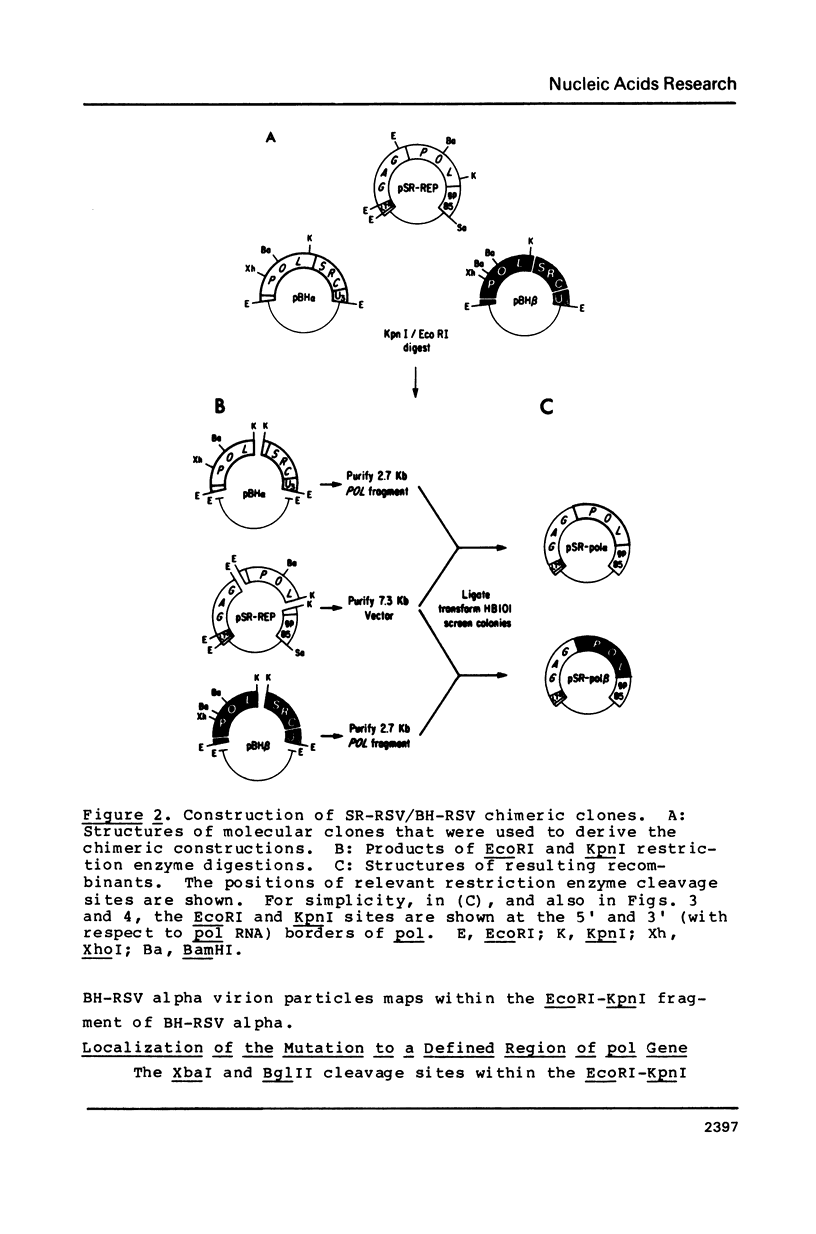
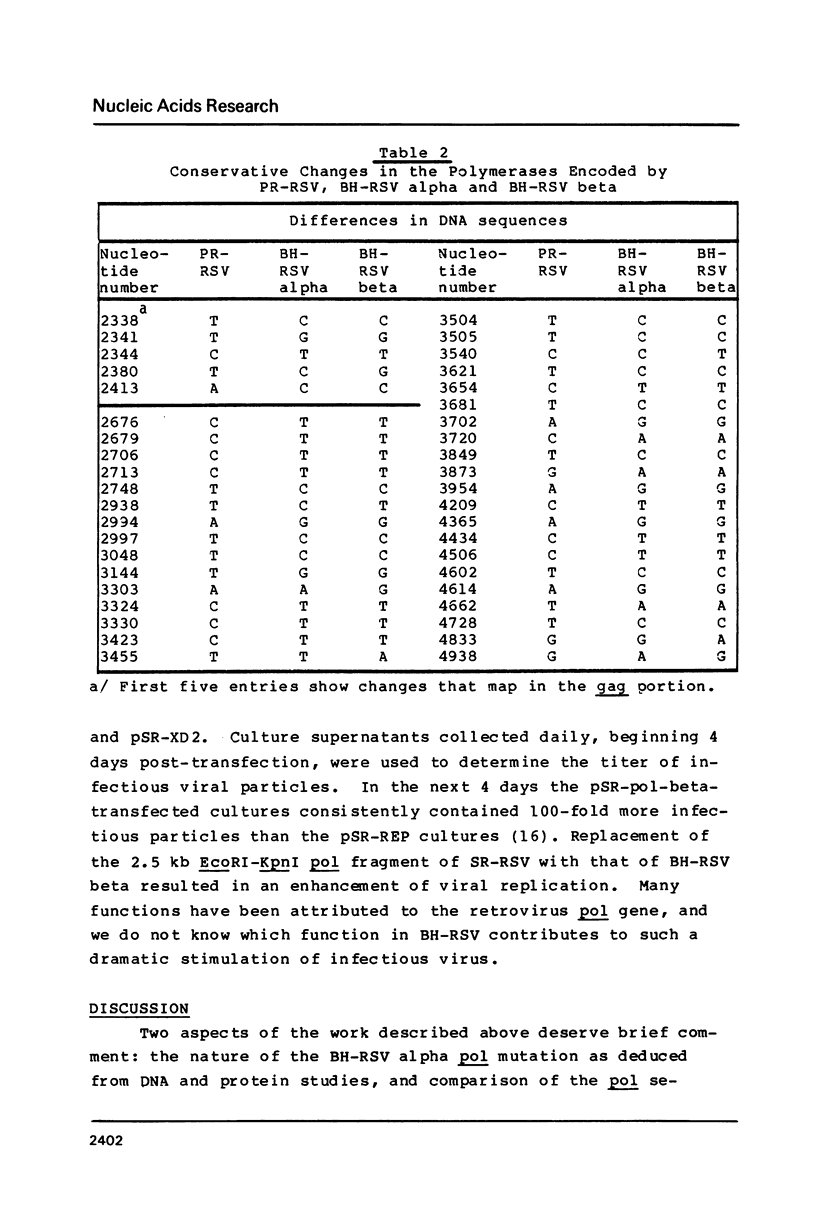
A mutant of the Bryan high-titer strain of Rous sarcoma virus defective in reverse transcriptase is known as type alpha (BH-RSV alpha). BH-RSV alpha virion particles do not contain any polymerase-related proteins but they direct the synthesis of a normal sized Pr180 gag-pol polyprotein precursor in infected cells. Using a bioassay for polymerase gene function that is based on the requirement of viral replication for transformation of transfected chicken cells, we have localized the defect to the 2.5 kb EcoRI-KpnI DNA fragment containing more than 90% of the polymerase gene by comparison with the corresponding DNA fragment from the wild-type polymerase-positive BH-RSV, called type beta. In vitro recombination experiments with the polymerase gene of Schmidt-Ruppin RSV allowed us to map the defect to the 0.86 kb XbaI-BglII DNA fragment of the BH-RSV alpha polymerase. DNA sequence analysis of the entire polymerase gene of BH-RSV alpha and beta has revealed one point mutation that maps within that XbaI-BglII fragment and substitutes leucine in BH-RSV alpha for glutamine in the wild-type BH-RSV beta.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYAN W. R., CALNAN D., MOLONEY J. B. Biological studies on the Rous sarcoma virus. III. The recovery of virus from experimental tumors in relation to initiating dose. J Natl Cancer Inst. 1955 Aug;16(1):317–335. [PubMed] [Google Scholar]
- BRYAN W. R. Enhancement of virus yield of Rous sarcomas. Acta Unio Int Contra Cancrum. 1959;15:764–767. [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Baltimore D., Smoler D., Watson K. F., Yaniv A., Spiegelman S. Absence of polymerase protein in virions of alpha-type rous sarcoma virus. Science. 1972 Sep 29;177(4055):1188–1191. doi: 10.1126/science.177.4055.1188. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Further studies on RSV production form transformed cells. Virology. 1968 Apr;34(4):630–636. doi: 10.1016/0042-6822(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner T. L., Hanafusa H. DNA sequence of the Bryan high-titer strain of Rous sarcoma virus: extent of env deletion and possible genealogical relationship with other viral strains. J Virol. 1984 Feb;49(2):549–556. doi: 10.1128/jvi.49.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]




