Abstract
The blood-cerebrospinal fluid barrier physiologically protects the meningeal spaces from blood-borne bacterial pathogens, due to the existence of specialized junctional interendothelial complexes. Few bacterial pathogens are able to reach the subarachnoidal space and among those, Neisseria meningitidis is the one that achieves this task the most constantly when present in the bloodstream. Meningeal invasion is a consequence of a tight interaction of meningococci with brain endothelial cells. This interaction, mediated by the type IV pili, is responsible for the formation of microcolonies on the apical surface of the cells. This interaction is followed by the activation of signaling pathways in the host cells leading to the formation of endothelial docking structures resembling those elicited by the interaction of leukocytes with endothelial cells during extravasation. The consequence of these bacterial-induced signaling events is the recruitment of intercellular junction components in the docking structure and the subsequent opening of the intercellular junctions.
Keywords: neisseria meningitidis, beta 2 adrenergic receptor, blood-brain barrier, brain endothelial cells, central nervous system infections, cerebrospinal meningitis
Introduction
Bacterial meningitis is the leading cause of central nervous system (CNS) infection. Although bacterial meningitis can be due to the dissemination of contiguous infections such as sinusitis or mastoiditis to the meningeal membranes, most cases are caused by blood-borne pathogens. The blood-brain barrier (BBB) protects the CNS from most bacteria that may have reached the bloodstream, thus restricting the etiology of bacterial meningitis to a few and predominantly extracellular pathogens: Escherichia coli K1 and Streptococcus agalactiae (Group B Streptococcus) in the newborn, Neisseria meningitidis, Haemophilus influenzae type b and Streptococcus pneumoniae in children and adults.1-3 Paradoxically, these bacteria are commensal of the nasopharynx (N. meningitidis, S. pneumoniae and H. influenzae) or of the digestive tract (E. coli and S. agalactiae).4
The pathophysiology of bacterial meningitis is a multistep process that reflects the ability of bacterial pathogens to cross the oropharyngeal or digestive mucosal barrier, survive and replicate in the bloodstream, and to eventually cross the BBB.4-6 It has been demonstrated that these extracellular pathogens express various virulence factors allowing them to survive in the extracellular compartments and to interact directly with the components of the blood-brain barrier. The main virulence factor expressed by all extracellular pathogens is a capsule that prevents bacterial phagocytosis or complement-mediated lysis.4,7-12 Once inside the cerebrospinal fluid (CSF), bacterial multiplication is thought to be uncontrolled, due to the local deficiency in complement and immunoglobulins, despite the influx of polymorphonuclear leukocytes induced by the local inflammatory response. However data obtained in primates showed that bacterial presence in the CSF can be transient if bacteremia is not sustained, reflecting the fact that bacterial entry into the CSF may not always lead to meningitis.13
The small number of bacterial species capable of invading the meninges suggests that specific virulence factors are required for bacteria to enter the subarachnoidal space. Among the above-mentioned extracellular bacteria, Neisseria meningitidis, the agent of cerebrospinal meningitis, is the pathogen that once in the bloodstream is able to invade the meninges the most constantly. It has been estimated that 63% of bacteremia due to N. meningitidis are associated with meningitis.14 In this review, Neisseria meningitidis will be used as a model to address the mechanisms by which a bacterial pathogen can cross the BBB and invade the meninges.
The Cerebrospinal Meningitis
Neisseria meningitidis is a frequent asymptomatic colonizer of the human nasopharynx, and only a very small proportion of infections proceed to a sustained bacteremia. Once in the bloodstream N. meningitidis can either be responsible for a deadly septic shock leading to purpura fulminans and/or cross the BBB to invade the meninges. The reasons why disease occurs in some individuals and not in others remains unclear, but human genetic polymorphism is likely to be important in determining the outcome of infection.15,16 In addition, all meningococci do not have the same pathogenic potential. Indeed, analysis of results from multilocus sequence typing (MLST) has demonstrated the existence of distinct phylogenetic groups (clonal complexes), some of which are more likely to be isolated from patients than others.17 These are the so-called “hyper-virulent” or “hyperinvasive” lineages. Recently, the presence of a prophage has been shown to be responsible for a large proportion of invasiveness of strains belonging to hyperinvasive lineages.18,19 This element inserted into the bacterial chromosome can be induced to produce a filamentous phage.
N. meningitidis interacts only with human cells and there is no animal model of meningococcal sepsis. In some circumstances mice and infant rats have been used to assess the ability of the bacteria to survive in the extracellular fluids,20 but these models are unable to assess the consequences of the interaction with endothelial cells. Most of the hypotheses regarding the pathogenesis of meningococcal infections have been obtained studying postmortem samples and/or biopsies of skin lesions.11,21N. meningitidis interacts with endothelial cells and form colonies on the apical surface of endothelial cell capillaries. Bacteria are also found inside cells and in intercellular spaces. In peripheral purpuric lesions, retraction of endothelial cells with capillary disruption can be observed, as well as hemorrhages, adhesion of leukocytes and formation of small thrombi. In the brain, bacteria are seen interacting with capillaries of the subarachnoidal space, the brain parenchyma and the choroidal plexuses, and inside brain vessels. When a low or moderate number of meningococci is present in the bloodstream, the bacteria interacting with peripheral capillaries cause only few localized purpuric lesions, whereas the interaction with brain endothelial cells is sufficient to lead to meningeal invasion. Adhesion of the bacteria to the meninges and meningeal cells,22 is then probably critical for N. meningitidis to disseminate through the meningeal spaces. In contrast, in case of high bacteremia, many peripheral endothelial cells are colonized by meningococci, leading to a significant increase of vascular permeability possibly associated with extensive thrombosis and purpura.
Where is the Blood-Brain Barrier Breached?
The blood-brain barrier is a highly specialized structural and functional component of the central nervous system that separates the circulating blood from the brain and spinal cord parenchyma. Among the different cellular types that make up the BBB, endothelial cells form the front defense line of the CNS parenchyma against invading pathogens. Schematically, the capillaries of the CNS parenchyma have two specific features that are not shared by those of other peripheral organs: (1) the presence of specialized junctional complexes and (2) a sparse pinocytotic vesicular transport activity that is counterbalanced by highly specialized transport systems that limit the entry of neuroactive blood-borne molecules.23 Junctional complexes are composed of adherens junctions and of multistranded belts of tight junctions that result in apparent membrane fusion of adjacent endothelial cells, forming a continuous blood vessel. Interendothelial tight junctions exclude the paracellular passage of hydrophilic macromolecules between the blood and the brain and account for the high endothelial electric resistance of brain capillaries.24-26 Recently the endothelial Wnt/β-catenin signaling pathway has been shown to regulate the induction and maintenance of the BBB during embryonic and postnatal development.27,28 In vitro, the specialization of brain capillary tight junctions has been shown to be under the control of paracrine factors produced by astrocytic end-feet unsheathing brain capillaries. In addition to astrocytes, other perivascular cells including pericytes, brain macrophages and microglial cells form a perivascular immunological barrier of the CNS. Some recent data have underlined the role of pericytes in the regulation of the blood-brain barrier.29,30 On the arterial side of the brain capillaries, the perivascular space localized between endothelial cells and astrocytes is virtual and both basal lamina produced by astrocytes and by endothelial cells are in very close contact and can be fused. For this reason, the blood-brain barrier at the capillary level is also called the “gliovascular” or “neurovascular” unit. The gliovascular unit is a dynamic structure whose main function is to actively regulate brain homeostasis and protect the brain from circulating blood-borne insults.
Brain post-capillary venules, venules and veins are also part of the blood-CNS interface. The structure and function of the brain venous network strikingly differs from that of brain capillaries. Electron microscopy studies showed that when venules turn into veins and exit the brain parenchyma the interendothelial tight junctions are leaky.31-33 A possible explanation for this is that astrocytic end-feet require intimate interactions with endothelial cells to induce a “full” BBB phenotype.34,35 Indeed, when capillaries turn into venules, endothelial cells of the brain draining system are progressively separated from astrocytic end-feet by the Virchow-Robin perivascular spaces. The space between endothelial cells and astrocytes, designated as the Virchow space, is even more important when veins exit from the brain parenchyma into the subpial space. The structure of brain post-capillary venules and veins and their ability to express leukocyte adhesion molecules36,37 likely reflect their role in mediating the entry of leukocytes and plasmatic molecules in the perivascular space during inflammatory processes.35,38 These findings highlight that, probably due to different physiological functions, both the anatomy and the structure of these brains vessels differ, and that the venous system of the CNS can be considered as a relatively vulnerable localization of the blood-brain barrier.
The precise site and mechanism of entry of N. meningitidis into the CSF is still enigmatic. As shown from the postmortem examination of patients who died of meningococcal infection,21 extracellular bacterial pathogens can interact directly with the components of the blood-brain barrier and do not need a Trojan horse, such as leukocytes, to cross the BBB. Initial work suggested that bacteria may preferentially use the choroids plexus (CPs) route to cross the blood-CSF barrier. However, in this case, meningitis should theoretically be associated with ventriculitis, which is not corroborated by clinical data. Because of their proximity to the subarachnoidal space and their “leaky” interendothelial structure, the brain postcapillary venules and veins of the subpial and subarachnoid spaces may be the site of passage of bacteria into the CSF. Indeed, once bacteria have crossed the endothelial monolayer of these vessels, they could be easily drained into the CSF using the Virchow-Robin perivascular spaces. Interaction with the brain endothelial cells is therefore a crucial step in CNS invasion by N. meningitidis.
How is N. meningitidis Breaching the Blood-Brain Barrier?
Following the interaction of the bacteria with the endothelial cells, at least four strategies are possible for a microorganism to cross a monolayer of brain endothelial cells: (1) transcellular transport by passive or adhesion-induced transcytosis, (2) paracellular passage through opened tight junctions, (3) disruption of the endothelial barrier due to a direct cytotoxic effect and (4) leukocyte-facilitated transport by infected phagocytes.
These routes are not exclusive, as shown for viruses that may both directly interact with the blood-brain barrier or be transported by infected leukocytes to penetrate into the CNS.39 However and as already mentioned, extracellular pathogens probably do not use leukocytes as vehicles to cross the blood-CSF barrier. A breakdown of the blood-CNS barrier due to apoptosis or bacterial cytotoxity is unlikely, since tissue lesions such as hemorrhages in the subarachnoidal space are uncommon during bacterial meningitis. Therefore, the entry of blood-borne pathogens most probably respects the architecture of the blood-CNS barrier.12 Accordingly, adhesion of bacteria to endothelial cells can induce an intracellular signaling pathway leading to disruption of intercellular tight junctions or, alternatively, bacteria may induce their own transcytosis through the cell monolayer. In vitro, transcytosis of bacteria through human brain endothelial cells has been demonstrated for S. pneumoniae,40 E. coli,41-43 S. agalactiae44 and through human umbilical vein endothelial cells for H. influenzae.45,46 Although N. meningitidis can readily be internalized in vitro within vacuoles in human brain microvascular endothelial cells,47 recent data clearly demonstrate that N. meningitidis can open gaps in a monolayer of brain endothelial as a consequence of the delocalization of junctional components whereas in the same cell line transcytosis was not observed. These in vitro data suggest that N. meningitidis cross the BBB using the paracellular route. The molecular events elicited by meningococcal interaction leading to the opening of the paracellular route will be discussed below.
Bacterial Attributes Required for Meningeal Invasion
The growth/survival in extracellular fluids
The level of bacteremia is directly correlated with meningeal invasion by N. meningitidis. The bacteremia is believed to favor meningeal invasion by directly increasing the likelihood of the interaction of the bacteria with the components of the blood-CSF barrier. Therefore, the bacterial attributes involved in the growth and/or the survival in the extracellular fluids are playing an essential role in meningeal invasion by N. meningitidis.
Some of these virulence factors are commonly observed in most extracellular pathogens. They participate in the prevention of bacterial killing by the host effectors of the innate immune systems such as polymorphonuclear neutrophils and complement. These bacterial attributes are the polysaccharidic capsule, the lipooligosaccharide (LOS), and iron chelation systems. A new virulence factor, the factor-H binding protein (fHBP), was recently identified.48 It is a 28 kDa surface-exposed lipoprotein that binds factor H, a key inhibitor of the alternative complement pathway. This protein is expressed by all N. meningitidis strains studied to date, although the level of expression varies between strains (high, intermediate or low expressers). Antibodies directed against fHBP are bactericidal and this protein is currently one of the best vaccine candidates.49 More recently, it has been shown that a mechanism by which N. meningitidis escape the killing by polymorphonuclear neutrophil leucocytes (PMNs) to survive in the bloodstream is due to its ability to uptake available l-glutamate and to convert it to glutathione: this key molecule by maintaining intracellular redox potential protects the bacterium from reactive oxygen species, such as hydrogen peroxide, that are produced by the oxidative burst of neutrophils.50
The interaction with brain endothelial cells
As mentioned above, interaction with endothelial cells is essential in meningococcal pathogenesis. In vivo, blood flow generates mechanical forces that vary depending on the vessels and that could prevent bacterial interaction with the endothelial cells. The ability of N. meningitidis to bind to endothelial cells in the presence of shear stress mimicking the bloodstream was recently investigated.51 These data revealed that, after initial attachment, bacteria have the ability to resist high blood velocities, to multiply and to form microcolonies onto the apical surface of the endothelial cells. This resistance to shear stress and the ability to grow at the luminal surface of endothelial cells in the presence of blood flow highlight the efficacy of the interaction between N. meningitidis and the host cells, for which N. meningitidis has evolved efficient attributes.
Various bacterial surface components have been described allowing the interaction of N. meningitidis with human cells. These are type IV pili (Tfp) and other attributes such as Opa or Opc proteins (for review, see Carbonnelle et al.52,53).
However, in capsulated bacteria, type IV pili are the main bacterial attributes capable of promoting adhesion since non-piliated capsulated bacteria are unable to adhere to any cell type. Early work performed with piliated capsulated meningococci has shown that meningococcal interaction with human cells can be divided in two steps. The first one allows the adhesion of single diplococci in a rather inefficient manner. The second step corresponds to the bacterial division onto the apical surface of the cells. Therefore the high number of bacteria that interact with cells is a consequence of bacterial division of the few meningococci that have initially succeeded to adhere. Type IV pili are required for both steps. They promote the initial interaction of diplococci with the endothelial cells, and then generate bacteria-bacteria interactions that lead to the spreading of the bacteria on the apical surface of the cells.
Pilus biogenesis
Type IV pili are polymeric filaments found on many Gram-negative bacteria.54 These structures correspond to the multimeric assembly of a pilin subunit. Functional Tfp are dynamic structures. Pilin subunits are constantly assembled into fibers from a platform in the inner-membrane. The fiber is then extruded through the outer membrane via the secretin PilQ.54 A remarkable property of Tfp is their ability to retract into the bacterium from which they originate, via the action of the force-generating ATPase PilT.55,56 Retraction is a consequence of the disassembly of pilin subunits that are then stored in the cytoplasmic membrane.57 Tfp retraction is essential for bacterial motility (twitching motility), competence for DNA transformation and pilus-associated signaling to host cells.58
The major neisserial pilus subunit, the pilin, is encoded by the pilE gene that is subject to antigenic variation following recombination with silent loci (pilS). Pilin is synthesized as a preprotein. A short leader sequence (5–6 residues) is cleaved by the prepilin peptidase PilD.59 PilD also methylates the N-terminal phenylalanine of the mature protein product. Pilins are packed through internal hydrophobic interactions between conserved N-terminal α helices, leaving hypervariable C-terminal globular regions exposed.60 PilE undergoes several post translational modifications of serine residues including glycosylation at position 63, whereas at position 68, the residue has been reported to be modified by phosphate, phosphoethanolamine or phosphorylcholine addition.61,62 Concerning the glycosylation, the structure of the sugar is different depending on the strain.63,64
In addition to PilE, pili also contain the low abundance proteins PilX, PilV and ComP. These are called minor pilins65,66 as they structurally resemble PilE and are likely to be assembled within the filaments in a similar way.67 Importantly, each minor pilin modulates Tfp-linked properties.65 PilX is crucial for the formation of bacterial aggregates and indirectly controls adhesion to human cells by promoting bacteria-bacteria interactions,66,68 ComP is essential for competence for DNA transformation,65,69,70 while PilV affects several pilus-linked properties such as signaling to endothelial cells (see below65).
The PilC proteins play a crucial but still enigmatic role.54,71-74 Two alleles were originally discovered.71 Expression of both variants is subject to phase variation as a result of frameshift in homopolymeric “G” tracts located in the open reading frames.71 PilC-null strains show impaired pilus expression and lack the ability for transformation competence. In N. meningitidis, only PilC1 is required for adhesion. PilC2, which is expressed independently of PilC1, fails to promote adhesion despite identical functions in pilus expression and transformation competence.72,75 Abolition of pilT in a PilC-null background restores piliation, confirming the hypothesis that PilC acts as an antagonist of PilT by preventing PilT-mediated retraction.57,76
Mechanism of pilus-mediated interaction with host cells
The molecular mechanism responsible for the first step of the adhesive process, i.e., the initial attachment of individual diplococci to the cells, is still not fully understood. One report suggested that the PilC1 protein could carry a cell binding domain,74 this hypothesis was based on inhibition of adhesion using purified PilC molecules. However, non-adhesive non-piliated isolates of a serogroup B strain with high PilC expression and piliated adhesive isolates with barely detectable PilC expression have been described.77 In addition, another PilC+/PilE- strain, in which PilC location has been demonstrated in the outer membrane, is unable to interact with eukaryotic cells (Nassif X, unpublished observations), thus raising doubts on the role of PilC as an adhesin. It is therefore likely that the cell binding domain on the type IV pili remain to be identified.
As mentioned above, the minor pilin PilX is essential to promote inter-bacterial interactions and pilX mutants, which are unable to form aggregates, are unable to multiply at the cell surface. The inter-bacterial interactions generated by the Tfp are therefore required for bacteria to form microcolonies at the cell surface. Bacterial spreading onto the host cells relies also on the ability of bacteria to retract their pili. Indeed, following the interactions between bacteria and target cells, in some systems, pili have been shown to retract and eventually adherent meningococci appear non-piliated.55,78 Measurements using optical tweezers showed that retraction of a single Tfp generates forces up to 110 pN, in a transient manner for each fiber. Bundles of Tfp, which result from the association of 8 to 10 pili, act as coordinated retractable units. Bundles can generate retraction forces in the nanonewton range.79 The successive extension, binding and retraction of Tfp enable bacteria to move by twitching motility and spread on the apical surface of the host cells. Furthermore, it has been recently shown that the addition of a phosphoglycerol on pilin, which is increased during bacterial cell interaction, enhances the ability of the bacteria to detach from the adherent aggregates and to disseminate.80
Consequences of the Type IV Pilus-Mediated Interaction with Brain Endothelial Cells
As mentioned above, bacterial adhesion onto endothelial cells is mediated by the type IV pili that are responsible for the bacterial-cell and bacteria-bacteria interactions leading to the formation of the microcolonies. Following bacterial adhesion on the apical surface of the host cells, N. meningitidis induces elongation of microvilli toward the bacteria.81,82 Interestingly, the formation of such protrusions was also observed in vivo onto brain endothelial cells by transmission electron microscopy analysis of brain sections from a child who died from fulminant meningitis.82 These cellular projections are likely to be required to allow the microcolonies to stand up to the shear stress of the bloodstream.51 These observations strongly suggest that such morphological modifications of the host cell membrane are essential in meningococcal pathogenesis.
N. meningitidis induces the clustering of cellular receptors
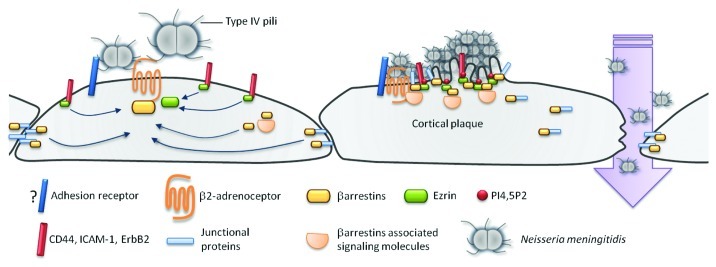
Following Tfp-mediated adhesion, N. meningitidis induces at the site of bacterial cell interaction the formation of a specific membrane domain enriched in structural proteins and membrane receptors designated the “cortical plaque.”83 This structure dramatically modifies the molecular equilibrium of the host cell, and leads to the opening of the blood-brain barrier (Fig. 1).84,85 The formation of such structure is a two steps process: (1) the initial recruitment of one or several receptors following pilus-mediated adhesion and (2) the activation of one or several of these receptors responsible for the recruitment of other transmembrane components and for the modification of the host cell cytoskeleton. This latter step is also mediated by the type IV pili (Fig. 1).83,85
Figure 1. Formation of the cortical plaque and transmigration of Neisseria meningitidis. Neisseria meningitidis adheres to brain microvascular endothelial cells by the interaction of Type IV pili with an unknown adhesion receptor.72 Following initial bacterial adhesion, type IV pili mediate the recruitment and the activation of the β2-adrenoceptor thus leading to the organization of specific cytoplasmic molecular complexes, referred to as cortical plaques.83,85 The formation of cortical plaques results (1) from the local production of PI4,5P294 that mediates the accumulation of ezrin and ezrin binding receptor such as ICAM-1 and CD4491 and (2) from the accumulation of β-arrestins and β-arrestin-binding molecules such as Src, p120-catenin and VE-cadherin.84,85,104 The formation of the cortical plaque induces the formation of microvilli like protrusions that protect bacterial colonies from the blood flow shear stress and dramatically modifies the molecular equilibrium of the host cell.92 One consequence is the opening of the cell-cell junctions that allows the transmigration of bacteria through the endothelium.84
The CD46 receptor was first proposed as being the adhesion receptor for both N. gonorrheae and N. meningitidis. However, this finding was not confirmed by subsequent studies.86,87 The Laminin receptor was also described as a potential receptor for N. meningitidis. Two bacterial ligands for this receptor have been reported, the PilQ secretin and the PorA protein.88 However considering that PilQ is expressed in PilE non-piliated mutant and that non-piliated non-capsulated strain are unable to interact with endothelial cells in flow conditions, its role remain to be précised. It should be pointed out that the I-domain-containing integrins were described to be essential for Neisseria gonorrhea adhesion to epithelial cells.89 However, similar data have not been reported for N. meningitidis and endothelial cells.
The initial cellular component of brain endothelial cell that interacts with the type IV pili remains unknown. On the other hand, among one of the receptors recruited following meningococcal initial adhesion, the β2-adrenergic receptor was recently described as an important signaling receptor for N. meningitidis. The β2-adrenergic receptor is a G protein coupled receptor (GPCR) that interacts with β-arrestins and the heterotrimeric Gαs protein. This receptor is also known for its implication in vascular homeostasis and disease. The expression of both the β2-adrenergic receptor and β-arrestins is sufficient to promote a N. meningitidis induced cell response in an incompetent cell line such as HEK293.85 It has been shown that PilE and PilV, the major pilin subunit and a minor pilin subunit, respectively, directly interact with the extracellular N-terminal domain of the β2-adrenergic receptor to transmit the signal.85 This interaction is believed to modify the conformation of the receptor, resulting in the activation of the β-arrestins pathway without activating the heterotrimeric Gαs protein and its downstream adenyl cyclase/cAMP pathway, a property referred to as biased activation.85 β-arrestins are scaffolding proteins involved in many cellular processes such as receptor internalization and actin polymerization.90 Following the activation of the β2-adrenergic receptor by N. meningitidis and the accumulation of β-arrestins, the signal leads to the formation of a “raft-like” membrane domain enriched in cholesterol and PIP2 in which several transmembrane receptors and structural proteins are sequestered, thus leading to the subsequent formation of the cortical plaque.91,92 It is likely that accumulated β-arrestins underneath bacteria plays a major role in the sequestration of these signaling molecules.
The interaction domain between the bacterial colony and the host cell can be compared with a synapse since (1) it is composed of adhesion receptors and signaling receptors (that are mostly immunoglobulin domain containing receptors and G protein coupled receptors, respectively) and (2) it transmits signals. One particularity of this “bacterial synapse” is that adhesion receptor and signaling receptor are not internalized but sequestrated underneath the meningococcus (Doulet et al.91 and personal observation). This “bacterial synapse” is maintained by a cortical network highly enriched in members of the ezrin-radixin-moesin (ERM) proteins family that are anchored to the plasma membrane by their PIP2 binding domain and control the organization of the cortical actin cytoskeleton through their C-terminal F-actin binding sites.93,94 ERM proteins also bind the cytoplasmic domain of several ERM binding transmembrane receptors such as CD44 and ICAM-1 through their C-terminal domain and act as linkers between the actin cytoskeleton and the plasma membrane.83,93,95 As a consequence of the recruitment of ERM proteins, many receptors known to be involved in leukocytes adhesion are sequestered underneath bacterial colonies.91 During leukocytes adhesion, these components that form the “endothelial docking structures” or “transmigratory cups” are essential to promote firm adhesion and extravasation of leukocytes through the endothelium. Transmigratory cups result from the dynamic redistribution of ICAM-1, VCAM-1, E-selectin and CD44 at the endothelial-leukocyte contact area, accompanied by the recruitment of activated ERM proteins, and leads to cortical actin polymerization.96-98 Since the same set of endothelial proteins is present within the membrane protrusions induced by N. meningitidis and within the docking structures promoted by leukocyte adhesion, it was suggested that N. meningitidis hijacks the leukocyte adhesion pathway.91
In summary, following the initial adhesion of the bacteria to a yet unknown receptor, the β2-adrenergic receptor is recruited and activated by components of the type IV pili, thus inducing the formation of the cortical plaque.
Consequences of N. meningitidis induced signaling
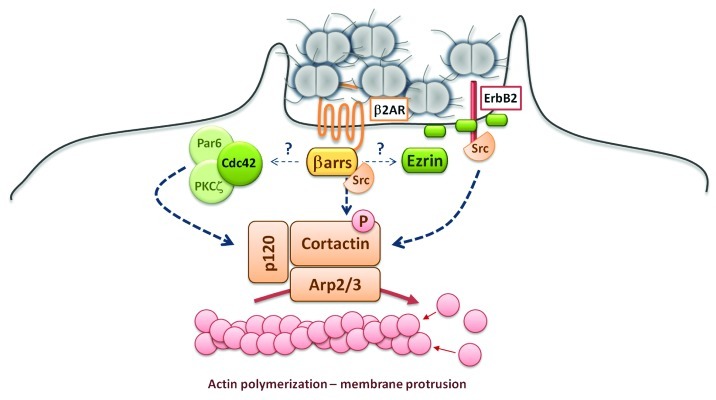
The formation of the cortical plaque is accompanied by a robust actin polymerization leading to the elongation of membrane protrusions around bacteria that, as mentioned above, are likely to play a role in the resistance of the colony to flow shear stresses.92 Actin polymerization relies on the activation of the small GTPases of the Rho family.93,94 In addition, it has been demonstrated that proper actin polymerization in these membrane protrusions relies on cortactin phosphorylation. Cortactin or cortical actin binding protein is a perinuclear cytoplasmic protein that is involved in the reorganization of the cell cortical actin cytoskeleton. It appears that cortactin recruitment and phosphorylation is finely tuned by N. meningitidis (Fig. 2):
Figure 2.Neisseria meningitidis regulates the polymerization of actin through phosphorylation of cortactin. Adhesion of N. meningitidis to endothelial cells leads to the formation of membrane protrusions that result from active actin polymerization. Type IV pili mediate the accumulation and activation of the β2-adrenoceptor/β-arrestins85 complex that allows (1) the activation of the Cdc42-Par6/PKCζ and the proper localization of p120-catenin and of the Cortactin/Arp 2/3 complex underneath the colony84; (2), the recruitment of the tyrosine kinase Src that phosphorylates and activates the cortactin/Arp 2/3 complex84,85,104; (3) the accumulation of Ezrin, that plays a key role in actin organization, and that of membrane receptor such as the ErbB2 receptor that serves as docking site for Src.91,94,104
(1) The recruitment of cortactin at the site of N. meningitidis adhesion is controlled by the Cdc42-Par6/PKCζ pathway.84 This pathway is involved in the proper tethering of microtubules at the leading edge of migrating cells.99,100 In the case of the meningococcal infection, the Cdc42-Par6/PKCζ pathway may tether microtubules to the site of bacterial adhesion, thus allowing p120-catenin dependent traffic of the cortactin along the microtubules network.84,101,102
(2) The activation of cortactin through phosphorylation is controlled by the tyrosine kinase Src that is itself sequestered and activated in the cortical plaque by direct interaction with the β-arrestins.85,103
(3) Finally, the ErbB2 tyrosine kinase receptor regulates Src activity and the subsequent cortactin phosphorylation.104 The ErbB2 tyrosine kinase receptor belongs to the family of epidermal growth factor (EGF) receptors. The interaction of N. meningitidis with human endothelial cells leads to its activation most likely via formation of ErbB2 homodimers in the cortical plaque. This is an example of a secondary signaling activated by the accumulation of a cellular receptor at site of bacterial adhesion that contributes to the formation of the cortical plaque.
Another consequence of the formation of the cortical plaque is the opening of the interendothelial junctions allowing the transmigration of bacteria by a paracellular route. Indeed, it has been shown that N. meningitidis recruits junctional proteins underneath the colony into the cortical plaque. This recruitment is due to the activation of the Cdc42-Par3/Par6/PKCζ pathway that is usually involved in the formation of adherens and tight junctions at cell-cell contact. Here, the ectopic activation of the polarity complex Par3/Par6/PKCζ leads to abnormal recruitment of junctional proteins that are sequestrated underneath bacterial colonies through their interaction with β-arrestins. As a consequence, these molecules are depleted at the intercellular junctions causing endothelium leakage. Adhesion of N. meningitidis lately promotes the cleavage of occludin (a component of the tight junction) by the metalloproteinase MMP-8,105 further altering the intercellular junctions.
Conclusion
Recent exciting findings have considerably expanded our understanding of the cellular events involved in meningococcal interaction with brain endothelial cells, thus leading to the opening of the paracellular route and meningeal invasion. However, in spite of recent advances in our understanding of these molecular mechanisms, much remains to be discovered about the complex molecular networks involved. Among major issues is the identification of the receptor for meningococcal adhesion, which would constitute a significant breakthrough in the field and the identification of animal models that would allow us to confirm these in vitro observations.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/18639
References
- 1.Pong A, Bradley JS. Bacterial meningitis and the newborn infant. Infect Dis Clin North Am. 1999;13:711–33. doi: 10.1016/S0891-5520(05)70102-1. [DOI] [PubMed] [Google Scholar]
- 2.Huang SH, Stins MF, Kim KS. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2000;2:1237–44. doi: 10.1016/S1286-4579(00)01277-6. [DOI] [PubMed] [Google Scholar]
- 3.van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 4.Nassif X, Bourdoulous S, Eugene E, Couraud PO. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 2002;10:227–32. doi: 10.1016/S0966-842X(02)02349-1. [DOI] [PubMed] [Google Scholar]
- 5.Rubin LG, Zwahlen A, Moxon ER. Role of intravascular replication in the pathogenesis of experimental bacteremia due to Haemophilus influenzae type b. J Infect Dis. 1985;152:307–14. doi: 10.1093/infdis/152.2.307. [DOI] [PubMed] [Google Scholar]
- 6.Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–72. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 7.Moxon ER, Vaughn KA. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981;143:517–24. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- 8.Winkelstein JA, Moxon ER. The role of complement in the host's defense against Haemophilus influenzae. J Infect Dis. 1992;165(Suppl 1):S62–5. doi: 10.1093/infdis/165-Supplement_1-S62. [DOI] [PubMed] [Google Scholar]
- 9.Austrian R. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev Infect Dis. 1981;3(Suppl 1):S1–17. doi: 10.1093/clinids/3.Supplement_1.S1. [DOI] [PubMed] [Google Scholar]
- 10.Craven DE, Peppler MS, Frasch CE, Mocca LF, McGrath PP, Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980;142:556–68. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- 11.Harrison OB, Robertson BD, Faust SN, Jepson MA, Goldin RD, Levin M, et al. Analysis of pathogen-host cell interactions in purpura fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo. Infect Immun. 2002;70:5193–201. doi: 10.1128/IAI.70.9.5193-5201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721–36. doi: 10.1016/S1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 13.Smith AL, Daum RS, Scheifele D, Syriopoulou V, Averil DR, Roberts MC, et al. Pathogenesis of Haemophilus influenzae meningitis. In: Sell SH, Wright PF, eds. Haemophilus influenzae: epidemiology, immunology and prevention of the disease. New-York: Elsevier Sciences Publishing Co., Inc, 1982:89-109. [Google Scholar]
- 14.InVS. Réseau EPIBAC. Surveillance des infections invasives à Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus pneumoniae, Streptococcus agalactiae (B) et Streptococcus pyogenes (A) en France métropolitaine. Paris: Institut National de Veille Sanitaire (InVs), 2009. [Google Scholar]
- 15.Brouwer MC, de Gans J, Heckenberg SGB, Zwinderman AH, van der Poll T, van de Beek D. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:31–44. doi: 10.1016/S1473-3099(08)70261-5. [DOI] [PubMed] [Google Scholar]
- 16.Brouwer MC, Read RC, van de Beek D. Host genetics and outcome in meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:262–74. doi: 10.1016/S1473-3099(10)70045-1. [DOI] [PubMed] [Google Scholar]
- 17.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bille E, Zahar JR, Perrin A, Morelle S, Kriz P, Jolley KA, et al. A chromosomally integrated bacteriophage in invasive meningococci. J Exp Med. 2005;201:1905–13. doi: 10.1084/jem.20050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bille E, Ure R, Gray SJ, Kaczmarski EB, McCarthy ND, Nassif X, et al. Association of a bacteriophage with meningococcal disease in young adults. PLoS ONE. 2008;3:e3885. doi: 10.1371/journal.pone.0003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun YH, Bakshi S, Chalmers R, Tang CM. Functional genomics of Neisseria meningitidis pathogenesis. Nat Med. 2000;6:1269–73. doi: 10.1038/81380. [DOI] [PubMed] [Google Scholar]
- 21.Pron B, Taha MK, Rambaud C, Fournet JC, Pattey N, Monnet JP, et al. Interaction of Neisseria maningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. J Infect Dis. 1997;176:1285–92. doi: 10.1086/514124. [DOI] [PubMed] [Google Scholar]
- 22.Hardy SJ, Christodoulides M, Weller RO, Heckels JE. Interactions of Neisseria meningitidis with cells of the human meninges. Mol Microbiol. 2000;36:817–29. doi: 10.1046/j.1365-2958.2000.01923.x. [DOI] [PubMed] [Google Scholar]
- 23.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981;77:349–71. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staddon JM, Rubin LL. Cell adhesion, cell junctions and the blood-brain barrier. Curr Opin Neurobiol. 1996;6:622–7. doi: 10.1016/S0959-4388(96)80094-8. [DOI] [PubMed] [Google Scholar]
- 27.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–17. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 30.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy Z, Peters H, Huttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984;50:313–22. [PubMed] [Google Scholar]
- 32.Ge S, Song L, Pachter JS. Where is the blood-brain barrier ... really? J Neurosci Res. 2005;79:421–7. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- 33.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–85. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–9. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 35.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 37.Cotran RS, Kumar V, Collins T, Robbins SL. Robbins pathological basis of disease. Philadelphia: Elsevier Science, 1999. [Google Scholar]
- 38.Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, et al. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–9. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 39.Huang SH, Jong AY. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell Microbiol. 2001;3:277–87. doi: 10.1046/j.1462-5822.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- 40.Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347–60. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasadarao NV, Wass CA, Stins MF, Kim KS. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect Immun. 1999;67:5775–83. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stins MF, Badger J, Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 43.Stins MF, Nemani PV, Wass C, Kim KS. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect Immun. 1999;67:5522–5. doi: 10.1128/iai.67.10.5522-5525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nizet V, Kim KS, Stins M, Jonas M, Chi EY, Nguyen D, et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–81. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virji M, Kayhty H, Ferguson DJ, Alexandrescu C, Moxon ER. Interactions of Haemophilus influenzae with human endothelial cells in vitro. J Infect Dis. 1992;165(Suppl 1):S115–6. doi: 10.1093/infdis/165-Supplement_1-S115. [DOI] [PubMed] [Google Scholar]
- 46.Virji M, Kayhty H, Ferguson DJ, Alexandrescu C, Moxon ER. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb Pathog. 1991;10:231–45. doi: 10.1016/0882-4010(91)90057-H. [DOI] [PubMed] [Google Scholar]
- 47.Nikulin J, Panzner U, Frosch M, Schubert-Unkmeir A. Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells. Int J Med Microbiol. 2006;296:553–8. doi: 10.1016/j.ijmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, et al. Factor H-binding protein is important for meningococcal survival in human whole blood and serum, and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77:292–9. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beernink PT, Leipus A, Granoff DM. Rapid genetic grouping of factor h-binding protein (genome-derived neisserial antigen 1870), a promising group B meningococcal vaccine candidate. Clin Vaccine Immunol. 2006;13:758–63. doi: 10.1128/CVI.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talà A, Monaco C, Nagorska K, Exley RM, Corbett A, Zychlinsky A, et al. Glutamate utilization promotes meningococcal survival in vivo through avoidance of the neutrophil oxidative burst. Mol Microbiol. 2011;81:1330–42. doi: 10.1111/j.1365-2958.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mairey E, Genovesio A, Donnadieu E, Bernard C, Jaubert F, Pinard E, et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood-brain barrier. J Exp Med. 2006;203:1939–50. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbonnelle E, Hill DJ, Morand P, Griffiths NJ, Bourdoulous S, Murillo I, et al. Meningococcal interactions with the host. Vaccine. 2009;27(Suppl 2):B78–89. doi: 10.1016/j.vaccine.2009.04.069. [DOI] [PubMed] [Google Scholar]
- 53.Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7:274–86. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 54.Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19:6408–18. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 56.Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA. 2001;98:6901–4. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morand PC, Bille E, Morelle S, Eugene E, Beretti JL, Wolfgang M, et al. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 2004;23:2009–17. doi: 10.1038/sj.emboj.7600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SW, Higashi DL, Snyder A, Merz AJ, Potter L, So M. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell Microbiol. 2005;7:1271–84. doi: 10.1111/j.1462-5822.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 59.Lory S, Strom MS. Structure-function relationship of type-IV prepilin peptidase of Pseudomonas aeruginosa–a review. Gene. 1997;192:117–21. doi: 10.1016/S0378-1119(96)00830-X. [DOI] [PubMed] [Google Scholar]
- 60.Parge HE, Forest KT, Hickey MJ, Christensen DA, Getzoff ED, Tainer JA. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378:32–8. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 61.Hegge FT, Hitchen PG, Aas FE, Kristiansen H, Lovold C, Egge-Jacobsen W, et al. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc Natl Acad Sci USA. 2004;101:10798–803. doi: 10.1073/pnas.0402397101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forest KT, Dunham SA, Koomey M, Tainer JA. Crystallographic structure reveals phosphorylated pilin from Neisseria: phosphoserine sites modify type IV pilus surface chemistry and fibre morphology. Mol Microbiol. 1999;31:743–52. doi: 10.1046/j.1365-2958.1999.01184.x. [DOI] [PubMed] [Google Scholar]
- 63.Virji M. Post-translational modifications of meningococcal pili. Identification of common substituents: glycans and alpha-glycerophosphate–a review. Gene. 1997;192:141–7. doi: 10.1016/S0378-1119(97)00082-6. [DOI] [PubMed] [Google Scholar]
- 64.Chamot-Rooke J, Rousseau B, Lanternier F, Mikaty G, Mairey E, Malosse C, et al. Alternative Neisseria spp. type IV pilin glycosylation with a glyceramido acetamido trideoxyhexose residue. Proc Natl Acad Sci USA. 2007;104:14783–8. doi: 10.1073/pnas.0705335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown DR, Helaine S, Carbonnelle E, Pelicic V. Systematic Functional analysis reveals that a set of seven genes is involved in fine-tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis. Infect Immun. 2010;78:3053–63. doi: 10.1128/IAI.00099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hélaine S, Carbonnelle E, Prouvensier L, Beretti JL, Nassif X, Pelicic V. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol Microbiol. 2005;55:65–77. doi: 10.1111/j.1365-2958.2004.04372.x. [DOI] [PubMed] [Google Scholar]
- 67.Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–77. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helaine S, Dyer DH, Nassif X, Pelicic V, Forest KT. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc Natl Acad Sci USA. 2007;104:15888–93. doi: 10.1073/pnas.0707581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aas FE, Wolfgang M, Frye S, Dunham S, Lovold C, Koomey M. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol Microbiol. 2002;46:749–60. doi: 10.1046/j.1365-2958.2002.03193.x. [DOI] [PubMed] [Google Scholar]
- 70.Wolfgang M, van Putten JP, Hayes SF, Koomey M. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol Microbiol. 1999;31:1345–57. doi: 10.1046/j.1365-2958.1999.01269.x. [DOI] [PubMed] [Google Scholar]
- 71.Jonsson AB, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–88. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nassif X, Beretti JL, Lowy J, Stenberg P, O'Gaora P, Pfeifer J, et al. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–73. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer TF. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–90. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudel T, Scheurerpflug I, Meyer TF. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–9. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 75.Morand PC, Tattevin P, Eugene E, Beretti JL, Nassif X. The adhesive property of the type IV pilus-associated component PilC1 of pathogenic Neisseria is supported by the conformational structure of the N-terminal part of the molecule. Mol Microbiol. 2001;40:846–56. doi: 10.1046/j.1365-2958.2001.02452.x. [DOI] [PubMed] [Google Scholar]
- 76.Wolfgang M, Lauer P, Park HS, Brossay L, Hebert J, Koomey M. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol. 1998;29:321–30. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 77.Virji M, Makepeace K, Peak I, Payne G, Saunders JR, Ferguson DJ, et al. Functional implications of the expression of PilC proteins in meningococci. Mol Microbiol. 1995;16:1087–97. doi: 10.1111/j.1365-2958.1995.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 78.Pujol C, Eugene E, Marceau M, Nassif X. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc Natl Acad Sci USA. 1999;96:4017–22. doi: 10.1073/pnas.96.7.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chamot-Rooke J, Mikaty G, Malosse C, Soyer M, Dumont A, Gault J, et al. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science. 2011;331:778–82. doi: 10.1126/science.1200729. [DOI] [PubMed] [Google Scholar]
- 81.Merz AJ, Rifenbery DB, Arvidson CG, So M. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med. 1996;2:745–54. [PMC free article] [PubMed] [Google Scholar]
- 82.Pujol C, Eugene E, de Saint Martin L, Nassif X. Interaction of Neisseria meningitidis with a polarized monolayer of epithelial cells. Infect Immun. 1997;65:4836–42. doi: 10.1128/iai.65.11.4836-4842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merz AJ, Enns CA, So M. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol Microbiol. 1999;32:1316–32. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 84.Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–7. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coureuil M, Lecuyer H, Scott MG, Boularan C, Enslen H, Soyer M, et al. Meningococcus hijacks a beta2-adrenoceptor/beta- um. Cell. 2010;143:1149–60. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 86.Källström H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–47. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 87.Kirchner M, Heuer D, Meyer TF. CD46-independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect Immun. 2005;73:3072–82. doi: 10.1128/IAI.73.5.3072-3082.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–46. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards JL, Apicella MA. I-domain-containing integrins serve as pilus receptors for Neisseria gonorrhoeae adherence to human epithelial cells. Cell Microbiol. 2005;7:1197–211. doi: 10.1111/j.1462-5822.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 90.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 91.Doulet N, Donnadieu E, Laran-Chich MP, Niedergang F, Nassif X, Couraud PO, et al. Neisseria meningitidis infection of human endothelial cells interferes with leukocyte transmigration by preventing the formation of endothelial docking structures. J Cell Biol. 2006;173:627–37. doi: 10.1083/jcb.200507128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mikaty G, Soyer M, Mairey E, Henry N, Dyer D, Forest KT, et al. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 2009;5:e1000314. doi: 10.1371/journal.ppat.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eugène E, Hoffmann I, Pujol C, Couraud PO, Bourdoulous S, Nassif X. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J Cell Sci. 2002;115:1231–41. doi: 10.1242/jcs.115.6.1231. [DOI] [PubMed] [Google Scholar]
- 94.Lambotin M, Hoffmann I, Laran-Chich MP, Nassif X, Couraud PO, Bourdoulous S. Invasion of endothelial cells by Neisseria meningitidis requires cortactin recruitment by a phosphoinositide-3-kinase/Rac1 signalling pathway triggered by the lipo-oligosaccharide. J Cell Sci. 2005;118:3805–16. doi: 10.1242/jcs.02514. [DOI] [PubMed] [Google Scholar]
- 95.Merz AJ, So M. Attachment of piliated, Opa- and Opc- gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–9. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barreiro O, Yanez-Mo M, Sala-Valdes M, Gutierrez-Lopez MD, Ovalle S, Higginbottom A, et al. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood. 2005;105:2852–61. doi: 10.1182/blood-2004-09-3606. [DOI] [PubMed] [Google Scholar]
- 97.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–88. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–80. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol. 2010;191:585–98. doi: 10.1083/jcb.201002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, et al. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci USA. 2007;104:10882–7. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 104.Hoffmann I, Eugene E, Nassif X, Couraud PO, Bourdoulous S. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J Cell Biol. 2001;155:133–44. doi: 10.1083/jcb.200106148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schubert-Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog. 2010;6:e1000874. doi: 10.1371/journal.ppat.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]