Abstract
Background
Mantle cell lymphoma accounts for 6% of all B-cell lymphomas and is generally incurable. It is characterized by the translocation t(11;14) leading to cyclin D1 over-expression. Cyclin D1 is downstream of the mammalian target of rapamycin threonine kinase and can be effectively blocked by mammalian target of rapamycin inhibitors. We set out to examine the single agent activity of the orally available mammalian target of rapamycin inhibitor everolimus in a prospective, multicenter trial in patients with relapsed or refractory mantle cell lymphoma (NCT00516412).
Design and Methods
Eligible patients who had received a maximum of three prior lines of chemotherapy were given everolimus 10 mg for 28 days (one cycle) for a total of six cycles or until disease progression. The primary endpoint was the best objective response. Adverse reactions, progression-free survival and molecular response were secondary endpoints.
Results
Thirty-six patients (35 evaluable) were enrolled and treatment was generally well tolerated with Common Terminology Criteria grade ≥3 adverse events (>5%) including anemia (11%), thrombocytopenia (11%) and neutropenia (8%). The overall response rate was 20% (95% CI: 8–37%) with two complete remissions and five partial responses; 49% of the patients had stable disease. At a median follow-up of 6 months, the median progression-free survival was 5.5 months (95% CI: 2.8–8.2) overall and 17.0 (6.4–23.3) months for 18 patients who received six or more cycles of treatment. Three patients achieved a lasting complete molecular response, as assessed by polymerase chain reaction analysis of peripheral blood.
Conclusions
Everolimus as a single agent is well tolerated and has anti-lymphoma activity in relapsed or refractory mantle cell lymphoma. Further studies of everolimus in combination with chemotherapy or as a single agent for maintenance treatment are warranted. (Clinicaltrials.gov identifier: NCT00516412)
Keywords: everolimus, RAD001, mantle cell lymphoma, relapsed, refractory
Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of B-cell lymphoma composed of small to medium sized lymphoid cells, which originate from CD5-positive follicle mantle B cells.1–3 It is characterized on a molecular level by the t(11;14)(q13;q32) translocation that results in deregulated aberrant expression of cyclin D1.4,5 MCL patients typically present with advanced-stage disease at a median age of 60 to 65 years and have a median survival of approximately 5 years.6
The first-line treatment of MCL frequently includes rituximab-containing immunochemotherapies which can be successful in achieving durable remissions but overall long-term survival still remains poor.7–9 Early aggressive therapy appears to provide an advantage to some young patients but the impact on overall survival is not yet defined.10 R-CHOP-like, R-HyperCVAD, R-DHAP or R-VAD+C polychemotherapy regimens are most frequently used as front-line therapies for young and/or fit MCL patients.7,9,11 Those patients achieving a good response to initial therapy should be considered for consolidation by high-dose chemotherapy followed by autologous stem cell transplantation.9,12,13
However, many patients will not be valid candidates for aggressive immunochemotherapy given that MCL is diagnosed in a substantial proportion of elderly patients. Additionally, even patients treated with intensive first-line treatment will relapse and require subsequent therapy. Drugs commonly used in relapsed patients include rituximab, fludarabine, bendamustine, bortezomib and chlorambucil, as well as other new investigational agents.14–16 Published results in the salvage setting are rare and response rates vary, but it is well accepted that the duration of response in this setting is usually short. There is, therefore, a clear need for additional novel drugs in this disease.
The presence of a genetic event - translocation t(11;14)(q13;q32) with subsequent over-expression of the cyclin D1 protein - has shifted the focus onto molecular targeted agents and identified the mammalian target of rapamycin (mTOR) threonine kinase as a potential candidate.17 The mTOR pathway is involved in intracellular pro-survival signaling and its activation leads to G1 to S phase cell cycle progression. Recent studies have demonstrated that mTOR inhibitors down-regulate the transcription of the cyclin D1 message18 which in turn leads to a decrease of cyclin D1 protein levels as shown in several solid cancer models.19,20 One can speculate that inactivation of mTOR may play a major role in decreasing cyclin D1 in MCL as well, since rapamycin treatment effectively induced cell cycle arrest and apoptosis in two MCL cell lines studied.21 Temsirolimus (CCI-779) was the first intravenously administered mTOR inhibitor to be studied in patients with relapsed or refractory MCL22 and has recently gained approval for this indication.
Everolimus (RAD001; 40-O-[2-hydroxyethyl]-rapamycin) is a potent, orally bioavailable inhibitor of the mTOR pathway which effectively inhibits the proliferation and growth of a number of cancer cell lines in vitro and a range of tumor types in experimental animal models.23 Moreover, everolimus exhibits an anti-angiogenic activity, which may also contribute to its anticancer activity. Everolimus has been approved for the treatment of advanced metastatic renal cell carcinoma24 and is under consideration for approval for other indications such as primitive neuroectodermal tumors. The preliminary efficacy of everolimus, given as a single agent to 77 patients suffering from a broad range of aggressive subtypes of relapsed lymphoma has recently been demonstrated.25 Apart from a reported overall response rate of 32% for 19 MCL patients, no detailed information on efficacy or toxicity was presented for the MCL population.
Here we report the toxicity and activity profile of everolimus in a phase II single agent everolimus trial performed by the European Union MCL network specifically restricted to patients with relapsed or refractory MCL.
Design and Methods
Patients
Patients at least 18 years of age were included in this trial if they had histologically confirmed relapsed or chemotherapy-resistant MCL and had a World Health Organization performance status ≤ 2. At most, three previous lines of chemotherapy were permitted. Induction chemotherapy followed by high-dose chemotherapy with autologous stem cell support was considered as one line of treatment. A complete medical evaluation within 3 weeks prior to treatment included history of previous treatments, a physical examination with classification of performance status, blood counts, liver and renal parameters. Adequate hematologic values were defined as a neutrophil count ≥ 1.5×109/L and platelet count ≥ 100×109/L or, in the case of bone marrow infiltration, neutrophil count ≥ 1.0×109/L and platelet count ≥ 75×109/L. Women of child-bearing potential had to use effective anti-contraceptive measures. Tumor assessments were carried out using computed tomography scans of the neck, thorax, abdomen and pelvis. At least one measurable lesion of 15 mm in its greatest transverse diameter had to be present. Bone marrow aspirates and biopsies were performed at the beginning and the end of treatment. Assessment after each cycle included physical examination and blood tests (hemoglobin, white blood cell, neutrophil, and platelet counts, aspartate amino transferase and/or alanine amino transferase, alkaline phosphatase, bilirubin, creatinine and lactate dehydrogenase).
The institutional review boards of all participating centers approved the study protocol. The study was conducted according to the international standards of good clinical practice. All patients had to provide their written informed consent. The trial was registered at the National Institute of Health (www.clinicaltrials.gov; identifier number: NCT00234026) and performed in collaboration with two Italian centers and two European groups [the Swiss SAKK and the French Groupe Ouest Est d’Etude des Leucémies et Autres Maladies du Sang (GOELAMS)] of the European Mantle Cell Lymphoma Network.
Treatment and follow-up
The study drug everolimus (RAD001) was provided by Novartis Switzerland and all patients were instructed to swallow a 10 mg dose (two 5 mg tablets) daily. For the sake of consistency, the drug had to be taken at the same time each day in a fasting state or with a light fat-free meal. If vomiting occurred, no replacement was given. Everolimus was taken daily for six cycles (1 cycle = 28 days) or until disease progression or discontinuation from the study for any other reason. Patients benefiting from treatment, i.e. achieving at least disease stabilization as defined by the response criteria given at the end of cycle 6 were allowed to continue treatment until disease progression or until medically indicated. However, patients were transferred to the follow-up phase after six cycles regardless of whether or not they continued with treatment.
Adverse events were defined according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Dose adjustments and interruptions of treatment had to be performed if CTCAE grade ≥ 2 occurred and were managed with a delay of treatment (CTCAE grade 2), a delay of treatment and a dose reduction to 5 mg (CTCAE grade 3), or discontinuation of treatment (CTCAE grade 4). If a patient had already decreased medication by two dose levels, no further dose reductions were permitted and the patient permanently discontinued treatment and was transferred to follow-up. In addition, the patient was transferred to follow-up if treatment was interrupted for a period of >14 days, or if more than 50% of the study medication was missed in a given cycle.
Tumor assessment was performed every three cycles as at baseline according to the International Working Group criteria published in 1999.26 Bone marrow aspirate, gastroscopy and a colonoscopy were only performed for the final evaluation in the case of initial involvement of the bone marrow, stomach or colon. All patients were followed up until either documented objective disease progression, the start of any other anticancer treatment or death.
Central pathology review and molecular follow-up
Patients’ biopsy samples underwent central pathology review including assessment of the MIB-1 index, immunophenotypic profiling and fluorescence in situ hybridization (FISH) analysis for the t(11;14)(q13;q32) translocation.27 The mutational status of the variable immunoglobulin heavy chain (IGHV) genes was determined as described elsewhere.27,28 Cases with a homology rate lower than 98% when compared to the closest germ-line IGHV-sequence in the IMGT database (https://imgt.cines.fr/) using the IMGT/V-QUEST software were considered mutated.29
In addition, bone marrow and peripheral blood samples were analyzed in a central laboratory by polymerase chain reaction (PCR) at baseline for the presence of the chromosomal translocation t(11;14)(q13;32) and, in negative cases, for monoclonal IGHV rearrangement, which were to be used as molecular markers during the follow-up. The t(11;14) was evaluated as described elsewhere,27 while monoclonal IGH rearrangements were assessed using the IGH Gene Clonality Assay targeting the FR3-JH segments (InVivoScribe Technologies, San Diego, CA, USA). Samples were scored according to the kit manufacturer’s guidelines. Briefly, at baseline, a sample was scored positive when a PCR product could be demonstrated within the size range expected from the set of primers in use. A sample was scored negative when it did not show a PCR product within the size range expected from the set of primers in use, in the presence of a positive control and if the same DNA sample had given the expected products using a set of primers to evaluate its quality. For follow-up samples, a sample was scored positive when it showed a PCR product of the same size as at baseline; a sample was scored negative as defined at baseline. Only samples positive at baseline were analyzed during the follow-up.
Statistical analysis
The primary endpoint was defined as the best objective response (complete remission and partial remission) as determined by the International Working Group criteria of 1999.26 Secondary endpoints included: (i) adverse drug reactions as assessed by NCI CTCAE v3.0; (ii) progression-free survival as calculated from registration until progression of disease or until death;26,27 (iii) molecular response as defined by a negative PCR after treatment in a patient with a previously positive PCR result at baseline.
The total number of patients was calculated using Simon’s optimal two-stage design. Everolimus was to be considered uninteresting if the objective response rate was ≤ 10% and promising if ≥ 30%. For a 5% significance level and a power of 90%, a total sample size of 35 treated patients assessable for the primary endpoint was required with 18 patients needed for stage I. At stage I, if there were two or fewer responders among the first 18 patients, then the trial would be closed and everolimus would be rejected for further investigations. If, at the end of stage II, there were fewer than six responders the trial therapy would be considered not promising.
Adverse events were summarized by event type and grade over the total number of therapy cycles administered as well as within patients (worst recorded adverse event grade per event type per patient). Best response while on treatment for all patients was considered as well as the best response for all patients completing all treatment cycles. For time-to-event endpoints such as progression-free survival patients not showing an event were censored at the time of the last follow-up or at the end of treatment when appropriate. The data were analyzed using SAS (Statistical Analysis Systems, version 9.2) and R 2.12.0 (www.r-project.org) based on the intention-to-treat principle.
Results
Patients’ characteristics
Between August 2007 and January 2010, a total of 36 patients (35 evaluable) were recruited from 19 centers in Switzerland, Italy and France (Table 1). One patient had progressive disease before receiving the first dose and was replaced according to the protocol. A central pathology review was performed in 33 cases and confirmed the diagnosis of non-blastoid MCL. Morphologically, the tumors corresponded to typical MCL with a proliferation index as measured by the nuclear MIB-1 immunoreactivity (31 patients) ranging from 0.3% to 72% (mean, 26%). All 33 cases were invariably positive for cyclin D1 protein and the presence of the t(11;14)(q13;q32) chromosomal translocation was shown in 30/30 cases by FISH analysis. Ten out of 22 (45%) cases had mutated IGHV.
Table 1.
Patients’ baseline characteristics.
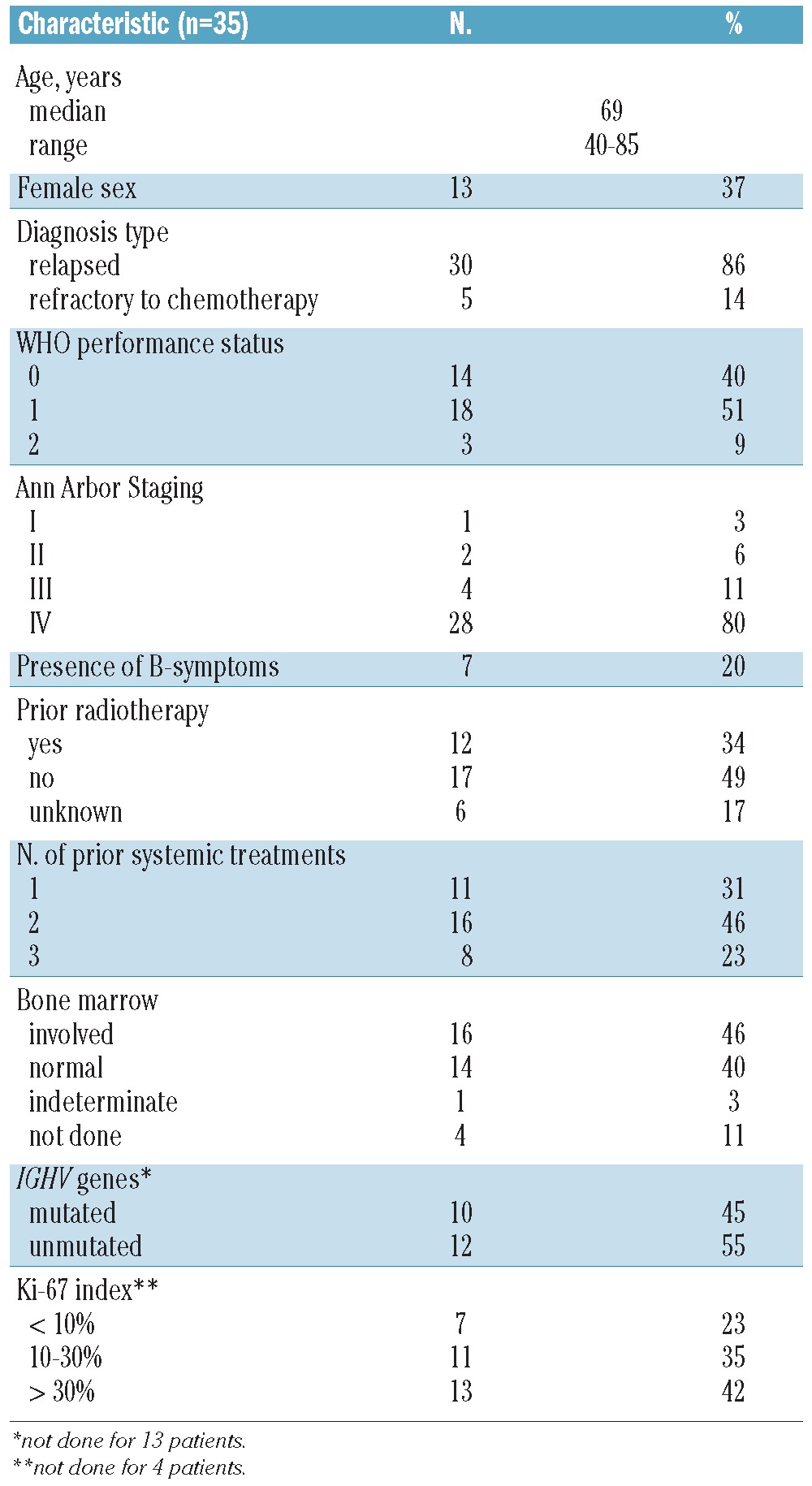
Our series of patients in this clinical trial was representative of patients with MCL in general with a median age of 69 years, a male predominance (63%), a World Health Organization performance status of predominantly 0–1 and the majority of patients (80%) presenting with advanced (stage IV) disease. Bone marrow involvement was diagnosed in 16 patients (52%) by histomorphological criteria and in 14 (56%) out of 25 evaluable bone marrow samples using the more sensitive PCR technology. Five patients presented with bulky disease, defined as a lymphoma mass bigger than 10 cm in its greatest diameter. Twenty-four patients (69%) had received at least two prior treatment regimens and almost all patients (31 of 35) had received at least one rituximab-containing regimen. Three patients had been previously treated with high-dose chemotherapy followed by autologous stem cell support. A detailed list of previous treatment regimens is given in Online Supplementary Table S1.
Clinical results
Primary endpoint
The predefined criterion of three responders at the interim analysis was met, which allowed the study to be continued and finished as planned. Seven out of 35 evaluable patients achieved either a complete remission (n=2, 6%) or partial remission (n=5, 14%) leading to an objective response rate of 20% (95% CI: 8–37%). In addition, 17 patients (49%) achieved disease stabilization while 10 patients (29%) did not respond to treatment at any time.
Secondary endpoints
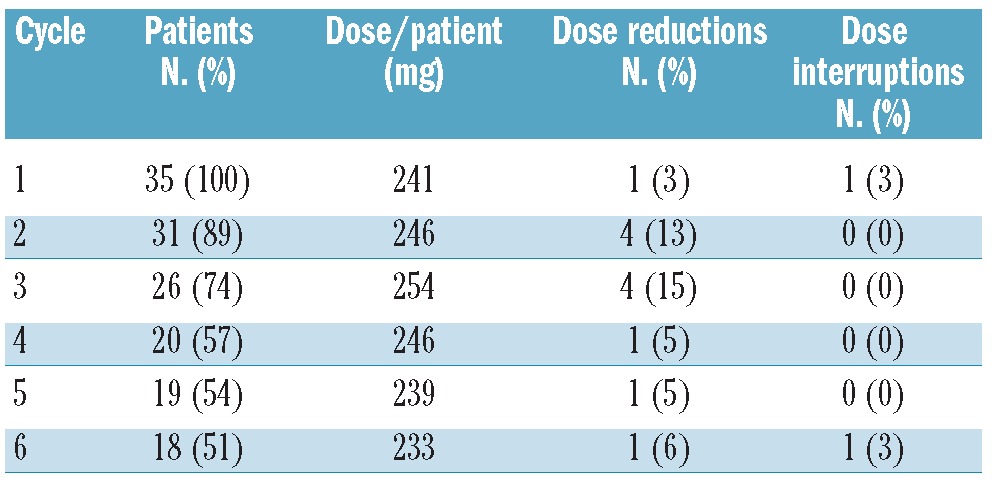
The study drug was well tolerated as the administered dose of everolimus was stable throughout all cycles for patients still on treatment (Table 2). Eighteen patients received six or more cycles of everolimus treatment, while 17 patients had to stop treatment prematurely. The major reason for treatment discontinuation was progressive disease (13 patients).
Table 2.
Number of cycles and dose administered.
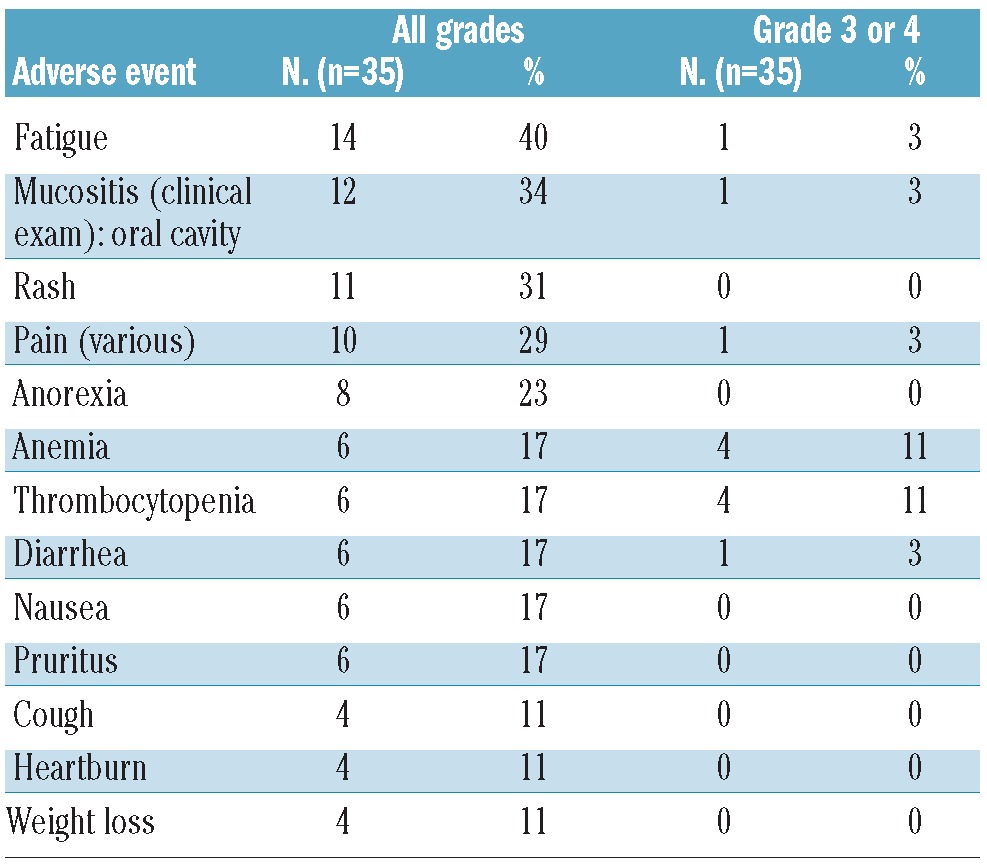
Analysis of adverse events per cycle showed that there was no clear link of toxicity to time of occurrence and adverse events were equally distributed throughout all cycles. Hematologic adverse events (Table 3) were generally mild with grade 3/4 anemia and thrombocytopenia (n=4, 14%) being the most frequent ones. Non-hematologic adverse events of any grade included fatigue (n=14, 40%), mucositis of the oral cavity (n=12, 34%), rash (n=11, 31%), pain (n=10, 29%), anorexia (n=8, 23%), diarrhea (n=6, 17%), nausea (n=6, 17%) and pruritus (n=6, 17%). None of the grade 3/4 non-hematologic adverse events occurred at a frequency higher than 3%. Pneumonitis/pneumonia was reported once (grade 2) as an adverse event and twice (grade 3) as a serious adverse event (out of a total of 11 serious adverse events) with likely relationship to the study drug.
Table 3.
Adverse events occurring in at least 10% of the patients.
With a median follow-up of 6 months, median progression-free survival on an intent-to-treat basis was 5.5 months (95% CI 2.8–8.2) (Figure 1) for the entire population. When divided into responders (including patients with complete or partial remission and stable disease) and non-responders, the median progression-free survival was 17.0 months (95% CI 6.4–23.3) for the former group and 2.7 months (95% CI 1–3) for the latter group. There were no significant differences regarding progression-free survival or objective response rate when patients were analyzed according to IGHV mutation status.
Figure 1.
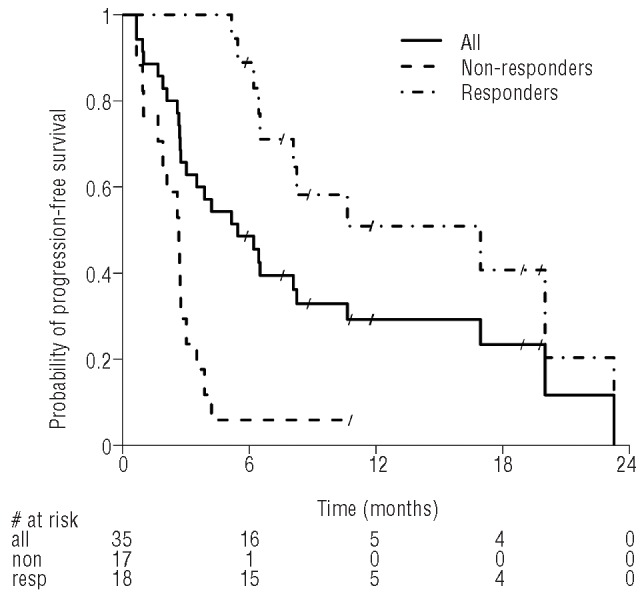
Kaplan Meier estimate of progression-free survival (PFS) for the whole population (––––) with a median PFS of 5.5 months (95% CI 2.8–8.2). The median PFS is 2.7 months (95% CI 1.0–3.0) for non-responders (– – –) and 17.0 months (95% CI 6.4–23.3) for responders (.–. –.). By definition, there can be no events within the first 6 months for the responders.
The peripheral blood of 30 patients was analyzed at baseline by PCR for the presence of a molecular marker, which was detected in 21/30 (70%) patients: t(11;14) in six cases and clonal IGHV rearrangement in 16 samples. Three out of 16 (19%) patients evaluated during follow-up achieved a molecular remission, defined as peripheral blood negative for the marker, which was maintained at the last follow-up (at 5, 6 and 7 months in the three patients). One of these patients had achieved a complete remission, one a partial remission and one stable disease according to nodal status analyzed by computed tomography. All the other 13 patients remained PCR-positive up to their last follow-up analysis (two at 2 months, five at 3, two at 4, three at 6, and one at 7 months).
Discussion
Here, we report on the results of a multicenter European prospective phase II trial of the orally available mTOR inhibitor everolimus in 35 patients with relapsed or refractory MCL. The treatment resulted in an objective response rate of 20% and 49% patients had stable disease. The median progression-free survival was 5.5 months for the entire population and 17.0 months for the patients who received six or more cycles of treatment. Three patients achieved a long-lasting complete molecular response, as assessed in the peripheral blood.
Inspired by the observation that the PI3K/AKT/mTOR pathway is constitutively activated in MCL, clinical trials using mTOR inhibitors as single agents have gained much attention in recent times. So far, temsirolimus is the most extensively studied mTOR inhibitor approved in Europe for the treatment of patients with refractory or relapsed MCL. This approval was based on a randomized, open-label phase III trial demonstrating that, in 54 patients who received a loading dose of temsirolimus with 175 mg weekly for 3 weeks followed by 75 mg weekly until disease progression, the objective response rate was 22%, including one complete remission and 11 partial responses (95% CI, 11–33, P=0.0019).22 Progression-free survival was significantly longer in the temsirolimus arm than in the control arm, defined as the investigator’s choice of therapy (4.8 versus 1.9 months, P<0.0009). These efficacy data correspond well with our results presented here on everolimus with an objective response rate of 20% and 5.5 months median progression-free survival in a similar setting. Importantly, everolimus has some properties that might render it more attractive than temsirolimus or the parent compound rapamycin.30 Everolimus is rapidly absorbed after oral administration and, in cancer patients, has a terminal half-life of 30 hours favoring daily oral intake.30 In contrast, temsirolimus is a naturally occurring water-soluble ester analog of rapamycin with a low oral bioavailability and is metabolized to rapamycin with both molecules exhibiting a similar potency of mTOR inhibition.30 However, the terminal half-life of rapamycin is about three times that of temsirolimus (54.6 hours versus 17.3 hours) indicating that both molecules are present in blood after intravenous administration at different levels over time.30 The long half-life of rapamycin suggests that a weekly dosing schedule for temsirolimus should be preferred in order to avoid accumulation of its derivative rapamycin. These differences in drug metabolism might be the reason for the slightly different toxicity profile seen for everolimus and temsirolimus in MCL patients. According to data from Hess et al.,22 the hematologic toxicity of temsirolimus, with grade 3/4 thrombocytopenia of 59% and anemia of 20%, seems to be higher than that of everolimus (11% for both adverse events) in our study. This difference might be advantageous when planning trials with combination treatments in the future. However, as a note of caution, the number of prior treatment regimens in the temsirolimus trial was not restricted which is reflected by a mean of three prior treatment regimens compared to two in our trial. A major concern related to both drugs is their potential to cause severe pneumonitis. Three patients in our study developed pneumonitis-like syndromes of grade 2 (n=1) or grade 3 (n=2) with a likely or more definitive attribution to the study drug.
The median progression-free survival observed in our trial was comparable to that reported for temsirolimus give at a dose of 250 mg or 25 mg weekly with median times to progression of 6.5 months (95% CI, 2.9–8.3 months) and 6 months (95% CI, 3–11 months), respectively.31,32 The small sample size of our study did not allow any meaningful correlation of response to treatment or duration of response to patients’ baseline characteristics. In addition, stratifying patients into three groups according to whether their MIB-1 index was less than 10% (n=7), 10% to 30% (n=11), or more than 30% (n=13) did not reveal any significant differences in the progression-free survival analysis (P=0.99).
The presence of minimal residual disease (MRD) after combined immunochemotherapy can be a powerful predictor of treatment outcome in MCL patients.33 Recent data suggest that the absence of MRD is a major goal for MCL therapy and the re-appearance MRD, as a sign of molecular relapse, could be counteracted by prompt rituximab treatment.34 In our trial, three patients became PCR negative in the peripheral blood under everolimus treatment. The clearance of circulating neoplastic cells occurred early during treatment (in one case during cycle 2 and in the other two cases during cycle 3) and lasted during the follow-up (up to 7 months). MRD negativity in the blood was not paralleled by a complete remission of nodal lesions in all three patients since only one achieved a nodal complete remission; one of the two other patients, one had a partial remission, the other had stable disease. Several study groups, including ours, reported similar observations for single-agent rituximab in MCL or follicular lymphoma;35,36 the clinical significance of these findings is unclear. The number of patients who achieved a negative MRD status was too small to correlate this occurrence with any other primary or secondary endpoint. However, to our knowledge this is the first report of such a result in lymphoma patients treated with an mTOR inhibitor. The demonstration of clearance of circulating neoplastic cells suggests that the drug might be able to remove MRD rendering it a candidate drug for consolidation regimens.
Previous data from the European Union MCL network showed that four to six cycles of standard CHOP chemotherapy did not significantly reduce levels of MRD and no patient achieved a molecular response after induction.37 When rituximab chemotherapy was introduced, the molecular response rate increased to 56% among all treated patients and increased molecular response rates were paralleled by superior clinical response rates. Achievement of a molecular response after induction treatment correlated strongly with prolonged duration of remission. A molecular response is, therefore, a desirable goal in the treatment of patients with MCL. Our observation that everolimus was able to remove circulating lymphoma cells should be further studied in future trials evaluating the drug either as part of a multi-drug regimen such as in combination with rituximab38 or as single-agent maintenance therapy.
mTOR inhibitors seem to have a similar efficacy profile as other drugs used as single agents for the same indication. The recently updated multicenter phase 2 PINNACLE study on bortezomib confirmed an objective response rate of 32% and a median time-to-progression of 6.7 months (95% CI, 4–7.3 months).39 Bortezomib was well tolerated with lymphcytopenia (34%) and neuropathy (13%) being the most frequently reported grade 3 or higher adverse events. A direct comparison with upcoming new drugs is appealing but still very difficult since preliminary data have only been reported so far. Immunomodulatory drugs such as lenalidomide seem to produce a high objective response rate (42%) but similar progression-free survival (5.7 months).40 When focusing on the mTOR pathway, interesting data have been reported for PI3K inhibitors (such as CAL101), which block the same PI3K/AKT/mTOR pathway upstream of mTOR. Six out of seven MCL patients with relapsed or refractory hematologic malignancies responded to PI3K inhibitor treatment indicating that this new class of compounds might be very active in this disease entity.41
In summary, our data suggest that everolimus is a therapeutic option worth further testing either as a single agent for consolidation treatment or in combination with other drugs to improve the still very poor survival of patients with MCL.
Acknowledgments
We would like to acknowledge the SAKK team, the data manager of the GOE-LAMS group, Mrs Valérie Rolland, and the local investigators/nurses for collecting and validating all the data. We would also like to thank Volker Thiel, Diana Förbs, Claudia Zimmermann and Doris Kradolfer for DNA and FISH analyses. Finally, we would like to thank all patients and physicians who participated in this trial.
Footnotes
Funding: this study was supported in part by Novartis Pharma Schweiz AG, the Swiss State Secretariat for Education and Research (SER) and the Fondazione per la Ricerca e la Cura sui Linfomi (FB).
This trial was partially presented at the 52nd ASH Annual Meeting, 2010.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78(2):259–63. [PubMed] [Google Scholar]
- 2.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7(10):750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985;315(6017):340–3. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- 5.Meeker TC, Sellers W, Harvey R, Withers D, Carey K, Xiao H, et al. Cloning of the t(11;14)(q13;q32) translocation breakpoints from two human leukemia cell lines. Leukemia. 1991;5(9):733–7. [PubMed] [Google Scholar]
- 6.Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–8. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 7.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23(26):6409–14. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 8.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114(8):1469–76. doi: 10.1182/blood-2009-02-179739. [DOI] [PubMed] [Google Scholar]
- 9.Gressin R, Caulet-Maugendre S, Deconinck E, Tournilhac O, Gyan E, Moles MP, et al. Evaluation of the (R)VAD+C regimen for the treatment of newly diagnosed mantle cell lymphoma. Combined results of two prospective phase II trials from the French GOELAMS group. Haematologica. 2010;95(8):1350–7. doi: 10.3324/haematol.2009.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thieblemont C, Antal D, Lacotte-Thierry L, Delwail V, Espinouse D, Michallet AS, et al. Chemotherapy with rituximab followed by high-dose therapy and autologous stem cell transplantation in patients with mantle cell lymphoma. Cancer. 2005;104(7):1434–41. doi: 10.1002/cncr.21313. [DOI] [PubMed] [Google Scholar]
- 11.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–23. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 12.Geisler C, Kolstad A, Laurell A, Räty R Nordic Lymphoma Group, Mantle Cell Lymphoma Subcommittee. Mantle cell lymphoma - does primary intensive immunochemotherapy improve overall survival for younger patients? Leuk Lymphoma. 2009;50(8):1249–56. doi: 10.1080/10428190903040030. [DOI] [PubMed] [Google Scholar]
- 13.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–93. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan J, Leonard JP. Mantle cell lymphoma: current concept in biology and treatment. Cancer Treat Res. 2006;131:141–59. doi: 10.1007/978-0-387-29346-2_5. [DOI] [PubMed] [Google Scholar]
- 15.Williams ME, Dreyling M, Winter J, Muneer S, Leonard JP. Management of mantle cell lymphoma: key challenges and next steps. Clin Lymphoma Myeloma Leuk. 2010;10(5):336–46. doi: 10.3816/CLML.2010.n.066. [DOI] [PubMed] [Google Scholar]
- 16.Kouroukis CT, Belch A, Crump M, Eisenhauer E, Gascoyne RD, Meyer R, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21(9):1740–5. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Samad N, Younes A. Temsirolimus in the treatment of relapsed or refractory mantle cell lymphoma. Onco Targets Ther. 2010;3:167–78. doi: 10.2147/ott.s8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipp S, Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90(10):1433–4. [PubMed] [Google Scholar]
- 19.Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59(15):3581–7. [PubMed] [Google Scholar]
- 20.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2(4 Suppl 1):S169–77. [PubMed] [Google Scholar]
- 21.Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108(5):1668–76. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–9. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 23.Lane HA, Lebwohl D. Future directions in the treatment of hormone-sensitive advanced breast cancer: the RAD001 (Everolimus)-letrozole clinical program. Semin Oncol. 2006;33(2 Suppl 7):S18–25. doi: 10.1053/j.seminoncol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 25.Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25(2):341–7. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 27.Hitz F, Martinelli G, Zucca E, von Moos R, Mingrone W, Simcock M, et al. A multicentre phase II trial of gemcitabine for the treatment of patients with newly diagnosed, relapsed or chemotherapy resistant mantle cell lymphoma: SAKK 36/03. Hematol Oncol. 2009;27(3):154–9. doi: 10.1002/hon.891. [DOI] [PubMed] [Google Scholar]
- 28.Bertoni F, Conconi A, Cogliatti SB, Schmitz SF, Ghielmini M, Cerny T, et al. Immunoglobulin heavy chain genes somatic hypermutations and chromosome 11q22-23 deletion in classic mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research. Br J Haematol. 2004;124(3):289–98. doi: 10.1046/j.1365-2141.2003.04763.x. [DOI] [PubMed] [Google Scholar]
- 29.Cogliatti SB, Bertoni F, Zimmermann DR, Henz S, Diss TC, Ghielmini M, et al. IgV H mutations in blastoid mantle cell lymphoma characterize a subgroup with a tendency to more favourable clinical outcome. J Pathol. 2005;206(3):320–7. doi: 10.1002/path.1781. [DOI] [PubMed] [Google Scholar]
- 30.Dudkin L, Dilling MB, Cheshire PJ, Harwood FC, Hollingshead M, Arbuck SG, et al. Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res. 2001;7(6):1758–64. [PubMed] [Google Scholar]
- 31.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347–56. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 32.Ansell SM, Inwards DJ, Rowland KM, Jr, Flynn PJ, Morton RF, Moore DF, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113(3):508–14. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Bottcher S, Asnafi V, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115(16):3215–23. doi: 10.1182/blood-2009-06-230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen NS, Pedersen LB, Laurell A, Elonen E, Kolstad A, Boesen AM, et al. Preemptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365–70. doi: 10.1200/JCO.2008.21.3116. [DOI] [PubMed] [Google Scholar]
- 35.Ghielmini M, Schmitz SF, Burki K, Pichert G, Betticher DC, Stupp R, et al. The effect of Rituximab on patients with follicular and mantle-cell lymphoma. Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2000;11(Suppl 1):123–6. [PubMed] [Google Scholar]
- 36.Czuczman MS, Grillo-Lopez AJ, McLaughlin P, White CA, Saleh M, Gordon L, et al. Clearing of cells bearing the bcl-2 [t(14;18)] translocation from blood and marrow of patients treated with rituximab alone or in combination with CHOP chemotherapy. Ann Oncol. 2001;12(1):109–14. doi: 10.1023/a:1008395214584. [DOI] [PubMed] [Google Scholar]
- 37.Pott C, Schrader C, Gesk S, Harder L, Tiemann M, Raff T, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood. 2006;107(6):2271–8. doi: 10.1182/blood-2005-07-2845. [DOI] [PubMed] [Google Scholar]
- 38.Ansell SM, Tang H, Kurtin PJ, Koenig PA, Inwards DJ, Shah K, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011;12(4):361–8. doi: 10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–5. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–7. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 41.Furman RR, Byrd JC, Flinn IW, Coutre SE, Benson DM, Jr, Brown JR, et al. Interim results from a phase I study of CAL-101, a selective oral inhibitor of phosphatidylinositol 3-kinase p110d isoform, in patients with relapsed or refractory hematologic malignancies. J Clin Oncol. 2010;28:15s. suppl; abstr 3032. [Google Scholar]


