Abstract
CD105 is an auxiliary receptor for the transforming growth factor beta superfamily, highly expressed on proliferating endothelial cells and adult hematopoietic stem cells. Because CD105 mRNA expression was reported in the developing aortic region, we further characterized its expression profile in the aorta and examined the hematopoietic potential of CD105+ cells. Aortic endothelial cells, intra-aortic hematopoietic cell clusters and the purified cell fraction enriched in progenitor/hematopoietic stem cell activity expressed CD105. Aortic hematopoietic short-term clonogenic progenitors were highly enriched in the CD105intermediate population whereas more immature long-term progenitors/hematopoietic stem cells are contained within the CD105high population. This places CD105 on the short list of molecules discriminating short-term versus long-term progenitors in the aorta. Furthermore, decreasing transforming growth factor beta signaling increases the number of clonogenic progenitors. This suggests that CD105 expression level defines a hierarchy among aortic hematopoietic cells allowing purification of clonogenic versus more immature hematopoietic progenitors, and that the transforming growth factor beta pathway plays a critical role in this process.
Keywords: CD105, aorta-gonad-mesonephros, hematopoietic, short-term, long-term, progenitors
Introduction
The aorta-gonad-mesonephros (AGM) region at embryonic Day 10.5 (E10.5) was shown to produce a cohort of hematopoietic cells composed of short-and long-term hematopoietic progenitors (HPs) as well as hematopoietic stem cells (HSCs).1 Much effort has been devoted to the identification and purification of the different sub-categories of AGM-derived hematopoietic cells (HCs) but so far no simple marker allows discriminating short-term committed versus long-term HP/HSC. In addition, little is known about the signaling pathways controlling AGM-derived HP/HSC emergence, survival and proliferation. Multiple lines of evidence support a role for CD105/endoglin in blood and vascular development. CD105 is an ancillary receptor for members of the transforming growth factor beta (TGFβ) superfamily including TGFβ1/3, activin and bone morphogenetic proteins 2/7.2 CD105 is expressed at the surface of endothelial cells (ECs)3 and adult bone marrow HSCs,4,5 being part of the markers identifying adult long-term repopulating HSCs and defining discrete subsets in the erythromyeloid lineage.6 Besides its role in cardiovascular development, CD105 was shown to be essential for hematopoietic commitment from the hemangioblast7,8 and implicated in embryonic hematopoiesis.9
We, therefore, further characterized CD105 expression during AGM hematopoiesis. At E11, CD105 was expressed on endothelial and intra-aortic hematopoietic cell clusters containing long-term HPs/HSCs. Phenotypic characterization revealed that the majority of CD34+c-kit+ and CD144+CD45+ cells, enriched in long-term HPs/HSCs, were CD105+. Hematopoietic cluster cells displayed variable levels of CD105, suggestive of differential biological effects according to the expression level. Functional analyses indeed showed that clonogenic progenitors expressed CD105 and were highly enriched in the CD105intermediate(int) population, whereas more immature progenitors segregated with the CD105high(hi) population. Taken together these data point to the crucial role of CD105 in AGM hematopoiesis and show that its expression level allows to define a hierarchy among AGM hematopoietic cells to be defined, placing CD105 on the very short list of molecules that can discriminate short-term versus long-term progenitors in the AGM.
Design and Methods
Animals and tissue preparation
Embryos were generated from C57BL/6 mice. Day 0 is day of vaginal plugging (E0). AGMs were dissected and dissociated as previously described.10
Human embryos
Human embryonic tissues were obtained following voluntary, spontaneous or therapeutic abortions. Informed consent for the use of the embryo in research was obtained from the patient, and tissue collection and use were performed according to the guidelines and with the approval of the French National Ethics Committee. Developmental age was estimated as previously described.11
Immunostaining and flow-cytometric analysis
Embryos were processed as previously described.12 Frozen sections (10 μm) were cut on a cryostat (Leica). Anti-mouse CD105-PE (1/50) and CD105 (1/100) (Biolegend), anti-human CD105 (1/40, R&D), anti-mouse CD31 (1/100), anti-mouse CD144 (11D4, 1/50, BD) and anti-IgG Rat-HRP (1/100, BD) antibodies were used for immunostaining followed by TSA amplification (Perkin Elmer). Nuclei were labeled with DAPI 1/1000 (Sigma).
Anti-CD105-PE, CD144-Alexa647 (BV13, eBiosciences), CD34-FITC, CD41-FITC, CD45-FITC, c-kit-APC (BD) were used for flow cytometry. Cells were sorted on an Influx 500 cell sorter (Cytopeia, BD Biosciences) or a FACSDiva cell sorter (BD Biosciences). Analysis was performed on a MoFlo (Beckman-Coulter).
RT-PCR
Total RNA was prepared from 10×103 sorted cells using Trizol reagent. To avoid genomic contamination, RNA samples were treated with RNAse-free DNAse I.
Oligo-dT primers and reverse transcriptase (SuperscriptII) were used for cDNA synthesis. All reagents are from Invitrogen, Carlsbad, USA. Specific primers are listed in the Online Supplementary Table S1.
Colony-forming-cell assay
Sorted cells were seeded in methylcellulose (Methocult®-StemCell Tech). Colonies were scored at Day 11 for AGM explant cultures and at Day 10 or 14 for sorted cells.
Long-term culture-initiating cells assay (LTC-IC) and lymphoid switch
Serial half-dilutions of sorted cells were seeded over MS-5 stromal feeder cells in LTC-IC medium in 96-well microplates as previously described.10 Cultures were maintained at 37°C with weekly half-medium changes. The frequency of cobblestone area forming cells (CAFC) and hematopoietic cells (HC) was determined on Days 7 and 35 (LTC-IC) of culture using L-calc software (StemCell technologies). In some experiments, a lymphoid switch was performed at Day 35.13
Statistical analysis
The Mann-Whitney test was used to determine statistical significance. P<0.05 was considered statistically significant.
Results and Discussion
CD105 expression in the developing AGM
We analyzed mouse (E9 to E11) and human (30 days of gestation) AGM regions for CD105 expression during aortic hematopoiesis. CD105 was expressed by all vascular ECs of the mouse embryo as testified by the co-expression of CD105 with either CD31 or CD144 at E9, the earliest stage examined (Figure 1A and B and Online Supplementary Figure S1) and E10 (Figure 1C-E and Online Supplementary Figure S1). On the E11 embryo, CD105 was expressed by most if not all vessels and by regions known to harbor HSCs, i.e. the fetal liver and the AGM (Figure 1F). In this latter, CD105 expression was restricted to endothelial and hematopoietic cell clusters attached to the endothelium confirming previous findings based on CD105 mRNA detection.9 Immunohistology suggested the presence of variable levels of CD105 on mouse embryo hematopoietic clusters at E11 (Figure 1G). Interestingly, the same pattern was revealed on human embryo hematopoietic clusters (Figure 1H).
Figure 1.
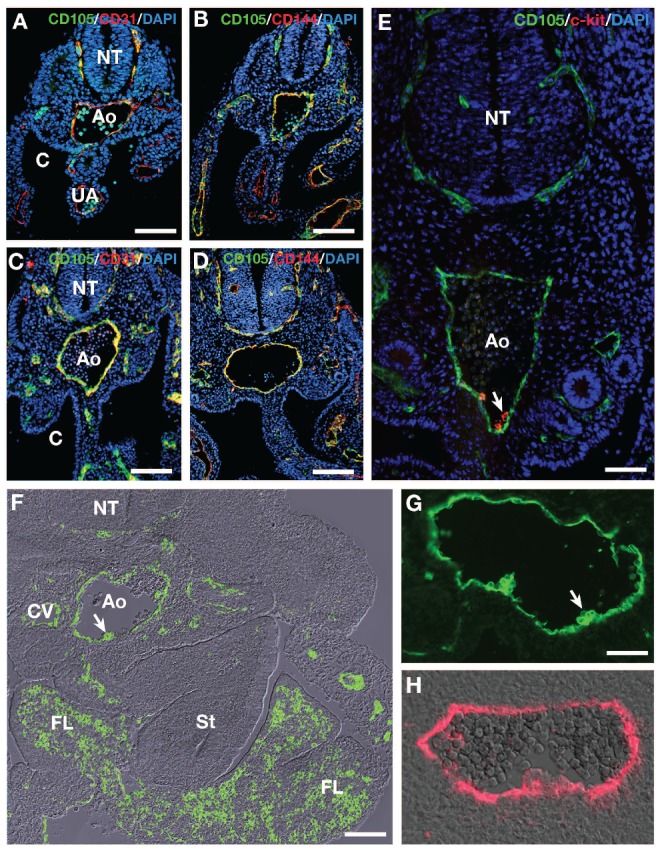
Immunofluorescence analysis of CD105, CD31, CD144 and c-kit expression in the mouse (A-G) AGM before and at the time of hematopoiesis and CD105 expression in the human AGM (H). (A and C) CD105/CD31/DAPI. (B-D) CD105/CD144/DAPI. (E) CD105/c-kit. Immunohistochemistry. Cross sections. (A and B) E9 embryos. Both CD31 (A) and CD144 (B) are co-expressed with CD105 in endothelial cells, as testified with the yellow color resulting from the overlay. Bar=30 μm C, (D) E10 embryos. Coexpression is similar to that seen at E9. Bar=50 μm. (E) E10 embryo. CD105 and c-kit are co-expressed at the level of the clusters. Bar=30 μm. (F) E11 mouse embryo; cross section. CD105 immunofluorescence merged with dichroic bright field. The signal is restricted to the endothelium and to hematopoietic cells of the fetal liver and the AGM. The white arrow points to a hematopoietic cluster in the aortic floor. Bar=100 μm. Ao: aorta; C: coelom; CV: cardinal vein; FL: fetal liver; NT: neural tube; St: stomach; UA: umbilical artery. (G) E11 mouse embryo; CD105 immunofluorescence. Close view of the aorta and hematopoietic clusters. The two hematopoietic cluster-composing cells display a variable level of CD105 expression. Bar=20 μm. (H) 30 day-old human embryo; cross section; CD105 immunofluorescence merged with dichroic bright field. A variable level of expression is also visible in the hematopoietic cluster. Bar=30 μm.
Most E11 AGM CD34+c-kit+ and CD144+CD45+ cells are CD105+
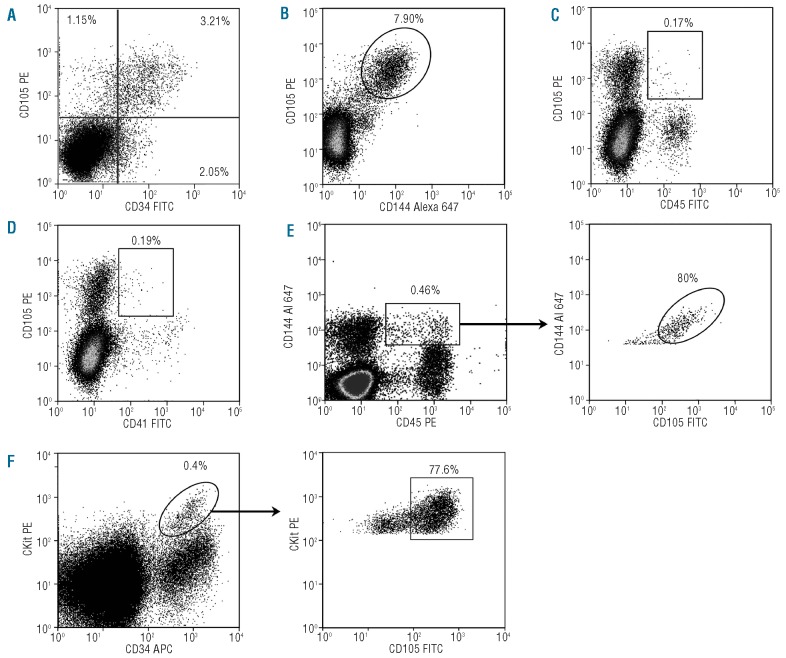
Flow cytometry was used to compare CD105 expression to known hematopoietic and endothelial markers; 4.91±1.56% of E11 AGM cells were CD105+ (n=7; data not shown). Double color FACS analysis showed that the majority of CD105+ cells expressed CD34 and CD144 (64.73±7.80% and 84.22±4.75%, respectively) (n=3; Figure 2A and B) while only 1.43±0.82% and 3.07±1.86% of CD105+ cells expressed CD45 and CD41, respectively (Figure 2C and D). We also analyzed CD105 expression in the CD144+CD45+ (Figure 2E) and CD34+c-kit+ (Figure 2F) AGM cell populations, known to be enriched in HSC activity.14,15 CD105 was present on the majority of CD144+CD45+ cells and CD34+c-kit+ cells at respectively 71.95±18.29% (n=2) and 78.72±10.09% (n=4).
Figure 2.
Endothelial and hematopoietic marker analysis of CD105 positive cells in the E11 AGM. Each pannel is the result of one representative experiment. Data in the text represent the mean of at least two to seven independent experiments. Whole E11 AGM cells were double stained with anti-CD105 and-CD34 (A), -CD144 (B), -CD45 (C), CD41 (D), and triple stained with either CD105, CD144 and CD45 (E) or CD105, c-kit and CD34 (F). Numbers indicate the percentage of positive cells in the corresponding gates.
CD105 expression levels discriminate long-term culture hematopoietic versus short-term clonogenic progenitor cells
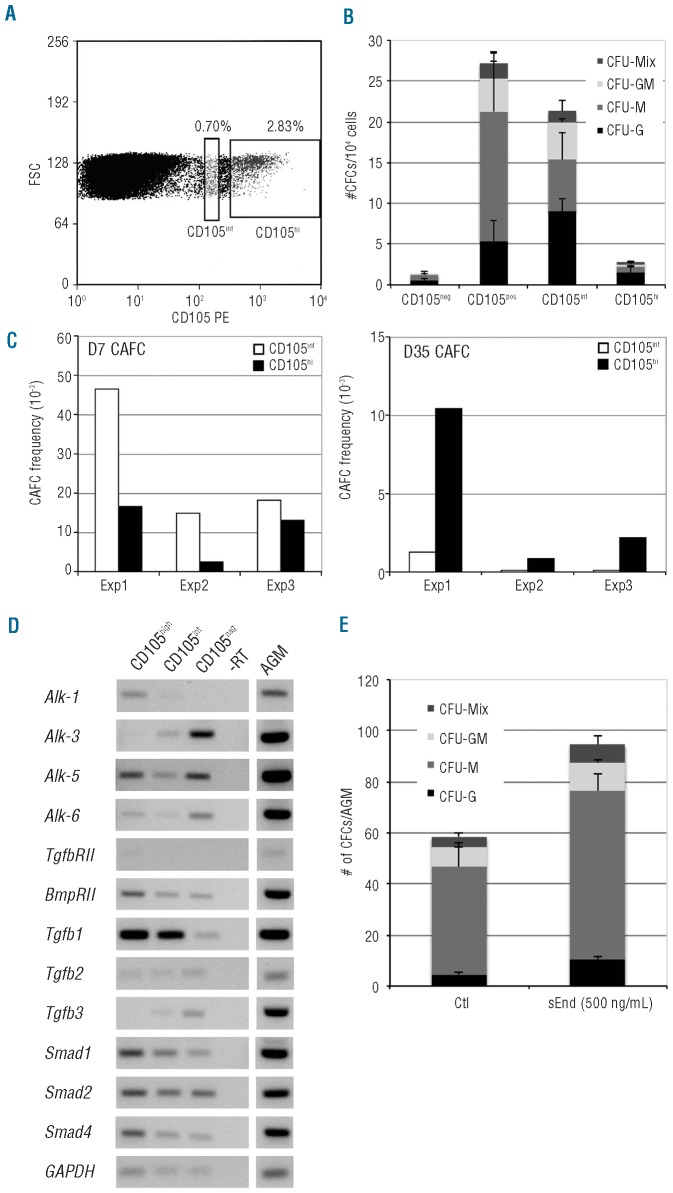
In keeping with the immunostainings on sections, AGM cells displayed a heterogeneous CD105 expression by FACS analysis (Figure 3A). CD105hi and CD105int populations represented 2.25±0.58% and 0.72±0.20% of cells, respectively (n=7). CD105int cells were smaller in size and less granular than CD105hi cells (Online Supplementary Figure S2). Under clonogenic conditions, hematopoietic progenitors were found mainly in the CD105 positive population (CD105− 1.33±0.35 CFU/AGM, CD105+ 27.21±4.43 CFU/AGM, P=0.001, n=3–6) (Figure 3B). We, therefore, decided to compare the in vitro potential of CD105hi and CD105int cells using clonogenic and LTC-IC assays. In this latter test, hematopoietic production (HC) and CAFCs were scored at Days 7 and 35 upon co-culture with MS5 stroma.16 Methylcellulose culture showed that the frequency of clonogenic progenitors was 4.6 to 10-fold higher in the CD105int population (CD105int 21.33±5.84, CD105hi 2.77±0.63, P=0.001, n=3) (Figure 3B). Enrichment of the CD105int population in hematopoietic progenitors (HP) was sustained by the fact that it also presented a higher Day 7 CAFC and HC frequency (3.4±1.8 and 3.8±0.9-fold, respectively, n=3) than the CD105hi population (Figure 3C). However, the LTC-IC potential was included in the CD105hi population (Figure 3C). Since Day 35 CAFC frequency was at least 8 to 10-fold higher than in the CD105int population (n=3) (Figure 3C). Furthermore, only the CD105hi population was able to produce B-lymphoid cells after 35 days of coculture, with 86% of HC being B220+CD19+ ten days after the lymphoid switch (Online Supplementary Figure S3A), and was endowed with long-term reconstitution ability when injected into lethally-irradiated mice (Online Supplementary Figure S3B). This last result is in keeping with the fact that the CD105hi population contains twice as much CD34+c-kit+ as CD105int population (CD105hi/CD105int=2.14±0.43, n=3). A highest expression of CD150 and CD105 mRNA levels was also reported in long-term versus short-term HSCs in adult bone marrow.6 Consistently, high levels of transcription factor or cell surface molecule expression, associated to immature hematopoietic progenitors or HSCs, have already been described in the bone marrow and in the AGM.17,18 Recently, a differential and spatial expression of CD41 (integrin GpIIb) has also been described. Contrary to CD105, the HSC potential was associated to the CD41int population in the E11 AGM.19
Figure 3.
Subpopulations of CD105 expressing cells in the E11 mouse AGM and analysis of their hematopoietic potentials. (A) FACS analysis of the CD105-expressing cells in the E11 mouse AGM. A CD105int (0.70%) and a CD105hi (2.83%) population are clearly visible. (B) Compared clonogenic potential of the CD105 sub-populations. After sorting, the CD105neg, CD105pos, CD105int and CD105hi sub-populations were plated in methylcellulose medium. Hematopoietic colonies were scored after 14 days of culture. Data represent the mean ± SEM from 3–6 independent experiments. (C) Comparison between Day 7 and Day 35 CAFC frequency in the CD105int and the CD105hi sub-populations. Exp1, Exp2, Exp3 are 3 independent experiments. (D) RT-PCR analysis of the BMP/TGFβ pathway in the CD105 populations in the E11 AGM. The presence of several TGFβ-specific receptors and the strong TGFβ1 expression suggest that a TGFβ pathway is active in CD105-positive cells. Primers and size of amplification products are given in the Online Supplementary Table S1. (E) Role of a soluble form of endoglin (sEnd) on the clonogenic potential of AGM cells. A 3-day explant culture of E11 AGM was performed in the absence or in the presence of 500ng/mL of sEnd. After collagenase dissociation, cells were plated in methylcellulose medium, and hematopoietic colonies were scored after 11 days. Data represent the mean ± SEM from 3 independent experiments.
Interestingly, we observed that co-expression of CD105 with c-kit led to the identification of an AGM cell population harboring a strong clonogenic potential highly enriched in CFU-GM and immature CFU-Mix progenitors (Online Supplementary Figure S4). Whereas the frequency of CFU-GM and CFU-Mix within the c-kit+CD105− populations is 1 for 652 and 1453, respectively, it reaches 1 for 64 for both in the c-kit+CD105+ fraction (Online Supplementary Figure S4B).
TGFβ signaling regulates AGM hematopoietic activity
In line with the study of Marshall et al. on the human embryo20 RT-PCR analysis showed that CD105+ cells from the AGM expressed members of the TGFβ signaling pathway, in particular TGFβ1 (Figure 3D and Online Supplementary Table S1).
We then performed AGM explant cultures with or without soluble recombinant endoglin (sEnd) that prevents TGFβ1-receptor binding.21 Addition of sEnd increased the total number of clonogenic progenitors by 1.61-fold (control 58.50±11.11, 500ng/mL sEnd 94.50±7.42, P=0,001, n=3), demonstrating that down-regulating the TGFβ pathway increased the survival/growth of hematopoietic progenitors. As shown in Figure 3E, the strongest effects were seen on CFU-M and CFU-G (control 42±8.12, sEnd 66±6.38, control 4.5±1.15, sEnd 10.5±0.72, P=0.001, n=3, respectively), whereas no significant effect was observed on CFU-GM and CFU-Mix (control 8±1.80, sEnd 11±0.88, P=0.2; control 4±1.60, sEnd 7±3.28, P=0.35, n=3, respectively). These results point to the critical role of the TGFβ pathway during AGM hematopoiesis which negatively regulates the survival/growth of already committed AGM hematopoietic progenitors. Taken together, these data place CD105 on the short list of molecules known to be differentially expressed in mature versus immature progenitors/HSCs and to exert a critical biological role in HPs/HSCs specification in the AGM.
Acknowledgments
We thank Dr E Oberlin for providing the human embryo and S Gournet for excellent image preparation. We are grateful to D Clay (Institut André Lwoff, Villejuif) and A Munier (IFR83, UPMC Paris) for cell sorting and analysis. We thank Dr N Boggetto (Institut Jacques Monod, ImagoSeine Bioimaging Core Facility, Paris, France) for cell sorting and expert Imagestream analysis.
Footnotes
The online version of this article has a Supplementary Appendix:
Funding: this work was supported by the CNRS, INSERM, UPMC, by a grant from ARC/INCA and by the Fondation pour la Recherche Médicale. MR was supported by the French Ministère de l’Education Nationale de la Recherche et de la Technologie (MENRT).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 2.Lebrin F, Mummery CL. Endoglin-mediated vascular remodeling: mechanisms underlying hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2008;18(1):25–32. doi: 10.1016/j.tcm.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–30. [PubMed] [Google Scholar]
- 4.Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265(15):8361–4. [PubMed] [Google Scholar]
- 5.Chen CZ, Li M, de Graaf D, Monti S, Gottgens B, Sanchez MJ, et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci USA. 2002;99(24):15468–73. doi: 10.1073/pnas.202614899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1(4):428–42. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Perlingeiro RC. Endoglin is required for hemangioblast and early hematopoietic development. Development. 2007;134(16):3041–8. doi: 10.1242/dev.002907. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Magli A, Catanese J, Xu Z, Kyba M, Perlingeiro RC. Modulation of TGF-β signaling by endoglin in murine hemangioblast development and primitive hematopoiesis. Blood. 2011;118(1):88–97. doi: 10.1182/blood-2010-12-325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimanda JE, Chan WY, Wilson NK, Smith AM, Kinston S, Knezevic K, et al. Endoglin expression in blood and endothelium is differentially regulated by modular assembly of the Ets/Gata hemangioblast code. Blood. 2008;112(12):4512–22. doi: 10.1182/blood-2008-05-157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petit-Cocault L, Volle-Challier C, Fleury M, Peault B, Souyri M. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development. 2007;134(16):3031–40. doi: 10.1242/dev.001818. [DOI] [PubMed] [Google Scholar]
- 11.Oberlin E, Fleury M, Clay D, Petit-Cocault L, Candelier JJ, Mennesson B, et al. VE-cad-herin expression allows identification of a new class of hematopoietic stem cells within human embryonic liver. Blood. 2010;116(22):4444–55. doi: 10.1182/blood-2010-03-272625. [DOI] [PubMed] [Google Scholar]
- 12.Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development. 2006;133(6):1013–22. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- 13.Lemieux ME, Rebel VI, Lansdorp PM, Eaves CJ. Characterization and purification of a primitive hematopoietic cell type in adult mouse marrow capable of lymphomyeloid differentiation in long-term marrow “switch” cultures. Blood. 1995;86(4):1339–47. [PubMed] [Google Scholar]
- 14.Sanchez MJ, Holmes A, Miles C, Dzierzak E. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity. 1996;5(6):513–25. doi: 10.1016/s1074-7613(00)80267-8. [DOI] [PubMed] [Google Scholar]
- 15.Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132(18):4179–91. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- 16.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89(5):1543–50. [PubMed] [Google Scholar]
- 17.Nutt SL, Metcalf D, D’Amico A, Polli M, Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J Exp Med. 2005;201(2):221–31. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA. 2007;104(22):9399–403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robin C, Ottersbach K, Boisset JC, Oziemlak A, Dzierzak E. CD41 is developmentally regulated and differentially expressed on mouse hematopoietic stem cells. Blood. 2011;117(19):5088–91. doi: 10.1182/blood-2011-01-329516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96(4):1591–3. [PubMed] [Google Scholar]
- 21.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]