Abstract
Background
FoxM1 has been shown to play a critical role in the pathogenesis of various epithelial malignancies. However, its role in lymphoid malignancies has not been fully clarified. We, therefore, investigated the role of FoxM1 expression in a large cohort of diffuse large B-cell lymphoma samples and panel of cell lines.
Design and Methods
FoxM1 expression was investigated in a large series of diffuse large B-cell lymphoma tissues in a tissue microarray format by immunohistochemistry. Apoptosis was measured by flow cytometry and protein expression was detected by immunoblotting using diffuse large B-cell lymphoma cell lines following treatment with either pharmacological inhibitor of FoxM1 or small interference RNA knockdown strategy. Invasion/migration and soft agar colony assays were also performed following treatment with FoxM1 inhibitor.
Results
FoxM1 expression was detected in 84.6% of diffuse large B-cell lymphoma tumors and found to be significantly associated with proliferative tumor marker Ki67 (P<0.0001), matrix metalloproteinases-2 (P=0.0008), matrix metalloproteinases-9 (P=0.0002), S-phase kinase associated protein-2 (P<0.0001) and inversely associated with p27 expression (P=0.0215). Expression of small interference RNA targeted against FoxM1 or treatment of diffuse large B-cell lymphoma cells with thiostrepton caused its downregulation accompanied by decreased expression of matrix metalloproteinases-2 and matrix metalloproteinases-9. Inhibition of FoxM1 in diffuse large B-cell lymphoma cells also decreased invasive and migratory capability, and induced caspase dependent apoptosis via activation of the mitochondrial apoptotic pathway. Finally, combined thiostrepton and bortezomib at sub-toxic doses led to efficient apoptosis in diffuse large B-cell lymphoma cells.
Conclusions
Altogether, these results suggest that FoxM1 is over-expressed in the majority of diffuse large B-cell lymphoma samples. These data also indicate that targeting FoxM1 signaling can serve as a potential therapeutic modality in the management of diffuse large B-cell lymphoma.
Keywords: diffuse large B-cell lymphoma, FoxM1, apoptosis, NHL therapy
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy making up approximately 40% of all cases. Despite improvement in treatment protocols, a large number of DLBCL cases remain refractory to treatment.1,2 Dysregulated survival pathways have been shown to contribute to the aggressiveness of DLBCL.3 The cause of most DLBCL remains unknown; however, dysregulation of apoptosis or defective repair plays a role in its pathogenesis.4,5
Forkhead box protein M1 (FoxM1) is a member of the FoxM family that consists of more than 50 proteins that are characterized by a conserved 100 amino acid DNA binding domain.6,7 Deregulation of FoxM1 has been implicated in the pathogenesis of many cancers because of its ability to drive cell cycle progression and prevention of growth arrest.8,9 FoxM1 is known to be a key regulator of transition from G1 to S phase by downregulation of p27kip1 through upregulation of S-phase kinase-associated protein 2 (SKP2) and CDK subunit.10–12 FoxM1 has also been known to regulate the transcriptional activity of a number of genes including cyclin B, cyclin A and Aurora B kinase which are very important for cell cycle progression and mitotic entry.13–15 Loss of FoxM1 expression has also been reported to generate mitotic spindle defects leading to mitotic catastrophe.13,16,17 FoxM1 signaling has been implicated in the carcinogenesis of tumor development in various cancers, including hepatocellular, prostate, lung, glioma, cervical and gastric cancers.14,18–22 Nakamura et al.23 have shown that FoxM1 promotes the proliferation of leukemia cells through the modulation of cell cycle progression. Recent studies have shown that downregulation of FoxM1 has resulted in inhibition of cell growth, migration and invasion in a number of cancer cells.9,24,25
FoxM1 has also been shown to be associated with Matrix metalloproteinases (MMPs) in a variety of cancers. MMPs are made up of a large family of related proteolytic enzymes that includes collagenases, gelatinases, stromelysins, elastases, and membrane-type (MT-MMPs).26 The expression and activity of MMPs increase in almost all human cancer and are associated with advanced tumor stage and poor survival.27,28 Expression of MMP-9 has also been found to be elevated in liver of FoxM1B transgenic mice.29
Since FoxM1 has been shown to play a critical role in the carcinogenesis and progression of several human cancers, its specific role in DLBCL has not been investigated. Therefore, in the present study, we first investigated the expression of FoxM1 in a large cohort of DLBCL using tissue microarray (TMA) analysis. Our study demonstrates that FoxM1 was over-expressed in 84.6% of DLBCL samples and was significantly associated with expression of Ki67, MMP-9, MMP-2 and SKP-2. We further demonstrate that pharmacological and small interference RNA (siRNA) mediated inhibition of FoxM1 negatively regulate the expression of cell cycle regulatory proteins in vitro. Finally, we show that the combination of proteasome inhibitor, bortezomib and FoxM1 inhibitor, has a potent anti-apoptotic effect. Altogether, our data show that downregulation of FoxM1 expression can lead to cell cycle arrest and inhibition of cell invasion/migration in DLBCL cells suggesting a possible potential therapeutic role of FoxM1 as novel therapeutic target for the treatment of DLBCL.
Design and Methods
Patient samples and construction of tissue microarray
Two hundred and thirty-one cases of de novo DLBCL, diagnosed between 1987 and 2006 and reclassified according to the WHO criteria,30 were collected from the department of pathology at King Faisal Specialist Hospital and Research Centre. Tissue microarrays (TMA) were constructed as described previously.31 The study was approved by the institutional review board of the King Faisal Specialist Hospital and Research Center (Project RAC# 2060008).
Immunohistochemistry (IHC)
TMA slides were processed and stained manually. The IHC protocol was followed as described.32 Primary antibodies used, their dilutions, and cut-off levels for evaluation are listed in the Online Supplementary Table S1. Immunohistochemical detection of FoxM1 was performed using antibody from Santa Cruz Bio Technology (SCBT, USA, clone K-19) which has been used previously and extensively validated.9 Antigen retrieval for SKP2, p27, MMP2 and MMP9 was performed for 10 min at 120°C in a pressure cooker at pH6 in Dakocytomation Target retrieval solution (code S2369) and at pH9 in Dako Target retrieval solution (code S2367).33
We also assessed expression of matrix MMP-9, MMP-2 and Aurora B kinase by immunohistochemistry using X-tile plots as previously described.9 X-tile plots were constructed for assessment of biomarker and optimization of cut-off points based on outcome as has been described earlier.32,34,35 The expression of CD10, BCL6 and MUM1 was used to classify tumors as germinal center B-cell (GC), like DLBCL and activated B cell (ABC), applying the decision tree described earlier.36
Statistical analysis
The JMP 9.0 (SAS Institute Inc., Cary, NC, USA) software package was used for data analyses. Comparisons between groups were made with the paired Student’s t-test. χ2 tests were used to examine the relationship between nominal variables. P=0.05 was The limit of significance for all analyses was defined as a P value of 0.05. Dose effect curves and combination indices were generated using calcusyn software applying Chou-Talalay methodology as previously described.37
Results
FoxM1 expression in diffuse large B-cell lymphoma patients
Immunohistochemical analysis of FoxM1 expression could be interpreted in 214 DLBCL spots and FoxM1 expression was predominantly seen in the nuclear compartment (Online Supplementary Figure S1A and B). Although FoxM1 expression was significantly higher (P=0.0126) in the germinal center B-cell (GCB) phenotype (43 of 45; 95.6%), a large proportion of the activated B-cell (ABC) phenotype (138 of 168; 82.1%) also showed high FoxM1 expression. Therefore, the overall incidence of FoxM1 expression was found to be 84.6% (181 of 214). However, FoxM1 expression showed no association with age, gender, LDH and IPI. In addition, FoxM1 expression showed a direct association with SKP-2 (P<0.0001) and an inverse association with p27 (P=0.0215). FoxM1 expression also correlated significantly with proliferative marker Ki-67 (P<0.0001) (Online Supplementary Figure S1C and D), MMP-2 (P=0.0008), MMP-9 (P=0.0002) and Aurora B kinase (P=0.0061). Therefore, FoxM1 expression is significantly associated with aggressive and proliferative markers in DLBCL. To compare the expression levels of FoxM1 in 36 non-neoplastic lymphoid tissues, we assessed FoxM1 expression in lymphoid tissue from lymph nodes and tonsils. Using Student’s t-test, the mean±SD of FoxM1 expression in lymphoma (103.2±18.2) was significantly higher than FoxM1 expression in normal lymphoid cells (21.9±16.9; P<0.0001) (Online Supplementary Figure S1E).
FoxM1 gene consists of three alternately spliced isoforms: FoxM1a, FoxM1c and FoxM1b. All three isoforms are expressed in DLBCL cell lines as detected by RT-PCR (data not shown).
Inhibition of FoxM1 expression leads to downregulation of MMP-2 and MMP-9 expression in diffuse large B-cell lymphoma cells
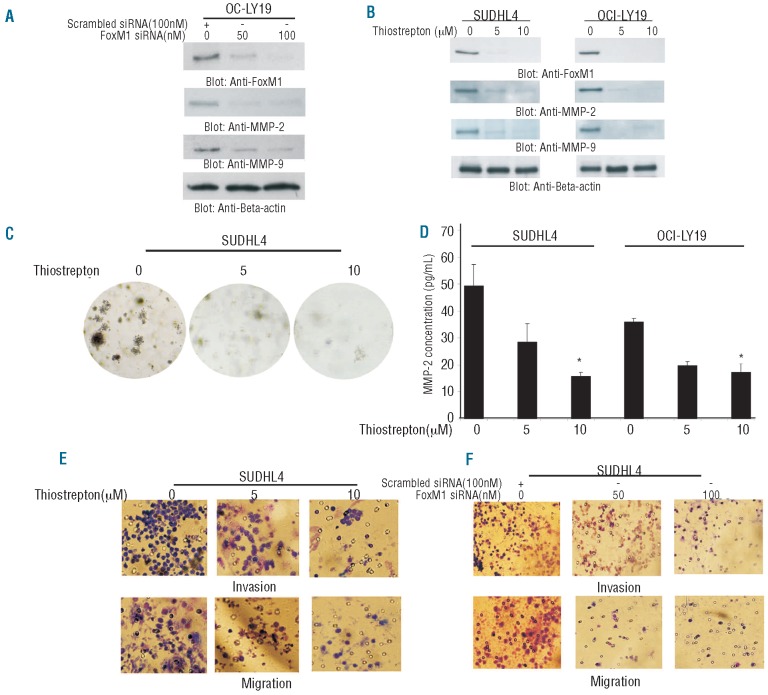
As our clinical data showed a significant association between expression of FoxM1 with MMP-2 and MMP-9, we investigated whether siRNA targeted against FoxM1 expression or inhibition of FoxM1 using either a selective FoxM1 inhibitor, thiostrepton,38 that has also been shown to have proteasomal inhibition activity39 leads to downregulation of MMP-2 and MMP-9 in DLBCL cells. SUDHL4 cells were transfected with 50 and 100 nM FoxM1 siRNA for 48 h and proteins were immunoblotted. As shown in Figure 1A, siRNA knockdown of FoxM1 down-regulated expression of FoxM1, MMP-2 and MMP-9 protein levels in a dose dependent manner. Interestingly, thiostrepton treatment of DLBCL cells produced similar results (Figure 1B), confirming that FoxM1 downregulation leads to their inhibition.
Figure 1.
Thiostrepton treatment causes downregulation of FoxM1 and its downstream targets in DLBCL cells. (A) OCI-LY19 cells were transfected with either 100 nM scrambled siRNA or 50 and 100 nM specific siRNA targeted against FoxM1 for 48 h. After incubation, cells were lysed and immunoblotted with antibodies against FoxM1, MMP-2 and MMP-9. Beta-actin was used as a loading control. (B) SUDHL4 and OCI-LY19 cells were treated with 5 and 10 μM thiostrepton for 48 h. After cell lysis, proteins were separated on SDS-Page and immunoblotted with antibodies against FoxM1, MMP-2, MMP-9 and beta-actin. (C) Clonogenic assays were performed as described in the Design and Methods section. SUDHL4 cells were treated with 5 and 10 μM thiostrepton for 48 h. Subsequently, cells were plated on Soft agar plates for four weeks. After four weeks, plates were stained and manually counted. (D) SUDHL4 and OCI-LY19 cells were treated with 5 and 10 μM thiostrepton for 48 h. Activity of MMP-2 was determined by enzyme-linked immunoabsorbent assay (ELISA). (E) SUDHL4 cells were treated with 5 and 10 μM thiostrepton for 24 h. Following treatment, Invasion-Migration assay were performed as described in the material and methods section. (F) SUDHL4 cells were transfected with either scrambled siRNA or FoxM1 specific siRNA for 48 h. Following treatment, Invasion-Migration assay were performed as described in the Design and Methods section.
FoxM1 expression has been shown to enhance anchorage dependent colony formation in tumors.9 We, therefore, sought to determine whether inhibition of FoxM1 expression affects the colony formation in DLBCL cells. As shown in Figure 1C, thiostrepton treatment of SUDHL4 cells led to decreased colony formation as compared to untreated cells. Next, we examined secretion of MMP-2 by ELISA following treatment of DLBCL cells with thiostrepton. Thiostrepton treatment caused decreased secretion of MMP-2 in media in SUDHL4 and OCI-LY19 cells (Figure 1D). MMP-2 and MMP-9 expressions have been known to play a major role in invasion and migration of cancer cells.28 Therefore, we were interested in determining whether thiostrepton treatment of DLBCL led to inhibition of invasion and migration of these cells. Thiostrepton treatment of DLBCL cells prevented penetration through the Matrigelcoated membrane for invasion/migration compared with the untreated sample suggesting that FoxM1 regulated invasion/migration of DLBCL cells via upregulation of MMPs (Figure 1E). These data were confirmed by siRNA knockdown of FoxM1 of DLBCL cells that showed similar data (Figure 1F) suggesting that FoxM1 modulates the activity of MMPs in DLBCL cells.
Downregulation of FOXM1 inhibits cell viability via induction of apoptosis in DLBCL cell lines
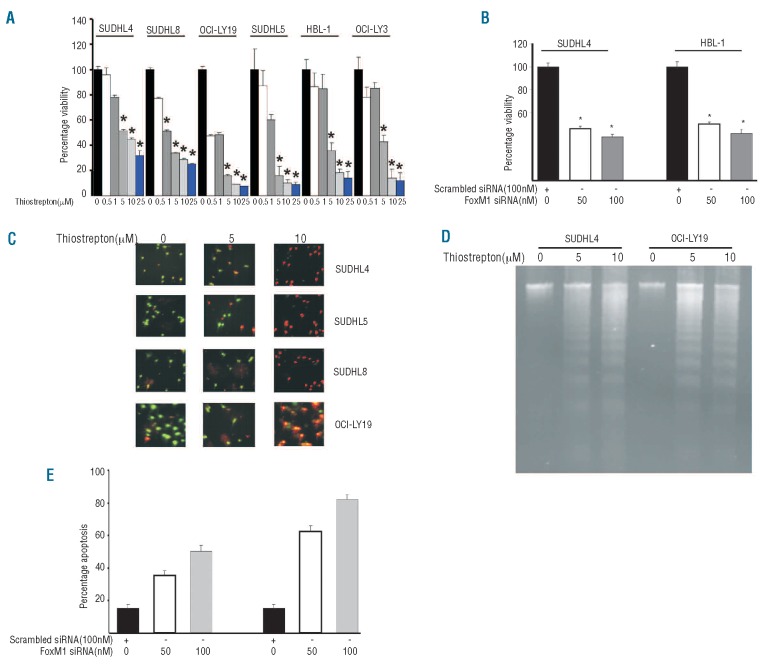
Our clinical data also demonstrated a significant association of FoxM1 expression with Ki67, a proliferative marker. We, therefore, sought to determine whether treatment with thiostrepton or siRNA knockdown of FoxM1 led to inhibition of cell viability in DLBCL cells as detected by MTT assay. Figure 2A shows that as the dose of thiostrepton increased from 0.5 to 25 μM, cell growth inhibition increased in a dose dependent manner in all the DLBCL cell lines. The growth inhibition induced by thiostrepton treatment was found to be statistically significant (P<0.01) (Student’s t-test) at most of the doses tested in all cell lines. Similar data were obtained using siRNA knockdown of FoxM1 (Figure 2B). We then examined whether inhibition of cell viability occurred due to DLBCL undergoing cell death. For this, we treated DLBCL cell lines with 5 and 10 μM thiostrepton for 48 h and, after staining them with calcein and ethidium homodimer, we examined the cells under a microscope to check for plasma membrane integrity. As shown in Figure 2C, untreated cells were stained green showing live cells with intact plasma membrane integrity while cells treated with thiostrepton showed an increase in red cells suggesting disruption of plasma membrane integrity, i.e. dead cells. Finally, we confirmed the response of DLBCL cell lines to thiostrepton by DNA laddering assay. SUDHL4 and OCI-LY19 cell lines were treated with 5 and 10 μM thiostrepton for 48 h and DNA fragmentation were observed in both the cell lines (Figure 2D). Interestingly, transfection of DLBCL cells with FoxM1 siRNA showed increased apoptosis after transfection with 50 and 100 nM siRNA targeted against FoxM1 confirming that FoxM1 downregulation leads to apoptosis in DLBCL cells (Figure 2E). To exclude off-target effects of FoxM1 specific siRNA in inhibition of cell viability and inducing apoptosis, we transfected DLBCL cells with another siRNA targeting a different sequence of the FoxM1 gene and found similar results (Online Supplementary Figure S2A and B). Finally, to assess the response of thiostrepton on normal PBMNC, we treated 3 samples of normal PBMNC with 5 and 10 μM thiostrepton for 48 h and analyzed the samples for apoptosis. As shown in the Online Supplementary Figure S2C, there was no apoptosis detected in any of the PBMNC samples following treatment with thiostrepton. Additionally, caspase-3 was not activated in PBMNC cells following treatment with thiostrepton (Online Supplementary Figure S2D).
Figure 2.
Downregulation of FoxM1 expression leads to inhibition of cell viability and induction of apoptosis in DLBCL cell lines. (A) DLBCL cells were incubated with 0.5, 1, 5, 10 and 25 μM thiostrepton for 48 h. Cell viability was assayed using MTT as described in Design and Methods section. The graph displays the mean ± SD (standard deviation) of 3 independent experiments with replicates of 6 wells for all the doses and vehicle control for each experiment * P<0.05, statistically significant (Student’s t-test.) (B) SUDHL4 and HBL-1 cells were transfected with scrambled siRNA or FoxM1 specific siRNA for 48 h. Following treatment, cell viability was measured by MTT. The graph displays the mean ± SD (standard deviation) of 3 independent experiments with replicates of 6 wells for all the doses and vehicle control for each experiment *P<0.05, statistically significant (Student’s t-test.) (C) SUDHL4, SUDHL5, SUDHL8 and OCI-LY19 cells were treated with 5 and 10 μM thiostrepton for 48 h and apoptosis was measured by Live/Dead Assay. (D) Thiostrepton-induced apoptosis detected by DNA laddering. DLBCL cells were treated with 5 and 10 μM thiostrepton (as indicated) for 48 h and DNA was extracted and separated by electrophoresis on 1.5% agarose gel. (E) SUDHL4 and HBL-1 cells were transfected with scrambled siRNA or FoxM1 specific siRNA for 48 h. Following treatment, cells were subsequently stained with flourescein-conjugated annexin-V and propidium iodide (PI) and analyzed by flow cytometry. Bar graph denotes standard deviation of 3 independent transfections.
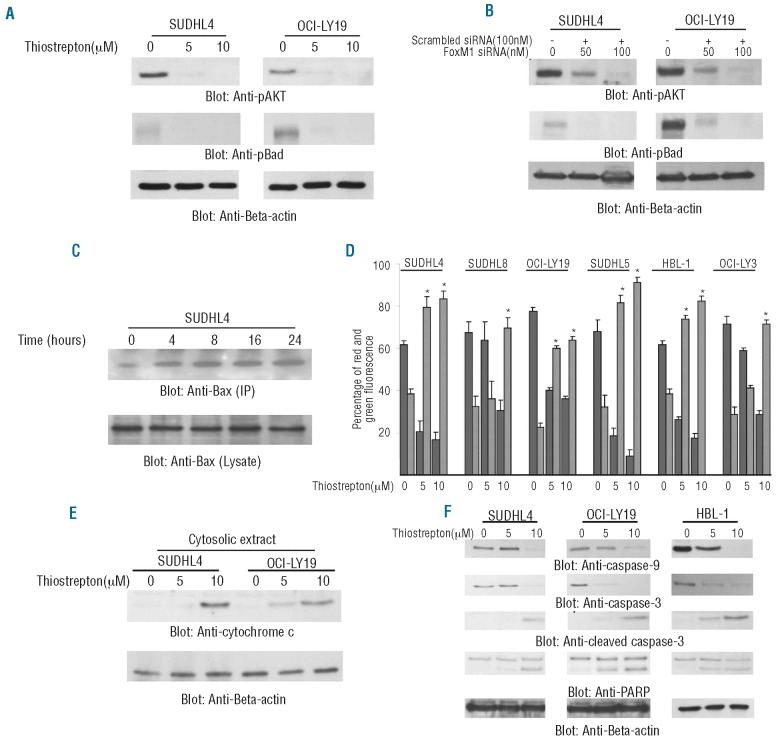
Park et al.40 have previously shown that tumor cells that express activated AKT are dependent on FoxM1 expression for survival. Therefore, we determined the phosphorylation (activation) status of AKT and its downstream target, BAD, following treatment with thiostrepton. As shown in Figure 3A, thiostrepton treatment of SUDHL4 and OCI-LY19 cell lines inactivated AKT and Bad in a dose dependent manner. These data were further confirmed by siRNA knockdown of FoxM1 that showed similar results (Figure 3B). It has been shown before that dephosphorylation of Bad leads to upregulation of pro-apoptotic Bax and activation of the mitochondrial apoptotic pathway.41 As shown in Figure 3C, inhibition of FoxM1 led to conformational changes of Bax protein starting within 4 h of thiostrepton treatment in the SUDHL4 cell line and peaking at 8 h as detected by immunoprecipitation with Bax 6A7 antibody that only recognizes the conformationally changed Bax. We further tested the effect of thiostrepton on the mitochondrial membrane potential and release of cytochrome c in these cells. Cells were treated with thiostrepton for 48 h and labeled with JC1 dye and mitochondrial membrane potential was measured by flow cytometry. As shown in Figure 3D, treatment of cells with thiostrepton resulted in loss of mitochondrial membrane potential in DLBCL cells as measured by JC1 stained green fluorescence depicting apoptotic cells as well as release of cytochrome c from mitochondria to cytosole (Figure 3E).
Figure 3.
Thiostrepton-induced mitochondrial apoptotic pathway in DLBCL cells. (A) SUDHL4 and OCI-LY19 cells were treated with 5 and 10 μM thiostrepton for 48 h and cells were lysed and equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with antibodies against p-AKT, p-Bad and Beta-actin. (B) SUDHL4 and OCI-LY19 cells were transfected with either 100 nM scrambled siRNA or 50 and 100 nM specific siRNA targeted against FoxM1 for 48 h. After incubation, cells were lysed and immunoblotted with antibodies against p-AKT, p-Bad and Beta-actin. (C) After treating with 10 μM thiostrepton for indicated time periods, SUDHL4 cells were lysed in 1% Chaps lysis buffer and subjected to immunoprecipitation with anti-Bax 6A7 monoclonal antibody and probed with specific polyclonal anti-Bax antibody for detection of conformationally changed Bax protein. In addition, the total cell lysates were applied directly to SDS–PAGE, transferred to immobilon membrane and immunoblotted with specific anti-Bax polyclonal antibody. (D) Loss of mitochondrial membrane potential by thiostrepton treatment of DLBCL cells. DLBCL cells were treated with and without 5 and 10 μM thiostrepton for 48 h. Live cells with intact mitochondrial membrane potential and dead cells with lost mitochondrial membrane potential were measured by JC-1 staining and analyzed by flow cytometry as described in Design and Methods section. The graph displays the mean ± SD (standard deviation) of 3 independent experiments. (E) Thiostrepton-induced release of cytochrome c. SUDHL4 and OCI-LY19 cells were treated with and without 5 and 10 μM thiostrepton for 48 h. Mitochondrial free, cytosolic fractions were isolated as described in Design and Methods section. Cell extracts were separated on SDS-PAGE, transferred to PVDF membrane, and immunoblotted with an antibody against cytochrome c. The blots were stripped and re-probed with an antibody against actin for equal loading. (F) SUDHL4, OCI-LY19 and HBL-1 cells were treated with and without 5 and 10 μM thiostrepton for 48 h. Cells were lysed and equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with antibodies against caspase-9, caspase-3, cleaved caspase-3, PARP and beta-actin.
Release of cytochrome c has been shown to activate the downstream caspases that are ultimately required to induce apoptosis.40,41 We, therefore, decided to determine whether thiostrepton-induced release of cytochrome c is capable of activation of caspase 9, caspase-3 and cleavage of PARP. Figure 3F shows that thiostrepton treatment of SUDHL4 and OCI-LY19 cell lines resulted in the activation of caspase-9, caspase-3 and cleavage of PARP in DLBCL cells after treatment with 5 and 10 μM thiostrepton for 48 h. Interestingly, expression of FoxM1 siRNA also activated caspases-9 and caspases -3 in DLBCL cells (Online Supplementary Figure S3A). In addition, pre-treatment of DLBCL cells with 80 μM z-VAD-fmk, a universal inhibitor of caspases, followed by thiostrepton treatment, abrogated apoptosis and prevented caspases-9 and caspases-3 activation induced by thiostrepton (Online Supplementary Figure S2B and C). Finally, thiostrepton treatment of DLBCL cells also inhibits the expression of inhibitor of apoptosis proteins (IAP) (Online Supplementary Figure S2D).
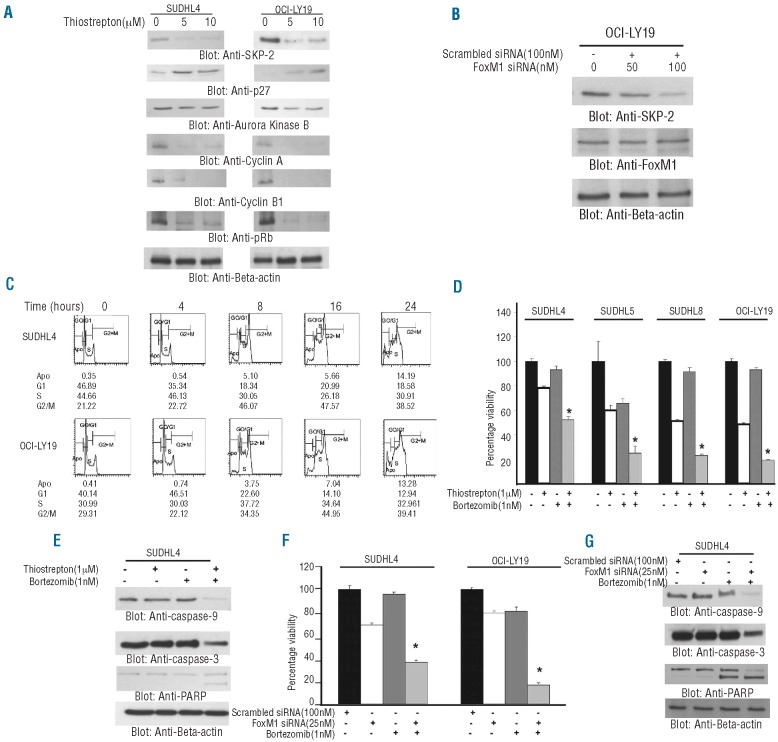
FoxM1 has also been shown to regulate the activity of cell cycle proteins in other cancers.10–12,18 Interestingly, our clinical data showed that FoxM1 was significantly associated with cell cycle regulatory proteins such as SKP-2 and Aurora kinase B. We, therefore, investigated the role of FoxM1 expression on cell cycle regulatory proteins following treatment with thiostrepton in DLBCL cells. Treatment of DLBCL cells led to downregulation of SKP-2, Aurora B kinase, Cyclin A, Cyclin B1 and pRb accompanied by upregulation of p27 (Figure 4A). We next performed transfection studies with siRNA targeted against SKP-2 and found that there was no effect on FoxM1 expression (Figure 4B). To further confirm the role of FoxM1 in cell cycle regulation, we analyzed cell cycle distribution in DLBCL cells in response to thiostrepton treatment. As shown in Figure 4C, thiostrepton treatment induced a gradual time dependent G2/M arrest in DLBCL cells of up to 16 h of treatment in the SUDHL4 cell line. Similar results were obtained in OCI-LY19 cells where the G2/M population of cells increased up to 16 h. These data suggest that FoxM1 plays an active role in cell cycle progression of DLBCL cells.
Figure 4.
Association of FoxM1 expression with cell cycle regulatory proteins. (A) SUDHL4 and OCI-LY19 cells were treated with and without 5 and 10 μM thiostrepton for 48 h. After cell lysis, equal amounts of proteins were separated by SDS-PAGE, transferred to Immobilon membrane, and immunoblotted with antibodies against SKP-2, p27Kip1, Aurora kinase B, Cyclin A, Cyclin B1, pRb and beta-actin as indicated. A representative of 3 different experiments is shown. (B) SKP-2 siRNA expression does not affect expression of FoxM1. OCI-LY19 cells were transfected with scrambled siRNA (100 nM) and SKP-2 siRNA (50 and 100 nM) with Lipofectamine as described in the Design and Methods section. After 48 h of transfection, cells were lysed and equal amounts of proteins were separated by SDS-PAGE, transferred to Immobilon membrane, and immunoblotted with antibodies against SKP-2, FoxM1 and beta-actin as indicated. (C) Thiostrepton treatment induces a G2/M cell cycle arrest at early time point in DLBCL. SUDHL4 and OCI-LY19 cells were treated with 10 μM thiostrepton for indicated time periods. Following incubation, cells were analyzed for cell cycle fractions by flow cytometry. (D) DLBCL cells were incubated with either 1 μM thiostrepton or 1nM bortezomib alone or in combination for 48 h. Cell viability was assayed using MTT as described in the Design and Methods section. The graph displays the mean ± SD (standard deviation) of 3 independent experiments with replicates of 6 wells for all the doses and vehicle control for each experiment *P<0.05, statistically significant (Student’s t-test.). (E) SUDHL4 cells were treated with either 1 μM thiostrepton or 1 nM bortezomib alone or in combination (as indicated) for 48 h. Cells were lysed and equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with antibodies against caspase-9, caspase-3, PARP and beta-actin. (F) SUDHL4 and OCI-LY19 cells were trans-fected with either 100 nM scrambled siRNA or 25 nM specific siRNA targeted against FoxM1 alone or in combination with 1 nM bortezomib for 48 h. Cell viability was assayed using MTT as described in Design and Methods section. The graph displays the mean ± SD (standard deviation) of 3 independent experiments with replicates of 6 wells for all the doses and vehicle control for each experiment. (G) SUDHL4 cells were transfected with either 100 nM scrambled siRNA or 25 nM specific siRNA targeted against FoxM1 alone or in combination with 1 nM bortezomib for 48 h. Cells were lysed and equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with antibodies against caspase-9, caspase-3, PARP and beta-actin.
Synergistic activity of thiostrepton and bortezomib to induce apoptosis in diffuse large B-cell lymphoma cell lines
Finally, we sought to determine whether co-treatment of thiostrepton and bortezomib, a proteasome inhibitor, at sub-toxic doses could induce a more potent apoptosis in DLBCL cells. Experiments were conducted to determine the optimal doses that could be used to induce a synergistic apoptotic response with combination of thiostrepton and bortezomib in DLBCL cells. As shown in the Online Supplementary Table S2 and Online Supplementary Figure S4, using the Chou-Talalay method,37 we found that 1 μM thiostrepton and 1 nM bortezomib exerted the maximum synergistic apoptotic response in SUDHL4 cells (combination index 0.182) and OCI-LY19 cells (combination index 0.108), both the values being less than 1.0 suggesting a strong synergistic response.37 We, therefore, co-treated DLBCL cells with sub-toxic doses of thiostrepton (1 μM) and bortezomib (1 nM) for 48 h and assessed the cell viability by MTT assay. As shown in Figure 4D, neither thiostrepton alone nor bortezomib could inhibit cell viability alone. However, combination treatment led to a significant inhibition of viability in all the cell lines tested. We followed up these experiments by examining whether this combination can drive DLBCL cells to undergo apoptosis. We found that there was efficient apoptosis following combination treatment for 48 h as assessed by activation of caspase-9, caspase-3 and PARP (Figure 4E). These data were confirmed by combining bortezomib treatment with transfection of siRNA targeted against FoxM1 that showed similar results (Figure 4F and G). These data clearly suggest that combined downregulation of FoxM1 and cell cycle regulating proteins induce efficient apoptosis in DLBCL cells.
Discussion
Recent studies have shown that deregulated expression of FoxM1 plays a critical role in the carcinogenesis of many cancers.8,9,14,18 However, the role of FoxM1 has not been fully clarified in DLBCL. We found that the FoxM1 was expressed in 84.6% of DLBCL samples and significantly associated with other aggressive molecular markers such as MMP-9, MMP-2, SKP-2 and Ki67. The expression of FoxM1 varied in a non-neoplastic reactive lymph node with maximal expression seen in proliferating cells of the germinal center and minimal expression in the majority of malignant lymphoid cells as compared to non-neoplastic reactive lymph nodes. These data agree with another report that showed absence or minimal expression of FoxM1 in normal tissue from various organ sites as compared to their cancer counterparts.42 Our data also showed that proliferative marker Ki67 was strongly associated with FoxM1 overexpression suggesting that FoxM1 deregulation may be driving the survival and proliferation of DLBCL cells.
FoxM1 is thought to be involved in metastasis and angiogenesis through the modulation of the activity of MMP-2, MMP-9 and vascular endothelial growth factor.9,24,25,29 MMP-2 and MMP-9 are crucial in the process of tumor invasion and metastasis, and are directly linked to degradation of basement membrane collagen leading to metastasis.25 Our clinical data showed that DLBCL tumor cells expressing FoxM1 showed a strong association with MMP-2 and MMP-9. We also found that depletion of FoxM1 by thiostrepton treatment or expression by siRNA resulted in reduced expression of MMP-2 and MMP-9 as well as decreased the invasive and migration capability of DLBCL cells. In addition, secretion of MMP-2 also decreased following thiostrepton treatment. These results strongly suggest that FoxM1 increases the aggressiveness of DLBCL via upregulation of MMPs.
FoxM1 inhibition has been shown to induce apoptosis in human cancer cells.9 In the current study, we found that thiostrepton treatment of DLBCL cells inhibited cell viability and induced apoptosis in a dose dependent and p53 independent manner. The exact mechanism of apoptosis following downregulation of FoxM1 is not known. However, our data suggested that FoxM1 inhibition led to inactivation of AKT, an important survival protein that was previously shown to be addicted to FoxM1 expression for survival.40 Inactivation of AKT caused dephosphorylation of pro-apoptotic protein Bad leading to conformational changes in Bax protein and induction of apoptosis via the mitochondrial apoptotic pathway. Once the mitochondrial apoptotic pathway is activated, it recruits and cleaves caspases-9 and caspases-3 ultimately leading to apoptosis. Furthermore, FoxM1 specific siRNA treatment of DLBCL cell lines caused activation and cleavage of caspases-9 and caspases-3 confirming the role of FoxM1 expression in prevention of caspase-dependent apoptosis.
FoxM1 activity has also been reported to induce p27kip1 degradation through the upregulation of specific subunits of the SKP1-Cullin1-F-box ubiquitin ligase complex involved in targeting proteins for degradation by proteasome.20,43 In addition, FoxM1 expression has also been shown to be associated with increased MYC oncogenic signaling as well as accelerated G2/M progression leading to cell survival.44 We also demonstrated a strong relationship between expression of FoxM1 and cell cycle regulating proteins by IHC and inhibition of FoxM1 expression with thiostrepton down-regulated SKP2 accompanied with the increased level of p27Kip1 in DLBCL cells leading to G2/M arrest. These data are consistent with the established notion that the FoxM1 is a key regulator of G2/M progression.8,9
Bortezomib has been approved for the treatment of multiple myeloma and mantle cell lymphoma and is currently being used in several clinical trials for the management of solid cancers.45 While the results in multiple myeloma and mantle cell lymphoma have been promising, bortezomib as a single agent has not been very successful in the management of solid tumors.46,47 Thrombocytopenia along with fatigue and edema have been the more common dose dependent toxicities associated with bortezomib in clinical trials.48 Therefore, we decided to use bortezomib at sub-toxic doses along with thiostrepton and hypothesized that administration of this sub-toxic dosage combination would potentially decrease drug-induced toxicity and would increase their anti-tumor effect. Our data clearly showed that the combination of bortezomib and thiostrepton profoundly inhibited cell viability via induction of apoptosis suggesting synergistic efficacy. This should, however, be confirmed by further studies in clinical trials in DLBCL.
In summary, we observed FoxM1 expression in the majority of DLBCL and a tight linkage with co-expression of MMP2, MMP9, Ki-67 and SKP2. We also presented experimental evidence that downregulation of FoxM1 caused inhibition of cell viability, and induced caspase dependent apoptosis in DLBCL cells. This study also confirms the strong association between expression of FoxM1 and proteins that play an important role in invasion and migration as well as cell cycle regulatory proteins in DLBCL cells. From these results, we conclude that the aberrant FoxM1 signaling pathway plays a critical role in the pathogenesis of DLBCL and identify FoxM1 as a potential therapeutic target in DLBCL.
Acknowledgments
We would like to thank Ms. Saeeda O Ahmed and Mr. Saravanan Thangavel for technical assistance and Mr. Zeeshan Qadri for assistance in statistics for the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Escalon MP, Lossos IS. Pharmacotherapy of large B-cell lymphoma. Expert Opin Pharmacother. 2008;9(13):2247–58. doi: 10.1517/14656566.9.13.2247. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RI. Cyclophosphamide, doxorubicin, vincristine, and prednisone versus intensive chemotherapy in non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol. 1997;40(Suppl):S42–6. doi: 10.1007/s002800051060. [DOI] [PubMed] [Google Scholar]
- 3.Elenitoba-Johnson KS, Jenson SD, Abbott RT, Palais RA, Bohling SD, Lin Z, et al. Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. Proc Natl Acad Sci USA. 2003;100(12):7259–64. doi: 10.1073/pnas.1137463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markovic O, Marisavljevic D, Cemerikic V, Perunicic M, Savic S, Filipovic B, et al. Clinical and prognostic significance of apoptotic profile in patients with newly diagnosed nodal diffuse large B-cell lymphoma (DLBCL) Eur J Haematol. 2011;86(3):246–55. doi: 10.1111/j.1600-0609.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 5.Staudt LM, Dave S. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol. 2005;87:163–208. doi: 10.1016/S0065-2776(05)87005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250(1):1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 7.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–6. [PubMed] [Google Scholar]
- 8.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 9.Uddin S, Ahmed M, Hussain A, Abubaker J, Al-Sanea N, AbdulJabbar A, et al. Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. Am J Pathol. 2011;178(2):537–47. doi: 10.1016/j.ajpath.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25(24):10875–94. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, et al. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem. 2003;278(39):37888–94. doi: 10.1074/jbc.M305555200. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277(46):44310–6. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA. 2002;99(26):16881–6. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, et al. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res. 2006;66(3):1712–20. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, et al. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol. 2004;276(1):74–88. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999;19(12):8570–80. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wonsey DR, Follettie MT. Loss of the fork-head transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res. 2005;65(12):5181–9. doi: 10.1158/0008-5472.CAN-04-4059. [DOI] [PubMed] [Google Scholar]
- 18.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18(7):830–50. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66(4):2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66(7):3593–602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 21.Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN, et al. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. J Pathol. 2008;215(3):245–52. doi: 10.1002/path.2355. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69(8):3501–9. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura S, Hirano I, Okinaka K, Takemura T, Yokota D, Ono T, et al. The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia. Carcinogenesis. 2010;31(11):2012–21. doi: 10.1093/carcin/bgq185. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67(17):8293–300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad A, Wang Z, Kong D, Ali S, Li Y, Banerjee S, et al. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res Treat. 2010;122(2):337–46. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- 26.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 27.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, et al. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol. 2002;187(1–2):39–45. doi: 10.1016/s0303-7207(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 28.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7(10):3113–9. [PubMed] [Google Scholar]
- 29.Wang X, Bhattacharyya D, Dennewitz MB, Kalinichenko VV, Zhou Y, Lepe R, et al. Rapid hepatocyte nuclear translocation of the Forkhead Box M1B (FoxM1B) transcription factor caused a transient increase in size of regenerating transgenic hepatocytes. Gene Expr. 2003;11(3–4):149–62. doi: 10.3727/000000003108749044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe ES, Stein HN, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 31.Bavi P, Jehan Z, Atizado V, Al-Dossari H, Al-Dayel F, Tulbah A, et al. Prevalence of fragile histidine triad expression in tumors from saudi arabia: a tissue microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1708–18. doi: 10.1158/1055-9965.EPI-05-0972. [DOI] [PubMed] [Google Scholar]
- 32.Bavi P, Abubaker J, Hussain A, Sultana M, Al-Dayel F, Uddin S, et al. Reduced or absent cyclin H expression is an independent prognostic marker for poor outcome in diffuse large B-cell lymphoma. Hum Pathol. 2008;39(6):885–94. doi: 10.1016/j.humpath.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Hussain AR, Uddin S, Ahmed M, Bu R, Ahmed SO, Abubaker J, et al. Prognostic significance of XIAP expression in DLBCL and effect of its inhibition on AKT signalling. J Pathol. 2010;222(2):180–90. doi: 10.1002/path.2747. [DOI] [PubMed] [Google Scholar]
- 34.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 35.Badve S, Turbin D, Thorat MA, Morimiya A, Nielsen TO, Perou CM, et al. FOXA1 expression in breast cancer--correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13(15 Pt 1):4415–21. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- 36.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 37.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Kwok JM, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther. 2008;7(7):2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 39.Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4(8):e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28(19):2908–18. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samovski D, Kalderon B, Yehuda-Shnaidman E, Bar-Tana J. Gating of the mitochondrial permeability transition pore by long chain fatty acyl analogs in vivo. J Biol Chem. 2010;285(10):6879–90. doi: 10.1074/jbc.M109.080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokomine K, Senju S, Nakatsura T, Irie A, Hayashida Y, Ikuta Y, et al. The forkhead box M1 transcription factor as a candidate of target for anti-cancer immunotherapy. Int J Cancer. 2010;126(9):2153–63. doi: 10.1002/ijc.24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng J, Wang L, Li Q, Li W, Bjorkholm M, Jia J, et al. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. J Pathol. 2009;218(4):419–27. doi: 10.1002/path.2530. [DOI] [PubMed] [Google Scholar]
- 44.Green MR, Aya-Bonilla C, Gandhi MK, Lea RA, Wellwood J, Wood P, et al. Integrative genomic profiling reveals conserved genetic mechanisms for tumorigenesis in common entities of non-Hodgkin’s lymphoma. Genes Chromosomes and Cancer. 2011;50(5):313–26. doi: 10.1002/gcc.20856. [DOI] [PubMed] [Google Scholar]
- 45.Escobar M, Velez M, Belalcazar A, Santos ES, Raez LE. The role of proteasome inhibition in nonsmall cell lung cancer. J Biomed Biotechnol. 2011;2011:806506. doi: 10.1155/2011/806506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruschak AM, Slassi M, Kay LE, Schimmer AD. Novel Proteasome Inhibitors to Overcome Bortezomib Resistance. J Natl Cancer Inst. 2011;103(13):1007–17. doi: 10.1093/jnci/djr160. [DOI] [PubMed] [Google Scholar]
- 47.Jatoi A, Foster NR, Egner JR, Burch PA, Stella PJ, Rubin J, et al. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: a pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol. 2010;36(3):601–6. doi: 10.3892/ijo_00000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bommakanti SV, Dudek AZ, Khatri A, Kirstein MN, Gada PD. Phase 1 Trial of Gemcitabine With Bortezomib in Elderly Patients With Advanced Solid Tumors. Am J Clin Oncol. 2011;34(6):597–602. doi: 10.1097/COC.0b013e3181f9441f. [DOI] [PubMed] [Google Scholar]