Abstract
Diverse fungal species are the cause of devastating agricultural and human diseases. As successful pathogenesis is dependent upon the ability of the fungus to adapt to the nutritional and chemical environment of the host, the understanding of signaling pathways required for such adaptation will provide insights into the virulence of these pathogens and the potential identification of novel targets for antifungal intervention. The cAMP-PKA signaling pathway is well conserved across eukaryotes. In the nonpathogenic yeast, S. cerevisiae, PKA is activated in response to extracellular nutrients and subsequently regulates metabolism and growth. Importantly, this pathway is also a regulator of pathogenesis, as defects in PKA signaling lead to an attenuation of virulence in diverse plant and human pathogenic fungi. This review will compare and contrast PKA signaling in S. cerevisiae vs. various pathogenic species and provide a framework for the role of this pathway in regulating fungal virulence.
Keywords: PKA, fungal virulence, metabolic adaptation, morphogenesis, stress response
Introduction
Species representing the major divisions of the fungal kingdom are responsible for devastating diseases of both plants and animals. Though pathogenic species may be highly diverged in terms of phylogeny or lifestyle, each must execute morphogenic and stress responsive programs that facilitate their invasion into host tissue and survival against host defenses. Accordingly, the fungal signaling pathways that promote growth and cellular homeostasis in response to environmental cues represent important determinants of pathogenesis and may prove to be ideal targets for the development of antifungals. The involvement of the cAMP-dependent Protein Kinase (PKA) pathway in regulating fungal virulence, through both conserved and species-specific mechanisms, will be the focus of this review.
The PKA holoenzyme exists as a heterotetramer consisting of two regulatory subunits that bind and inactivate two catalytic subunits. PKA becomes activated when the second messenger, cyclic adenosine 3′,5′ monophosphate (cAMP), binds to the regulatory subunits and induces a conformational change that releases the active kinases.1 The intracellular concentration of cAMP is regulated by the relative activities of two enzymes: adenylate cyclase (AC), which synthesizes the cyclic nucleotide from ATP, and phosphodiesterases, which catalyze cAMP hydrolysis. Although environmental signaling inputs and downstream effectors of the cAMP-PKA pathway may differ among species, the core canonical pathway is maintained from yeast to humans.
In mammals, AC activity is primarily regulated by heterotrimeric G-proteins, which consist of an α, β and γ subunit. When an extracellular ligand binds to a seven-transmembrane receptor at the plasma membrane, a conformational change of the receptor promotes dissociation of the Gα subunit, which then activates AC.2 Whereas growth factors, for example, hormones, serve as the extracellular initiator of the cAMP-PKA pathway in mammals, cumulative data suggest that environmental nutrients play an analogous role in lower eukaryotes. The most detailed analysis of PKA input and output among fungal organisms has been performed in the budding yeast Saccharomyces cerevisiae, a discussion of which will be provided as a reference for the evolution of the pathway across pathogenic fungal species.
PKA and S. cerevisiae: A Paradigm for Environmental Nutrient Signaling
When grown on a non-fermentable carbon source, e.g., glycerol or ethanol, the cells of S. cerevisiae arrest at the cell cycle start and subsequently enter stationary phase (G0). The addition of a readily fermentable carbon, such as glucose or fructose, to those de-repressed cells leads to cell cycle reactivation and the resumption of growth. Classical genetics and biochemical studies over the past several decades have shown that the cAMP-PKA pathway serves as the major intermediate by which glucose, as well as multiple nutrient inputs, regulates cell cycle progression.
Two G-protein modules are involved in the glucose-induced activation of AC in S. cerevisiae. The first is Gpa2, which is a homolog of the G-αs subunits of mammalian heterotrimeric G-proteins.3,4 In this pathway, glucose itself appears to be a ligand for the seven-transmembrane receptor, Gpr1p.5 Upon glucose binding, Gpr1 activates Gpa2, which then associates with, and activates yeast AC, Cdc35. Interestingly, Gpa2 differs from mammalian G-α proteins, as it does not appear to form a heterotrimeric complex with a β or γ subunit. Instead, two proteins with kelch repeat domains (Krh1/Krh2) have been shown to interact with Gpa2 and were initially believed to serve as G-β mimics.6 Subsequent studies revealed, however, that Krh1/2 are direct inhibitors of PKA by strengthening the interaction between the regulatory and catalytic subunits: activated Gpa2 blocks this action by Krh1/2.7,8 Therefore, Gpa2 promotes the activation of PKA in S. cervisiae in two ways: (1) activating Cdc35 to produce cAMP and (2) inhibiting Krh1/2, thereby sensitizing the PKA holoenzyme to the activity of cAMP.
The second G-protein module involved in AC activation involves the small GTPases, Ras1 and Ras2. In mammalian systems, the small GTPase superfamily is not involved in cAMP signaling. The role of these Ras proteins in glucose signaling in S. cerevisiae is still enigmatic, as the mechanism by which Ras responds to glucose is not well understood. However, both basal Cdc35 activity and its glucose-induced activation are dependent upon a functional Ras protein, thereby underscoring the importance of these proteins in the pathway.9 It has been demonstrated that glucose phosphorylation is required for the increase in GTP-bound Ras (active state), suggesting that Ras may serve as an indicator of proper glucose transport and metabolism.10 A current model proposes that low level sugar-phosphorylation serves as a trigger for a Ras-mediated localization of Cdc35 to the plasma membrane, where the cyclase would be accessible for activation by the membrane anchored Gpr1-Gap2 pathway described above.3
In addition to the glucose induction pathway, intracellular acidification also stimulates Ras-dependent Cdc35 activation.9 It is thought that under starvation conditions, the ATP-ADP ratio drops within the cell, resulting in higher levels of free phosphate and, as a result, lower intracellular pH. Consequently, the Ras-cAMP pathway leads to activation of PKA and subsequent catabolism of storage carbohydrates, such as glycogen. Glycolytic activity then restores ATP levels, which leads to a rise in intracellular pH and a consequent downregulation of the pathway.3,11 In this way, the Ras-PKA pathway may serve to maintain internal energy homeostasis under starvation conditions in S. cerevisiae.
Although the presence of a fermentable carbon source is sufficient to activate PKA via the cAMP pathway, PKA activity is not maintained in S. cerevisiae unless a full complement of essential nutrients is present in the environment. Rather, nitrogen or phosphate starvation, even in the presence of glucose, will result in an inactivated PKA pathway and arrest in G1 of the cell cycle. However, the addition of the limiting nutrient to the glucose medium will lead to the rapid activation of PKA by a cAMP- and regulatory subunit-independent mechanism. This mode of PKA regulation has been termed the ‘Fermentable-Growth Medium’ (FGM) pathway.12 The involvement of specific nitrogen and phosphate permeases that play dual roles as receptors have been reported as important upstream elements in the FGM pathway, though the mechanisms by which they ultimately regulate PKA remain unclear.4 In summary, the PKA pathway in S. cerevisiae is centrally positioned to signal multiple nutritional cues from the environment, via both classical G-protein cascades that mimic mammalian hormonal pathways, as well as through Ras or cAMP-independent mechanisms. Once activated, the effector functions of the pathway may be performed by any, or all, of three PKA catalytic subunits encoded by the yeast genome; Tpk1, Tpk2 and Tpk3. Each isoform is constitutively expressed and displays both partially redundant and unique functionalities with one another.13-16
S. cerevisiae is unique among most eukaryotes as it preferentially ferments glucose to ethanol, even in the presence of sufficient oxygen levels. Despite the substantially lower net ATP generated during fermentation compared with respiration, it is believed that this is beneficial to the organism because (1) ATP generation through the fermentative pathway is faster than respiration, allowing for a more rapid utilization of the glucose and (2) the ethanol produced can inhibit the growth of competing organisms.17 Upon its activation by glucose, PKA plays a major role in regulating this fermentative growth program by phosphorylating and activating a variety of glycolytic enzymes, such as phosphofructokinase, while concurrently inhibiting the activity of various proteins involved in the TCA cycle and oxidative phosphorylation. Moreover, PKA is a major mediator of carbon catabolite repression, in which pathways involved in alternative carbon assimilation, e.g., ethanol utilization by alcohol dehydrogenase or acetate via the glyoxylate pathway, are downregulated in the presence of glucose.3
PKA regulates other aspects of cellular physiology upon its activation, beyond carbon catabolism. For instance, yeast cells grown in the presence of glucose display increased sensitivity to various stresses, including oxidative stress and heat shock. PKA is a major regulator of this phenomenon, largely through its antagonistic influence on stress responsive transcription factors. The Msn2 and Msn4 transcription factors, for example, induce expression of genes with stress response elements (STREs) in their promoters, and deletion of Msn2/4 leads to a hypersensitivity to oxidative stress.18 PKA phosphorylation of Msn2/4 blocks their nuclear translocation, thereby reducing the expression of STRE genes.19,20 Similarly, PKA inhibits the activity of the protein kinase, Rim15, which also regulates STRE genes, promotes high temperature resistance, and is required for entry into stationary phase.21 The deletion of Msn2/4 or Rim15 overcomes the growth arrest caused by PKA inactivation, indicating that PKA’s influence on cell physiology is largely mediated through these proteins.
Depending upon the complement of environmental nutrients, PKA may also promote or inhibit developmental programs, such as sexual development or filamentation.15,22 Filamentation occurs in S. cerevisiae when diploid cells are starved for nitrogen; the cells become elongated and divide in a polarized manner, leading to the formation of cells connected end-on-end, called pseudohyphae. The function of pseudohyphal formation is analogous to that of true hyphal extension of filamentous fungi, as both allow the organism to grow into unexplored substrates. The observation that expressing a constitutively active GPA2 allele leads to pseudohyphal growth, even in the presence of nitrogen concentrations that are normally repressive of filamentation, provided the first line of evidence that the cAMP pathway is involved with morphogenesis.23 Subsequent studies have revealed that the three PKA isoforms of S. cerevisiae play disparate roles in filamentation. Tpk2, has a specific role in positively regulating this process, whereas the other isoforms, Tpk1 and Tpk3, have a repressive role.15,16 Tpk2 participates in morphogenesis, in part, through positively regulating the transcription factor Flo8. Flo8 positively regulates the expression of the flocculin protein Flo11, which is required for cell-cell adhesion.2,24,25 Further studies will be required to describe how Tpk2 activity is maintained during nitrogen starvation conditions.
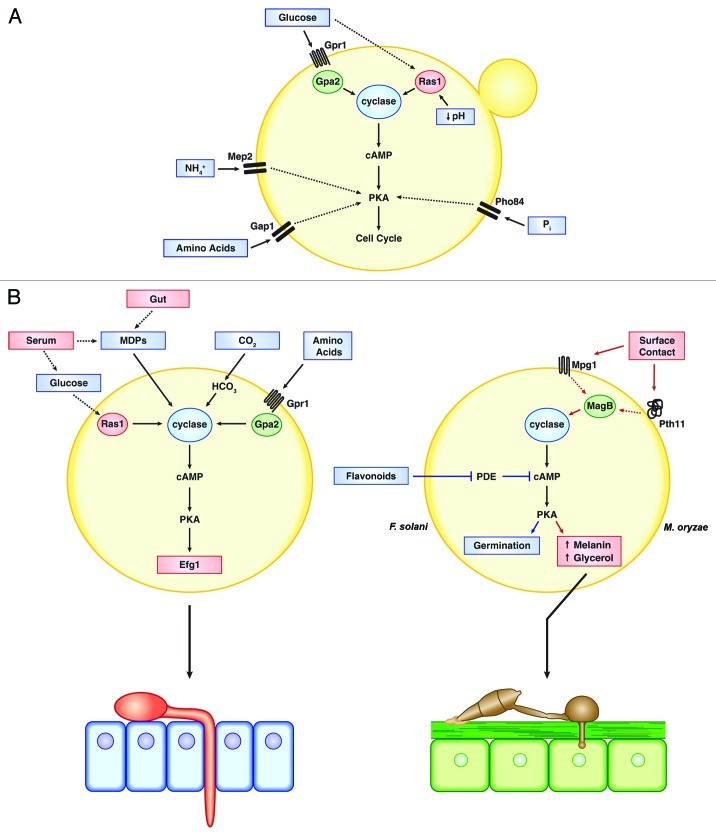
To summarize in S. cerevisiae, PKA is activated within a favorable nutrient environment, determined largely by the presence of glucose. Under such conditions, PKA facilitates the downregulation of stress responsive and reproductive pathways and re-directs its energy expenditure toward the rapid assimilation of an important, but potentially transient nutrient. Additional nutritional inputs, such as the presence or absence of nitrogen, also regulate PKA activity by cAMP-independent mechanisms (summarized in Fig. 1A). These non-glucose signaling inputs likely influence the PKA-dependent control of pseudohyphal growth, thereby promoting the acquisition of limiting nutrients. The role of PKA in relaying environmental nutritional cues to various physiological processes appears to be a unifying theme across diverse fungal species. These parallels, as well as how this conserved signaling pathway regulates virulence among pathogenic fungi will be discussed next.
Figure 1. (A) Schematic of various signaling inputs and regulatory pathways that govern PKA activity in S. cerevisiae. The cAMP-independent activation of PKA by ammonium (NH4+), amino acids and phosphate (Pi) make up the ‘fermentable-growth medium (FGM)’ pathway. (B) Left: Signaling inputs that activate PKA in the human fungal pathogen, C. albicans (top). Following activation, PKA induces the yeast-to-hypha transition that promotes invasion of the gut epithelium (bottom). MDPs, muramyl dipeptides. Right: Novel signaling inputs and regulatory mechanisms in the plant pathogenic species F. solani (blue pathway) and M. oryzae (red pathway) (top). In M. oryzae, the activation of PKA leads to the formation of the appressorium (AP), which promotes penetration through the outer plant cuticle into the underlying tissue (bottom).
PKA and Fungal Pathogenesis
The involvement of cAMP-PKA signaling in environmental sensing and growth is well conserved across the fungal kingdom. However, a wide range of environmental niches and lifestyles of fungal pathogens has allowed for the evolution of organism-specific PKA contributions to pathogenesis. In the following sections, a conceptual framework for PKA-mediated virulence attributes will be presented and important parallels and distinctions between cAMP signaling in S. cerevisiae will be suggested. The primary focus will be on three major human pathogens Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus, but examples among plant pathogenic species will be included when informative.
Control of morphogenesis
Morphogenesis (the change in cell shape) is a pervasive fungal process. Even fungi that maintain a single growth pattern, e.g., budding yeast or hyphae, may undergo morphological transitions in the form of germination (all molds) or titan cell formation (Cryptococcus). Changing morphological forms facilitates tissue invasion or host immune evasion and is, consequently, a crucial pathogenic process subject to a high degree of regulation.26 Just as the cAMP-PKA pathway is a key regulator of filamentation in S. cerevisiae, PKA similarly contributes to regulation of cell shape transitions in diverse plant and human pathogens.
Candida albicans is the predominant human fungal pathogen, causing a spectrum of disease states ranging from superficial mucosal infections to life-threatening systemic disease, primarily in immunocompromised patients.27,28C. albicans is polymorphic, capable of growing as budding yeast or as filaments, the latter of which may include pseudohyphae or true, septate hyphae. Filamentous growth has long been considered an important pathogenicity determinant by promoting tissue invasion and escape from phagocytic cells.29,30 Indeed, C. albicans mutants locked in the yeast morphology are routinely hypovirulent.31,32 Conversely, data concerning hyperfilamentous mutants have been conflicting, with such strains displaying phenotypes ranging from avirulent to hypervirulent.33-36 Though many of the mutants referenced may have defects that affect growth programs beyond morphogenesis, the data cumulatively support the view that regulated morphological transitions are more important than any particular morphology alone. As such, signaling pathways involved in such transitions are likely crucial for virulence.
The cAMP-PKA pathway positively regulates filamentation in C. albicans, at least partly through its direct influence on the transcription factors, Efg1 and Flo8.37,38 Consequently, reduced AC or PKA activity leads to an inability to grow in the hyphal form.39-43 Both PKA isoforms, Tpk1 and Tpk2, are involved in hyphal growth, whereas Tpk2 appears to uniquely promote pseudohyphal elongation, similar to what is seen in S. cerevisiae.44 Importantly, attenuated PKA signaling is associated with reduced virulence, a finding that may be strongly related to the loss in morphogenic flexibility. For example, a strain lacking CaTPK2 is defective in invasive growth on epithelial cells and is attenuated for virulence in a model of oropharyngeal candidiasis.45 Conversely, a histone deacetylase null mutant displays increased PKA activity, and is thus hypersensitive to hypha inducing signals. The mutant displays increased hyphal growth in vivo and is attenuated for virulence in a systemic model, again underscoring the importance of tight morphogenic control in vivo.34
The yeast-to-hypha transition is induced by a variety of environmental stimuli in vitro; including serum, glucose, amino acids, changes in pH, growth at 37°C, physiologic levels of CO2, and certain modified sugars, such as N-acetylglucosamine.46,47 C. albicans is a major commensal of human mucosal surfaces, primarily the gut, where the fungus likely encounters many of these stimuli regularly without incidence of infection. Therefore, it is likely a shift in the balance of these signals within the appropriate host context (e.g., immune deficiency or specific peptide or hormonal influences) that promotes tissue invasion. As will be outlined, cyclase is responsive to many of the above mentioned in vitro inducers in C. albicans, implicating a role for PKA as a key regulator in the morphogenic “decision making” in vivo.
Given the drastically different niches of S. cerevisiae (surface of fruits) and C. albicans (a human commensal), it may be anticipated that disparate environmental cues induce PKA in these respective organisms. Interestingly then, glucose induces AC in both species via a Ras1 sensing mechanism.48 However, although the signaling elements are largely conserved in the two species, the biological relevance of glucose itself may be highly diverged. In C. albicans for instance, the major in vitro inducer of hyphal growth is serum, in which glucose concentrations are high relative to other body tissues. In this way, the in vivo detection of glucose by C. albicans may serve as a signal that it has entered the bloodstream (e.g., as a consequence of intravenous catheterization), rather than a strict indicator of the nutritional status of its niche environment, as is likely the case for S. cerevisiae. Slight differences in the genetic pathway do exist, however, as the Gpr1-Gpa2 module in C. albicans is not involved in glucose sensing, but rather amino acid sensing.48
Beyond nutrients, C. albicans may have developed novel AC activation mechanisms that may reflect a specific adaptation to its host niche. For instance, muramyl dipeptides (MDPs) originating from bacterial peptidoglycan can also activate AC, in this case, through a direct interaction with the cyclase leucine-rich repeat domains.49 Given the abundance of bacteria in the gut, this additional mechanism for PKA activation may serve as an important microbial interaction response. A well-known risk factor for candidiasis is the administration of broad-spectrum antibiotics.50 Therefore, it is tempting to speculate that certain anti-bacterial agents lead to the release of MDPs into the gut lumen and trigger hyphal growth via PKA. In addition, the C. albicans AC respond directly to physiological levels of carbon dioxide. CO2 is hydrated by carbonic anhydrase to form bicarbonate, which then activates AC.51 As one would predict, C. albicans carbonic anhydrase mutants are defective for both CO2 induced filamentation and virulence.51 While these AC activation mechanisms may have evolved independently in C. albicans, there are currently no reports of them being tested in S. cerevisiae.
The ability of serum, pH and CO2 to serve as inducers of filamentation is dependent on physiologic temperature (37°C), a requirement that was recently shown to be dependent on the molecular chaperone, Hsp90.52,53 Interestingly, the authors showed that Hsp90 functions through an interaction with a component of the Ras1-cAMP-PKA pathway.52 More specifically, Hsp90 interacts with and inhibits an upstream pathway component, possibly Ras1 itself, at lower temperatures and releases the client at physiologic temperatures. This represents a potentially novel mechanism by which the cAMP pathway may also integrate temperature cues from the environment to induce filamentation in vivo. In summary, both S. cerevisiae and C. albicans share conserved nutrient sensing pathways that activate PKA. However, C. albicans may have evolved novel signaling mechanisms that facilitate its lifestyle as a human commensal and pathogen. For further reading on the role of PKA in C. albicans virulence, the reader is also referred to a recent review.54
The formation of specialized morphologic structures during pathogenesis is well illustrated by phytopathogenic fungi. Plant leaves are comprised of a waxy outer layer, called the cuticle, which offers protection from physical and chemical assaults from the environment. To bypass this plant defense, many plant pathogenic fungi form a specialized infective structure on the plant leaf called the appressorium. In the appressorium, a large amount of turgor pressure is generated, which is then used to propel a small infection peg through the cuticle and epidermis of the leaf and into the underlying host tissue.55 Signaling through cAMP-PKA plays a critical role in this early infectious process in many species.56 In the rice blast fungus, Magnaporthe oryzae (previously M. grisea), for example, appressorium formation is induced upon physical contact with a hydrophobic surface, such as polystyrene in vitro or the cuticle in the wild. Deletion of the G-α encoding gene, MAGB, leads to a loss in contact-induced appressorium formation, as does loss of a surface hydrophobin, Mpg1, or a transmembrane protein, Pth11.57-59 The surface-induced appressorium defect in all three deletion mutants can be bypassed by the addition of cAMP, suggesting that failure to activate AC in response to a physical interaction with a substrate underlies the mutant phenotypes. This is notably different than the previously discussed cyclase activation pathways in S. cerevisiae and C. albicans, in which chemical cues predominate as the environmental stimulants. Although the loss of the major PKA catalytic subunit gene in M. oryzae, CPKA, does not result in the inability of the organism to produce appressoria, appressorial development in the ΔcpkA mutant is delayed and they are smaller than those produced by the wild-type organism. The ΔcpkA mutant is only capable of infecting rice leaves with prior physical damage, suggesting that the virulence phenotype is, indeed, due to defective host penetration.60 PKA activity is known to play a major role in the breakdown of storage carbohydrates, e.g., glycogen or trehalose, in both yeast cells and fungal spores.61-63 Therefore, it seems likely that the involvement of PKA in appressorium development and function is largely at the level of glycogen breakdown, which is required for the increase in intracellular glycerol concentration and the subsequent generation of the turgor pressure.64 This reinforces the concept that a conserved role for PKA in carbohydrate metabolism can be utilized for the purpose of host invasion during pathogenesis, similar to what has been discussed in C. albicans. The involvement of PKA signaling in appressorium development is conserved in the phytopathogenic Colletotrichum spp and Erisyphie graminis as well as the entomopathogenic fungus Metarhizium anisopliae.65-67
Before host invasion can begin, all filamentous fungi must initiate growth from the dormant spore in the process of germination. Defects in cAMP-PKA signaling are associated with abnormal conidial germination phenotypes in a number of species, with Fusarium solani and A. fumigatus serving as examples. F. solani f sp pisi is a legume pathogen and germination is stimulated by flavenoid compounds released by the host plant. Interestingly, the flavenoids appear to increase intracellular cAMP levels through inhibition of phosphodiesterase activity, rather than by stimulating AC through a G-protein module.68 This flavenoid-mediated influence on cAMP levels may represent another unique mechanism by which the PKA pathway responds to niche specific environmental cues in order to regulate development and virulence.
A. fumigatus is the most common mold pathogen of immunocompromised hosts, causing both pulmonary and systemic infections with mortality rates between 50–90%.69,70 The importance of PKA in the germination of A. fumigatus has been demonstrated via several mutants in the PKA holoenzyme. Deletion of the PKA regulatory-subunit, leading to constitutive PKA activity, results in precocious germination in the absence of environmental nutrients.71A. fumigatus encodes two divergently related PKA isoforms, pkaC1 and pkaC2 and recently it was reported that both isoforms work cooperatively to regulate conidial germination, as a germination defect was only observed upon deletion of both genes. The delay in germination of the ΔpkaC1ΔpkaC2 mutant correlated with a reduced onset of fungal burden and reduced cumulative mortality in mice infected with the mutant.71 These data indicate that the proper onset of germination, mediated by PKA, is important for virulence, although the pleiotropic nature of the pathway must be considered.
The environmental basidiomycetous yeast Cryptococcus neoformans is an important human pathogen that causes a life-threatening meningoencephalitis among immunocompromised patients.73 Though the fungus grows solely as budding yeast in the host, C. neoformans can form an enlarged cell morphotype within the lung, called titan cells. Titan cells are 5–10 times the diameter of normal yeast and may account for 20% of the population in vivo. They are resistant to phagocytosis by host immune cells as well as to both oxidative and nitrosative stresses.74,75 Titan cell formation was found to be under the control of two G-protein coupled receptors, Gpr5 and the Ste3 pheromone receptor. Once activated, both of these pathways activate PKA, which then promotes titan cell formation through the transcription factor Rim101.76
Taken together, PKA signaling is involved in relaying specific environmental cues to the morphogenic machinery in many human and plant pathogenic fungi. Many of the PKA activation systems described are conserved nutrient detection pathways that may have been co-opted by the fungus to detect such stimuli as indicators of the host milieu, for example glucose activation in C. albicans. However, others appear to be novel host detection pathways that lack a known analog in S. cerevisiae, as in the case of flavonoid detection in F. solani (Fig. 1B).
Regulation of resistance to host defenses
A successful pathogen must adapt to a variety of stresses encountered within the host. In S. cerevisiae, PKA activity leads to a generalized downregulation of various stress responses, including oxidative, osmotic and starvation related responses. In contrast, PKA signaling in other fungal organisms, including various pathogenic species, may actually facilitate resistance to environmental and/or host derived assaults.
In addition to cell gigantism, PKA regulates at least two additional aspects of C. neoformans physiology that promote host cell invasion and stress resistance. First is the polysaccharide capsule, which has both anti-phagocytic and immunosuppressive properties.77 Mutants that are acapsular generally display a marked attenuation of virulence in murine infection models. Moreover, mutants with defects in cAMP signaling or PKA activity display reduced capsule formation and are hypovirulent. Conversely, loss of the PKA regulatory subunit leads to an enlarged capsule and a hypervirulent phenotype.78 Transcriptional profiling of cAMP-PKA mutants has also revealed a number of capsule biosynthetic genes that are under the positive influence of PKA.79
Given the in vitro data described, it seems likely that PKA is positioned to regulate capsule formation in the host. For example, capsule biosynthesis is induced upon phagocytosis by macrophages, and strains deficient in PKA or the upstream G-α protein, Gpa1, are defective in this response.80 Interestingly though, the gene expression profile of C. neoformans isolated from macrophages is suggestive of nutrient starvation, an environment in which PKA activity is low in most fungal species.80 Therefore, the exact upstream signal that induces G-protein signaling within the macrophage remains to be identified.
Iron limitation is another inducer of capsule formation. Rim101 is a conserved transcription factor in many fungi that serves as a pH sensor and plays the predominant role in regulating growth under conditions of alkaline pH and iron limitation.81 Recent reports have demonstrated that PKA regulates capsule biosynthesis in response to iron limitation by activating Rim101 in C. neoformans.82 This appears to be a novel interaction between two conserved signaling pathways that ultimately promotes fitness within the acidic and iron poor microenvironment of the phagosome.82
Of note, physiologic levels of CO2 also represent a potent capsule inducer. Similarly to C. albicans, CO2 can activate AC in C. neoformans via the formation of bicarbonate by carbonic anhydrase.51 Although required for growth ex vivo, the C. neoformans carbonic anhydrases are not required for capsule formation or growth under CO2 concentrations found within the host and are, therefore, dispensable for virulence.83 Therefore, the activation of AC by bicarbonate is conserved in two diverged fungal pathogens, C. albicans and C. neoformans, although the contribution of this signaling mechanism during infection is distinct.
The second important virulence factor of C. neoformans to be discussed is melanin, which is believed to impart resistance to UV stress ex vivo, while scavenging reactive oxygen species (ROS) and promoting survival within macrophages in the host. Melanin has also been demonstrated to inhibit phagocytosis, interfere with the activity of antimicrobial peptides and drugs, and inhibit pro-inflammatory cytokine production during infection.84 PKA positively regulates the expression of several genes involved in the melanin biosynthetic pathway, including two genes encoding the enzyme laccase, LAC1 and LAC2.85 Accordingly, a pka1 mutant defective in a PKA catalytic subunit displays a hypo-melanized phenotype under melanin inducing conditions.86 Interestingly, upstream elements of the cAMP pathway involved in melanin production appear to be distinct from those involved in capsule biosynthesis. For example, deletion of GPA1 leads to both capsule and melanin defects; however, loss of the G-protein-coupled receptor (GPCR) that signals upstream of Gpa1, Gpr4, leads only to a reduction in capsule size, with no defect in melanization.86,87 Similarly, the glucose-induced activation of AC is dependent on Gpa1, but not Gpr4.81 Therefore, Gpa1 appears to interact with multiple, distinct sensory molecules, perhaps undiscovered GPCRs, which detect diverse external cues.
It is known that the Gpr4-Gpa1 module is responsive to amino acid stimulation, similar to the Gpr1-Gpa2 pathway of C. albicans.87 However and in contrast to S. cerevisiae and C. albicans, Ras does not appear to influence the cAMP pathway in C. neoformans.88 Moreover, unlike S. cerevisiae, C. neoformans does not contain PKA inhibitory kelch repeat proteins; instead, it contains a more mammalian-like G-β protein, Gib2, which interacts with Gpa1 and serves as a positive regulator of AC.89 In this way, the C. neoformans AC activation pathway more closely resembles that of mammalian cells. Notably, functional G-β proteins have been characterized in filamentous fungi, but their involvement in AC regulation is not well described.90-92
The involvement of PKA in melanin production appears to be conserved across diverse fungal species, including A. fumigatus and the plant pathogenic fungi, M. oryzae and Ustilago hordei.93,94 The effect of stimulation of the pathway differs among these organisms, however, as increased cAMP reduces melanization in U. hordei.94 Conidia of A. fumigatus contain a green melanin pigment that imparts resistance to oxidative stress. Mutants lacking an important melanin biosynthetic enzyme, polyketide synthase (PksP), are hypersensitive to ROS and are killed more readily by human monocyte-derived macrophages.95,96 Likewise, A. fumigatus deletion mutants of AC (acyA), G-α (gpaB), or a PKA catalytic subunit (pkaC1) each display reduced pksP expression and enhanced killing by macrophages.97,98 Conversely, deletion of the PKA regulatory subunit gene, pkaR, leads to aberrant melanization of the hyphal wall and a slight increase in resistance to hydrogen peroxide treatment.99 However, in each of the cAMP-PKA mutants described, melanin-independent basis for the phenotypes cannot be excluded.
The two isoforms of PKA in C. albicans, Tpk1 and Tpk2, appear to play opposite roles in regulating stress responses. Deletion of TPK1 leads to decreased resistance to osmotic, heat and oxidative stresses, whereas deletion of TPK2 either results in unchanged or increased levels of resistance.100 Transcriptional profiling of C. albicans following growth in vivo has indicated that the fungus is experiencing both heat and oxidative stress, suggesting that a Tpk1 mediated stress response may be operative in the host.101 Future studies will be needed to reveal how these two PKA subunits are differentially regulated in vivo to balance morphogenic and stress response related processes. Nevertheless, the positive role for a PKA isoform in heat, osmotic or oxidative stress response is in apparent contrast to S. cerevisiae and may reflect divergent evolution of conserved orthologs.
In summary, the cumulative data support a conserved role in cAMP-PKA signaling in the adaptation of fungal pathogens to host-associated stresses. Many of these stress responses appear to be conserved across divergent species, such as the regulation of melanization. Moreover, those processes controlled by PKA that are important for the stress response may be highly connected to the morphogenic processes described previously. For example, PKA-dependent melanization is also an important aspect of proper appressorium development in M. oryzae, while filamentation may be an important survival response for C. albicans upon phagocytosis.
Metabolic adaptation
All pathogenic microbes must employ the appropriate metabolic pathways for the rapid acquisition and utilization of host derived nutrients. As discussed, the PKA pathway plays a predominant role in carbon metabolism in S. cerevisiae. Therefore, while most studies involving PKA in fungal pathogens have focused on morphogenesis or ‘virulence factor’ production, a major contribution of the pathway to virulence may be related to bioenergetics. In this section, the metabolic output of the PKA pathway in some pathogenic fungi will be briefly reviewed. Moreover, the relevance of PKA in this context will be discussed in light of the emerging in vivo data that addresses the metabolic programs used by fungi during infection.
In S. cerevisiae, PKA is activated in response to glucose and promotes glycolysis and fermentation, while concurrently inhibiting the use of alternative carbon sources. This appears to be well conserved in those organisms in which it has been investigated. In C. albicans, for instance, glucose is known to activate AC via the Ras1 pathway. Upon activation, both Tpk1 and Tpk2 influence the breakdown of the glucose monomer, glycogen.100
In A. fumigatus, measurable activity of PKA is higher when the fungus is grown in the presence of glucose compared with glycerol and artificially inducing PKA signaling through the addition of exogenous cAMP reduces growth of the organism on glycerol.102 Similarly, overexpression of pkaC1 leads to an inability to grow on acetate as the sole carbon source.103 The loss of PKA activity in A. fumigatus, conversely, leads to the reduced capacity to grow on reduced sugar concentrations and a reduced expression of at least one ethanol fermentation gene, pyruvate dehydrogenase.72 Together, these data suggest that PKA activity promotes glycolysis/fermentation while negatively regulating the metabolism of alternative carbon sources in A. fumigatus, similar to the carbon catabolite repression pathway of S. cerevisiae. Additional studies will be needed to identify the signaling components that lie both upstream and downstream of PKA within the glucose sensing pathway.
While the influence of PKA on metabolic pathways remains to be described in many fungi, the ability of glucose to activate the pathway is highly conserved and is, therefore, likely a conserved function. Accordingly, if glucose utilization is a requirement during host infection, PKA may be central to metabolic adaptation. What data support such a view?
Transcriptional profiling of fungi isolated from host tissue has implicated the importance of glucose catabolism in vivo. For example, A. fumigatus germlings isolated from bronchoalveolar lavage fluid of infected mice revealed an upregulation of several high-affinity hexose transporters.104 The pkaC1/pkaC2 deletion mutant of A. fumigatus is unable to grow on glucose media containing reduced glucose concentrations, which was correlated with reduced fungal burden and avirulence in vivo.72 Again, though, PKA could be affecting other processes to influence a complex phenotype such as virulence. Moreover, the transcriptional upregulation of sugar transporters may reflect a generalized response to sugar starvation, rather than a specific requirement for glucose utilization in vivo. Indeed, tissue glucose concentrations may be below 0.05 mM (lung), compared with the relatively glucose-rich blood (6–8 mM).105 In C. neoformans, a similar upregulation of a hexose transporter was observed following growth in the lung, but so too were several genes involved in acetate uptake and metabolism.106 Seemingly contradictory findings were also observed in C. albicans: fungus isolated from murine liver demonstrated an upregulation of genes involved in glycolysis, acetyl-CoA biosynthesis and the TCA cycle.107 In contrast, there was a reported downregulation of glycolytic genes in C. albicans isolated from the murine kidney, which was associated with a concomitant increase in the level of the glyoxylate pathway genes, consistent with glucose starvation.101 However, upon single cell analysis, a heterogeneous population within kidney tissue was observed; some organisms appeared to be undergoing glycolysis, while others were utilizing the glyoxylate cycle and gluconeogenesis.108 These findings support a view in which individual cells of the infecting fungus experience unique microenvironments in the host, even in the same organ. Importantly, defects in the glycolytic pathways of both C. albicans and C. neoformans leads to reduced virulence in their respective animal models.109-111
Though glucose may be a limiting substrate in vivo, additional environmental factors may accentuate the need to metabolize sugar. For example, most fungi are obligate or facultative aerobes and generate most of their ATP via the respiratory pathway. However, oxygen levels within host tissue are considerably lower than atmospheric levers (21%) and may not be sufficient to support a respiratory mode of growth. Within the perenchyma of healthy lungs, for example, the oxygen level is around 14%. However, following diffusion to surrounding tissues, levels may drop to 2–4%.112 Emerging evidence suggests that fungal organisms are under hypoxic stress in vivo and may, therefore, require the use of a fermentative mode of metabolism for sustained energy production.
Several lines of evidence support the sensing of hypoxia by the infecting fungus and the subsequent need for fermentation in vivo. For instance, sterol-response element binding proteins (SREBs) are a conserved family of transcription factors involved in sterol biosynthesis and the hypoxic response. The deletion of the SREB homolog in either A. fumigatus or C. neoformans leads to attenuated growth under hypoxia as well as a reduction in virulence in pulmonary and systemic model, respectively.113-115 This indirectly suggests that both organisms are under hypoxic stress, as the SREB proteins regulate a myriad of processes that could affect virulence. A hypoxic tissue stain was recently used to directly detect hypoxic microenvironments within A. fumigatus lesions in the lung, supporting the hypothesis that oxygen may become drastically reduced during infection due to tissue necrosis and/or extensive inflammation.116 Moreover, the same group detected ethanol within the lung of infected mice and the deletion of an alcohol dehydrogenase gene, alcC, led to reduced fungal burden in a murine model, demonstrating the importance of ethanol fermentation for A. fumigatus in vivo. Additional studies will be required to specifically determine the importance of fermentation in other fungal pathogens.
Along with reduced oxygen, there is also a higher concentration of CO2 in the host, relative to those found in the atmosphere. The activation of AC by CO2 has been described for C. albicans and C. neoformans, but remains to be tested in other pathogenic fungi. It is tempting to speculate that the bicarbonate pathway would be the mechanism by which the PKA pathway could indirectly sense a hypoxic microenvironment. In this model, PKA would become activated to influence glucose utilization, even if glucose levels themselves were insufficiently high to activate fermentation.
In addition to carbon metabolism, PKA signaling may also play a role in micronutrient metabolism during infection. For example, iron is an essential nutrient that is highly limiting in the host environment.117 Therefore, pathogenic microorganisms must employ a variety of iron uptake pathways to sustain growth in the host, including expression of ferrous and ferric transporters and the secretion of high-affinity iron siderophores that can compete for host-bound iron.118 In response to low iron conditions, C. neoformans PKA regulates capsule biosynthesis and also induces the expression of various iron permeases and reductases.79 The latter appears to be a conserved function for PKA, as the positive regulation of high-affinity iron transporters by the pathway in S. cerevisiae has also been described.
In summary, emerging in vivo data from a variety of fungal pathogens has implicated the importance of glycolysis and fermentation during infection, the requirement for which is likely accentuated by the reduced oxygen levels found in most mammalian tissues. Accordingly, the highly conserved role for PKA in positively regulating glucose transport and catabolism likely makes the pathway important in the metabolic response in vivo. In addition, the involvement of PKA in integrating multiple stresses, including iron limitation and physiologic levels of CO2, might allow the cAMP pathway to respond to multiple host signals. Therefore, the virulence defect associated with loss of PKA signaling among fungal pathogens could largely be due to its role in facilitating nutrient acquisition and energy production in the host.
PKA: a pleiotropic regulator of virulence
Thus far, the contributions of PKA to fungal virulence have been categorized as distinct processes for the sake of exposition. However, PKA is a pleiotropic regulator of fungal cell physiology and its role in promoting pathogenesis must be seen in a broader, interconnected context. The involvement of PKA and cell wall homeostasis provides a good example of the interrelatedness between morphogenesis, stress response and metabolism.
The cell wall plays a vital role in the interaction between the fungus and the environment. It provides a rigid scaffold that protects the cell from various chemical and physical stresses and enables the organism to penetrate or invade insoluble substrates.119 Not surprisingly, proper cell wall homeostasis is a critical determinant of fungal pathogenesis. The infecting fungus must constantly remodel its cell wall to facilitate morphogenesis and growth, and must be able to respond properly to cell wall stresses encountered within the host. For example, secreted chitinases that degrade the fungal cell wall are important antifungal defenses of both plants and mammals.120 Additionally, echinocandins are a major class of antifungals that inhibit the synthesis of cell wall glucans. Important fungal resistance mechanisms to these drugs may include the induction of salvage pathways that promote chitin synthesis to preserve cell wall stability.121,122 The major conserved cell wall-integrity pathway in fungi is the Protein Kinase C- MAP Kinase (MAPK) pathway, and defects in MAPK signaling lead to hypersensitivity to osmotic stress and cell wall modulating agents, including Congo red, sodium dodecyl sulfate and cell wall targeting antifungals.123 Mutations in the cell wall integrity pathway lead to attenuation of virulence in numerous pathogens, including C. albicans, C. neoformans and Magnaporthe oryzae.124-126
Beyond the MAPK pathway, PKA also contributes to cell wall integrity in various species. For example, deletion of S. cerevisiae PDE2, encoding a high affinity phosphodiesterase, leads to elevated cAMP levels and constitutive PKA activity. The pde2Δ mutant displays altered expression of genes involved in both cell wall biogenesis and the cell wall stress response. These transcriptional differences likely contribute to the mutant’s phenotype of increased sensitivity to cell wall perturbation.127 Deletion of the PDE2 ortholog of C. albicans leads to similar transcriptional and cell wall sensitivity profiles, thereby demonstrating a conserved role for the pathway.128 Interestingly, the C. albicans Tpk1 and Tpk2 isoforms appear to play opposite roles in cell wall homeostasis; tpk1 null mutants are hypersusceptible to the echinocandin caspofungin, and to osmotic stress, whereas tpk2 mutants display increased resistance to these stresses.100,129
The composition of the fungal cell wall is greater than 90% carbohydrate, consisting of interconnecting chains of modified glucose (glucan) or amino-glucose (chitin) polymers.119 The proper synthesis and maintenance of the cell wall is, therefore, dependent upon a continual flow of glucose monomers to the site of cell wall assembly. Accordingly, those pathways that control glucose uptake and utilization are not only important to support cellular bioenergetics, but they also play a central role in cell wall biogenesis. The initial steps in glucose utilization are its uptake and activation to a sugar-phosphate. Recently, an A. fumigatus mutant deficient in the glucose phosphorylating enzymes, glucokinase and hexokinase, was shown to be hypersensitive to cell wall perturbation, which underscores the relationship between glucose utilization and the cell wall.130 Furthermore, the ΔpkaC1 mutant of A. fumigatus displays increased sensitivity to both Congo red and SDS. This hypersensitivity phenotype is recovered by elevating the glucose concentrations of the medium, suggesting that the defect is, in part, due to reduced flow of glucose monomers into the cell wall biosynthetic pathway.72
Conclusions and Future Perspectives
The contribution of the cAMP-PKA pathway to fungal virulence cannot likely be attributed to its involvement in a single, isolated process. Rather, PKA centrally coordinates multiple, interconnected processes that cumulatively promote overall fitness of the organism in the host environment. Although the pleiotropic nature of the pathway may complicate basic research on PKA-mediated processes (e.g., dissecting cell wall regulation from carbon metabolism), it is the pleiotropy that makes this and other signaling pathways ideal candidates for antifungal intervention. For example, it is the potential to simultaneously inhibit multiple physiological processes through a single target that has made the calcineurin pathway one of recent interest as an antifungal target.
Like PKA, calcineurin is a highly conserved eukaryotic signaling protein that regulates growth and virulence in numerous fungal pathogens, including A. fumigatus, C. neoformans and C. albicans.131 Due to its high conservation, the fungal homolog can be targeted with mammalian calcineurin inhibitors that are already utilized clinically as immunosuppressants. Although it is perhaps paradoxical that an immunosuppressant drug would be used as an anti-infective, patients specifically taking calcineurin inhibitors were found to have reduced incidence of both cryptococcosis and aspergillosis.132,133 Calcineurin controls the transcription of cell wall biosynthetic genes in response to cell wall perturbation and, consequently, pathway mutants are hypersensitive to cell wall targeting drugs, including the echinocandins.134,135 Similarly, calcineurin inhibitors display a synergistic activity with both the echinocandins and the azoles, both in vitro and in in vivo animal models. This suggests that combination therapy could be a valuable treatment strategy, particularly against species that are partially refractory to certain antifungal classes (e.g., C. neoformans and the echinocandins).
As the attenuation of PKA signaling affects a multitude of cellular processes required for a full virulence phenotype in many species, the pharmacological inhibition of the pathway also seems to be a promising approach for antifungal therapy. Moreover, because PKA mutants demonstrate hypersensitivity to cell wall modulating agents, PKA pathway inhibitors could be used to augment echinocandin efficacy, as has been suggested with calcineurin. The high conservation of the PKA pathway will likely allow such studies to be performed with PKA inhibitors already used for mammalian research. Indeed, the inhibitor MyrPKI, which directly targets the PKA enzyme, has been shown to inhibit the C. albicans pathway.136 Such PKA inhibitors negatively influence mammalian cell proliferation and, as a result, have been pursued as treatment for many cancers.137 Accordingly, a major concern for their usage in antifungal therapy would be the potential for adverse effects on healthy host tissue. As such, further detailed analyses will be needed to identify more fungal-specific targets that lie up- or downstream of PKA itself. This will ultimately require a greater integration of systems-based methodologies (e.g., comparative transcriptomics and proteomics) into studies that look at PKA mutants grown in vitro and in association with the host. Such work promises to enhance our knowledge of both fungal physiology and pathobiology, while potentially identifying novel therapeutic targets that could be exploited for clinical or agricultural use.
Glossary
Abbreviations:
- cAMP
cyclic adenosine monophosphate
- PKA
Protein Kinase A
- AC
adenylate cyclase
- MDPs
muramyl dipeptides
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/19396
References
- 1.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza CA, Heitman J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol Rev. 2001;25:349–64. doi: 10.1111/j.1574-6976.2001.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 3.Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–18. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Texeira M, Van Zeebroeck G, Voordeckers K, Thevelein JM. Saccharomyces cerevisiae plasma membrane nutrient sensors and their role in PKA signaling. FEMS Yeast Res. 2010;10:134–49. doi: 10.1111/j.1567-1364.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 5.Lemaire K, Van de Velde S, Van Dijck P, Thevelein JM. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol Cell. 2004;16:293–9. doi: 10.1016/j.molcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Harashima T, Heitman J. Galpha subunit Gpa2 recruits kelch repeat subunits that inhibit receptor-G protein coupling during cAMP-induced dimorphic transitions in Saccharomyces cerevisiae. Mol Biol Cell. 2005;16:4557–71. doi: 10.1091/mbc.E05-05-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeters T, Louwet W, Geladé R, Nauwelaers D, Thevelein JM, Versele M. Kelch-repeat proteins interacting with the Galpha protein Gpa2 bypass adenylate cyclase for direct regulation of protein kinase A in yeast. Proc Natl Acad Sci U S A. 2006;103:13034–9. doi: 10.1073/pnas.0509644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters T, Versele M, Thevelein J. Directly from G-alpha to protein kinase A: the kelch repeat protein bypass of adenylate cyclase. Trends Biochem Sci. 2007;10:134–49. doi: 10.1016/j.tibs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Thevelein JM. Fermentable sugars and intracellular acidification as specific activators of the RAS-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol Microbiol. 1991;5:1301–7. doi: 10.1111/j.1365-2958.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 10.Colombo S, Ronchetti D, Thevelein JM, Winderickx J, Martegani E. Activation state of the Ras2 protein and glucose-induced signaling in Saccharomyces cerevisiae. J Biol Chem. 2004;279:46715–22. doi: 10.1074/jbc.M405136200. [DOI] [PubMed] [Google Scholar]
- 11.Colombo S, Ma P, Cauwenberg L, Winderickx J, Crauwels M, Teunissen A, et al. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:3326–41. doi: 10.1093/emboj/17.12.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thevelein JM, Geladé R, Holsbeeks I, Lagatie O, Popova Y, Rolland F, et al. Nutrient sensing systems for rapid activation of the protein kinase A pathway in yeast. Biochem Soc Trans. 2005;33:253–6. doi: 10.1042/BST0330253. [DOI] [PubMed] [Google Scholar]
- 13.Toda T, Cameron S, Sass P, Zoller M, Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987;50:277–87. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- 14.Robertson LS, Causton HC, Young RA, Fink GR. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc Natl Acad Sci U S A. 2000;97:5984–8. doi: 10.1073/pnas.100113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–87. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson LS, Fink GR. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci U S A. 1998;95:13783–7. doi: 10.1073/pnas.95.23.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002;2:183–201. doi: 10.1111/j.1567-1364.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 18.Hasan R, Leroy C, Isnard AD, Labarre J, Boy-Marcotte E, Toledano MB. The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol Microbiol. 2002;45:233–41. doi: 10.1046/j.1365-2958.2002.03011.x. [DOI] [PubMed] [Google Scholar]
- 19.Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–97. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A, Ward MP, Garrett S. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 1998;17:3556–64. doi: 10.1093/emboj/17.13.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinders A, Bürckert N, Boller T, Wiemken A, De Virgilio C. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 1998;12:2943–55. doi: 10.1101/gad.12.18.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagee S, Sherman A, Shenhar G, Robzyk K, Ben-Doy N, Simchen G, et al. Multiple and distinct activation and repression sequences mediate the regulated transcription of IME1, a transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1985–95. doi: 10.1128/mcb.18.4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz MC, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 1997;16:7008–18. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–78. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo WS, Dranginis AM. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:161–71. doi: 10.1091/mbc.9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengeler KB, Davidson RC, D’souza C, Harashima T, Shen WC, Wang P, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–85. doi: 10.1128/MMBR.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odds FC. Epidemiological shifts in opportunistic and nosocomial Candida infections: mycological aspects. Int J Antimicrob Agents. 1996;6:141–4. doi: 10.1016/0924-8579(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 28.Weig M, Gross U, Mühlschlegel F. Clinical aspects and pathogenesis of Candida infection. Trends Microbiol. 1998;6:468–70. doi: 10.1016/S0966-842X(98)01407-3. [DOI] [PubMed] [Google Scholar]
- 29.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–33. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 30.Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–54. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 31.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 32.Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–56. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–9. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 34.Hnisz D, Majer O, Frohner IE, Komnenovic V, Kuchler K. The Set3/Hos2 histone deacetylase complex attenuates cAMP/PKA signaling to regulate morphogenesis and virulence of Candida albicans. PLoS Pathog. 2010;6:e1000889. doi: 10.1371/journal.ppat.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–60. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, López-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci U S A. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bockmühl DP, Ernst JF. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics. 2001;157:1523–30. doi: 10.1093/genetics/157.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, et al. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonneborn A, Bockmühl DP, Gerads M, Kurpanek K, Sanglard D, Ernst JF. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol. 2000;35:386–96. doi: 10.1046/j.1365-2958.2000.01705.x. [DOI] [PubMed] [Google Scholar]
- 40.Bahn YS, Sundstrom P. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J Bacteriol. 2001;183:3211–23. doi: 10.1128/JB.183.10.3211-3223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, et al. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–87. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 42.Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005;16:1971–86. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bockmühl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol. 2001;42:1243–57. doi: 10.1046/j.1365-2958.2001.02688.x. [DOI] [PubMed] [Google Scholar]
- 44.Giacometti R, Kronberg F, Biondi RM, Passeron S. Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast. 2011;28:293–308. doi: 10.1002/yea.1839. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards J, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 46.Ernst JF. Transcription factors in Candida albicans - environmental control of morphogenesis. Microbiology. 2000;146:1763–74. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 47.Cottier F, Mühlschlegel FA. Sensing the environment: response of Candida albicans to the X factor. FEMS Microbiol Lett. 2009;295:1–9. doi: 10.1111/j.1574-6968.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 48.Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005;16:1971–86. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu XL, Lee RTH, Fang HM, Wang YM, Li R, Zou H, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Bouza E, Muñoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008;32(Suppl 2):S87–91. doi: 10.1016/S0924-8579(08)70006-2. [DOI] [PubMed] [Google Scholar]
- 51.Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schröppel K, Naglik JR, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–6. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–9. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shapiro RS, Cowen L. Coupling temperature sensing and development: Hsp90 regulates morphogenetic signalling in Candida albicans. Virulence. 2010;1:45–8. doi: 10.4161/viru.1.1.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009;4:1263–70. doi: 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- 55.Wang ZY, Jenkinson JM, Holcombe LJ, Soanes DM, Veneault-Fourrey C, Bhambra GK, et al. The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea. Biochem Soc Trans. 2005;33:384–8. doi: 10.1042/BST0330384. [DOI] [PubMed] [Google Scholar]
- 56.Lee N, D’Souza CA, Kronstad JW. Of smuts, blasts, mildews, and blights: cAMP signaling in phytopathogenic fungi. Annu Rev Phytopathol. 2003;41:399–427. doi: 10.1146/annurev.phyto.41.052002.095728. [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Dean RA. G protein alpha subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol Plant Microbe Interact. 1997;10:1075–86. doi: 10.1094/MPMI.1997.10.9.1075. [DOI] [PubMed] [Google Scholar]
- 58.Beckerman JL, Ebbole DJ. MPG1, a gene encoding a fungal hydrophobin of Magnaporthe grisea, is involved in surface recognition. Mol Plant Microbe Interact. 1996;9:450–6. doi: 10.1094/MPMI-9-0450. [DOI] [PubMed] [Google Scholar]
- 59.DeZwaan TM, Carroll AM, Valent B, Sweigard JA. Magnaporthe grisea pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell. 1999;11:2013–30. doi: 10.1105/tpc.11.10.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell TK, Dean RA. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995;7:1869–78. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thevelein JM. Regulation of trehalase activity by phosphorylation-dephosphorylation during developmental transitions in fungi. Exp Mycol. 1988;12:1–7. doi: 10.1016/0147-5975(88)90011-4. [DOI] [Google Scholar]
- 62.d'Enfert C. Fungal spore germination: Insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet Biol. 1997;21:163–72. doi: 10.1006/fgbi.1997.0975. [DOI] [Google Scholar]
- 63.Carrillo D, Vicente-Soler J, Gacto M. Cyclic AMP signalling pathway and trehalase activation in the fission yeast Schizosaccharomyces pombe. Microbiology. 1994;140:1467–72. doi: 10.1099/00221287-140-6-1467. [DOI] [PubMed] [Google Scholar]
- 64.Thines E, Weber RWS, Talbot NJ. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell. 2000;12:1703–18. doi: 10.1105/tpc.12.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Dickman MB. Colletotrichum trifolii mutants disrupted in the catalytic subunit of cAMP-dependent protein kinase are nonpathogenic. Mol Plant Microbe Interact. 1999;12:430–9. doi: 10.1094/MPMI.1999.12.5.430. [DOI] [PubMed] [Google Scholar]
- 66.Takano Y, Komeda K, Kojima K, Okuno T. Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol Plant Microbe Interact. 2001;14:1149–57. doi: 10.1094/MPMI.2001.14.10.1149. [DOI] [PubMed] [Google Scholar]
- 67.Fang W, Pava-ripoll M, Wang S, St Leger R. Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fungal Genet Biol. 2009;46:277–85. doi: 10.1016/j.fgb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Bagga S, Straney D. Modulation of cAMP and phosphodiesterase activity by flavonoids which induce spore germination of Nectria haematococca MP VI (Fusarium solani) Physiol Mol Plant Pathol. 2000;56:51–61. doi: 10.1006/pmpp.1999.0247. [DOI] [Google Scholar]
- 69.Segal BH. Aspergillosis. N Engl J Med. 2009;360:1870–84. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 70.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 71.Fuller KK, Zhao W, Askew DS, Rhodes JC. Deletion of the protein kinase A regulatory subunit leads to deregulation of mitochondrial activation and nuclear duplication in Aspergillus fumigatus. Eukaryot Cell. 2009;8:271–7. doi: 10.1128/EC.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuller KK, Richie DL, Feng X, Krishnan K, Stephens TJ, Wikenheiser-Brokamp KA, et al. Divergent Protein Kinase A isoforms co-ordinately regulate conidial germination, carbohydrate metabolism and virulence in Aspergillus fumigatus. Mol Microbiol. 2011;79:1045–62. doi: 10.1111/j.1365-2958.2010.07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casadevall A, Perfect JR. Cryptococcus neoformans ASM Press, Washington, DC 1998. [Google Scholar]
- 74.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chrétien F, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okagaki LH, Wang Y, Ballou ER, O’Meara TR, Bahn YS, Alspaugh JA, et al. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell. 2011;10:1306–16. doi: 10.1128/EC.05179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janbon G. Cryptococcus neoformans capsule biosynthesis and regulation. FEMS Yeast Res. 2004;4:765–71. doi: 10.1016/j.femsyr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 78.D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–91. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu G, Steen BR, Lian T, Sham AP, Tam N, Tangen KL, et al. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 2007;3:e42. doi: 10.1371/journal.ppat.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan W, Kraus PR, Boily MJ, Heitman J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot Cell. 2005;4:1420–33. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol. 2009;12:365–70. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 82.O’Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahn YS, Cox GM, Perfect JR, Heitman J. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol. 2005;15:2013–20. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 84.Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17:406–13. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, et al. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–17. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue C, Bahn YS, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17:667–79. doi: 10.1091/mbc.E05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maeng S, Ko YJ, Kim GB, Jung KW, Floyd A, Heitman J, et al. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot Cell. 2010;9:360–78. doi: 10.1128/EC.00309-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer DA, Thompson JK, Li L, Prat A, Wang P. Gib2, a novel Gbeta-like/RACK1 homolog, functions as a Gbeta subunit in cAMP signaling and is essential in Cryptococcus neoformans. J Biol Chem. 2006;281:32596–605. doi: 10.1074/jbc.M602768200. [DOI] [PubMed] [Google Scholar]
- 90.Yu HY, Seo JA, Kim JE, Han KH, Shim WB, Yun SH, et al. Functional analyses of heterotrimeric G protein G alpha and G beta subunits in Gibberella zeae. Microbiology. 2008;154:392–401. doi: 10.1099/mic.0.2007/012260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seo JA, Han KH, Yu JH. Multiple roles of a heterotrimeric G-protein gamma-subunit in governing growth and development of Aspergillus nidulans. Genetics. 2005;171:81–9. doi: 10.1534/genetics.105.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Q, Poole SI, Borkovich KA. A G-protein beta subunit required for sexual and vegetative development and maintenance of normal G alpha protein levels in Neurospora crassa. Eukaryot Cell. 2002;1:378–90. doi: 10.1128/EC.1.3.378-390.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol. 2003;38:143–58. doi: 10.1016/S1087-1845(02)00526-1. [DOI] [PubMed] [Google Scholar]
- 94.Lichter A, Mills D. Control of pigmentation of Ustilago hordei: the effect of pH, thiamine, and involvement of the cAMP cascade. Fungal Genet Biol. 1998;25:63–74. doi: 10.1006/fgbi.1998.1087. [DOI] [PubMed] [Google Scholar]
- 95.Langfelder K, Jahn B, Gehringer H, Schmidt A, Wanner G, Brakhage AA. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 96.Jahn B, Langfelder K, Schneider U, Schindel C, Brakhage AA. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell Microbiol. 2002;4:793–803. doi: 10.1046/j.1462-5822.2002.00228.x. [DOI] [PubMed] [Google Scholar]
- 97.Liebmann B, Gattung S, Jahn B, Brakhage AA. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol Genet Genomics. 2003;269:420–35. doi: 10.1007/s00438-003-0852-0. [DOI] [PubMed] [Google Scholar]
- 98.Liebmann B, Müller M, Braun A, Brakhage AA. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus. Infect Immun. 2004;72:5193–203. doi: 10.1128/IAI.72.9.5193-5203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao W, Panepinto JC, Fortwendel JR, Fox L, Oliver BG, Askew DS, et al. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect Immun. 2006;74:4865–74. doi: 10.1128/IAI.00565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giacometti R, Kronberg F, Biondi RM, Passeron S. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non-redundant roles in stress response and glycogen storage. Yeast. 2009;26:273–85. doi: 10.1002/yea.1665. [DOI] [PubMed] [Google Scholar]
- 101.Walker LA, Maccallum DM, Bertram G, Gow NA, Odds FC, Brown AJ. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol. 2009;46:210–9. doi: 10.1016/j.fgb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oliver BG, Panepinto JC, Askew DS, Rhodes JC. cAMP alteration of growth rate of Aspergillus fumigatus and Aspergillus niger is carbon-source dependent. Microbiology. 2002;148:2627–33. doi: 10.1099/00221287-148-8-2627. [DOI] [PubMed] [Google Scholar]
- 103.Grosse C, Heinekamp T, Kniemeyer O, Gehrke A, Brakhage AA. Protein kinase A regulates growth, sporulation, and pigment formation in Aspergillus fumigatus. Appl Environ Microbiol. 2008;74:4923–33. doi: 10.1128/AEM.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDonagh A, Fedorova ND, Crabtree J, Yu Y, Kim S, Chen D, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154. doi: 10.1371/journal.ppat.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Prost N, Saumon G. Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol. 2007;159:331–7. doi: 10.1016/j.resp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Hu G, Cheng PY, Sham A, Perfect JR, Kronstad JW. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol Microbiol. 2008;69:1456–75. doi: 10.1111/j.1365-2958.2008.06374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol. 2007;63:1606–28. doi: 10.1111/j.1365-2958.2007.05614.x. [DOI] [PubMed] [Google Scholar]
- 108.Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–71. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, et al. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog. 2009;5:e1000612. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature. 2001;412:83–6. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 111.Price MS, Betancourt-Quiroz M, Price JL, Toffaletti DL, Vora H, Hu G, et al. Cryptococcus neoformans requires a functional glycolytic pathway for disease but not persistence in the host. MBio. 2011;2:e00103–11. doi: 10.1128/mBio.00103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grahl N, Cramer RA., Jr Regulation of hypoxia adaptation: an overlooked virulence attribute of pathogenic fungi? Med Mycol. 2010;48:1–15. doi: 10.3109/13693780902947342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Willger SD, Puttikamonkul S, Kim KH, Burritt JB, Grahl N, Metzler LJ, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chun CD, Liu OW, Madhani HD. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2007;3:e22. doi: 10.1371/journal.ppat.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, et al. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen aspergillus fumigatus. PLoS Genet. 2011;7:e1002374. doi: 10.1371/journal.pgen.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, et al. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 118.Kosman DJ. Molecular mechanisms of iron uptake in fungi. Mol Microbiol. 2003;47:1185–97. doi: 10.1046/j.1365-2958.2003.03368.x. [DOI] [PubMed] [Google Scholar]
- 119.Latgé JP. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–90. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 120.Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci. 2006;63:3018–29. doi: 10.1007/s00018-006-6269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee KK, Maccallum DM, Jacobsen MD, Walker LA, Odds FC, Gow NA, et al. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother. 2012;56:208–17. doi: 10.1128/AAC.00683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–91. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diez-Orejas R, Molero G, Navarro-García F, Pla J, Nombela C, Sanchez-Pérez M. Reduced virulence of Candida albicans MKC1 mutants: a role for mitogen-activated protein kinase in pathogenesis. Infect Immun. 1997;65:833–7. doi: 10.1128/iai.65.2.833-837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeon J, Goh J, Yoo S, Chi MH, Choi J, Rho HS, et al. A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol Plant Microbe Interact. 2008;21:525–34. doi: 10.1094/MPMI-21-5-0525. [DOI] [PubMed] [Google Scholar]
- 126.Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell. 2008;7:1685–98. doi: 10.1128/EC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones DL, Petty J, Hoyle DC, Hayes A, Ragni E, Popolo L, et al. Transcriptome profiling of a Saccharomyces cerevisiae mutant with a constitutively activated Ras/cAMP pathway. Physiol Genomics. 2003;16:107–18. doi: 10.1152/physiolgenomics.00139.2003. [DOI] [PubMed] [Google Scholar]
- 128.Wilson D, Tutulan-Cunita A, Jung W, Hauser NC, Hernandez R, Williamson T, et al. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol Microbiol. 2007;65:841–56. doi: 10.1111/j.1365-2958.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- 129.Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 2010;6:e1000752. doi: 10.1371/journal.ppat.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fleck CB, Brock M. Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation. Eukaryot Cell. 2010;9:1120–35. doi: 10.1128/EC.00362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steinbach WJ, Reedy JL, Cramer RA, Jr., Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–30. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 132.Singh N, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, et al. Cryptococcal Collaborative Transplant Study Group Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756–64. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- 134.Steinbach WJ, Cramer RA, Jr., Perfect BZ, Henn C, Nielsen K, Heitman J, et al. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob Agents Chemother. 2007;51:2979–81. doi: 10.1128/AAC.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother. 2010;54:1555–63. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cloutier M, Castilla R, Bolduc N, Zelada A, Martineau P, Bouillon M, et al. The two isoforms of the cAMP-dependent protein kinase catalytic subunit are involved in the control of dimorphism in the human fungal pathogen Candida albicans. Fungal Genet Biol. 2003;38:133–41. doi: 10.1016/S1087-1845(02)00520-0. [DOI] [PubMed] [Google Scholar]
- 137.Tortora G, Ciardiello F. Protein kinase A as target for novel integrated strategies of cancer therapy. Ann N Y Acad Sci. 2002;968:139–47. doi: 10.1111/j.1749-6632.2002.tb04332.x. [DOI] [PubMed] [Google Scholar]