Abstract
Purpose
Oxidative damage has been implicated in carcinogenesis. We hypothesized that elevated systemic oxidative status would be associated with later occurrence of colorectal adenomatous polyps, a precursor of colorectal cancer.
Methods
We examined the prospective association between four systemic markers of oxidative status and colorectal adenomatous polyps within a non-diabetic sub-cohort of the Insulin Resistance Atherosclerosis Study (IRAS) (n=425). Urine samples were collected from 1992–1994 and colorectal adenomas prevalence were assessed in 2002–2004. Oxidative status markers were assessed, which included four F2-isoprostanes (F2-IsoPs) from the classes III and IV: iPF2α-III, 2,3-dinor-iPF2α-III (a metabolite of iPF2α-III), iPF2α-VI, and 8,12-iso-iPF2α-VI. All biomarkers were quantified using liquid chromatography–tandem mass spectrometry. Prospective associations were assessed using multivariate logistic regression analysis.
Results
The adjusted ORs (95% CIs) for occurrence of colorectal adenomatous polyps and scaled to 1 SD of F2-IsoP distribution were 1.16 (0.88–1.50), 0.88 (0.63–1.17), 1.04 (0.80–1.34), and 1.16 (0.90–1.48) for iPF2α-III, iPF2α-VI, 8,12-iso-iPF2α-VI, and 2,3-dinor-iPF2α-III, respectively.
Conclusion
The lack of association between F2-IsoPs and adenomatous polyps does not support the hypothesis that elevated oxidative status is associated with colorectal adenomatous polyp occurrence during a 10-year period of follow-up.
Keywords: oxidative stress, biomarkers, F2-isoprostanes, adenomatous polyps, adenoma, colorectal cancer, epidemiology
Introduction
External oxidative exposures (e.g. smoking, ionizing radiation, diet) are known to act as carcinogenic risk factors, damaging DNA, lipids, and proteins (1–5). These exposures are thought to interact with an individual’s internal sources of reactive oxygen species, such as metabolism, and inflammation, to define one’s oxidative status (6, 7). How this reductive-oxidative (redox) balance influences individual cancer predisposition is not well established. Several prospective studies of lung, breast, and prostate cancers have so far yielded inconsistent results (8–10). These studies assessed individual oxidative status at the systemic level (urinary excretion or plasma levels) of lipid peroxidation biomarkers, the F2-isoprostanes (F2-IsoPs) (11). Whether these inconsistent findings present spurious associations or reflect a complicated relationship between individual oxidative status and cancer risk remains an open question.
A common cancer outcome that may be related to redox balance is colorectal cancer (CRC), because CRC risk has been associated with oxidizing exposures, such as ionizing radiation and alcohol consumption (12–13). Adenomas or adenomatous polyps are common precursors to CRC (14–17). Oxidizing exposures, such as smoking and alcohol use have been associated with adenoma risk (18–20). Therefore, markers of redox balance may also be associated with adenoma risk. We hypothesized that individuals with elevated levels of systemic oxidative status markers would have a higher risk of colorectal adenomatous polyps, a precursor of colorectal cancer. We examined our hypothesis in a prospective study, using four urinary F2-IsoPs to assess individual oxidative status.
F2-IsoPs are formed during the non-enzymatic oxidation of arachidonic acid by different types of free radicals (21, 22). Depending on the position where the oxygen molecule is added to arachidonic acid, four regioisomers are formed, giving four F2-IsoPs series. Furthermore, each series comprises 16 stereoisomers, which yields a final total of 64 possible isomers.
Arachidonic acid is ubiquitously integrated into the phospholipids comprising biological membranes and lipoproteins. Formed within these phospholipids, F2-IsoPs are hydrolyzed from esterified lipids and metabolized via the beta-oxidation pathway. Both the original F2-IsoPs and their metabolites are excreted in urine, with exertion of the metabolites being proportional to the formation of the original F2-IsoPs (22). Urinary measurements of F2-IsoPs have several advantages as compared to blood measurements, namely they present a time-integrated index of total body F2-IsoP production, whereas the half-life of F2-IsoPs in blood is measured in minutes and are not liable to autooxidation due to low lipid content of urine. Previous work has shown that F2-IsoPs demonstrate sufficiently low (approximately 30%) intra-individual variation, making them potentially good biomarkers for assessing inter-individual variability in systemic redox status (23).
To examine our hypothesis, we measured multiple F2-isoprostanes. Two F2-IsoPs were selected from the III-series: iPF2α-III was selected because it is the first isomer proposed as an index of lipid peroxidation in vivo and, therefore, is the most frequently measured isomer (24). 2,3-dinor-iPF2α-III was selected as a beta-oxidation metabolite of iPF2α-III, addressing a theoretical concern that renal tissues may contribute disproportionally to the total production of iPF2α-III. In addition, we selected two F2-IsoPs from the VI-series, iPF2α-VI and 8,12-iso-iPF2α-VI, because they are most abundant in human urine (25). Due to their abundance, the VI-series F2-IsoPs may be more sensitive biomarkers than the III-series. Furthermore, as shown by previous studies, associations may vary depending on the specific F2-IsoP being measured (26, 27). By including multiple F2-IsoP isomers, we increase sensitivity of the study to detect a possible association. Importantly, these four F2-IsoP species have been validated as sensitive markers of oxidative stress in a clinical model (22).
Materials and Methods
Study Population
The Insulin Resistance Atherosclerosis Study (IRAS) was a multi-ethnic cohort. The subjects were recruited from four U.S. communities in 1992–1994 with the primary goal of assessing the relationship between insulin resistance, insulinemia, glycemia, other components of the insulin resistance syndrome, and prevalent cardiovascular disease. A total of 1626 individuals ages 40 to 69 years of age participated in the IRAS. (28). The colon study was nested in the IRAS cohort, where colonoscopies were conducted in the subcohort between 2002 and 2004; details are described elsewhere (29). Briefly, eligibility for colonoscopy was the following: surviving IRAS cohort participants who were ≥49 years of age, mentally eligible, and without serious concurrent illnesses (e.g. recent heart attack, oxygen dependent pulmonary disease, renal failure, prosthetic heart valve, or colon cancer). Participants who reported having adenomatous polyps at least five years prior to the study period of 2002–2004 were included, if their next colonoscopy exam was due within the study period.
A total of 600 IRAS participants had a colonoscopy, including those who had diabetes at baseline. This analysis excluded participants with diabetes at baseline, as increased oxidative status may be a consequence of this condition (24). Of the 600 IRAS participants, the analytical cohort for this study was comprised of 425 IRAS participants who were free of diabetes at baseline and had available baseline (1992–1994) urine sample for measurements of oxidative stress markers. All participants provided signed informed consent and the study was approved by the Institutional Review Boards of all collaborating organizations.
Colonoscopy
Experienced physicians performed the colonoscopies, reaching the cecum in 96% of participants. Size and location of all visible polyps were recorded and the polyps were removed. A standard histologic assessment was done by the local clinical laboratory. Within the analytical cohort, 331 participants (77.9%) had no adenomatous polyps or had hyperplastic polyps (further referred to as “no adenoma”). 94 participants (22.1%) had adenomatous polyps (referred to as “adenoma”). No carcinomas were diagnosed in this study population.
Urinary F2-isoprostanes
At the baseline examination, morning spot urine samples were collected and stored at −70 °C. Four F2-IsoPs (iPF2α-III, 2,3-dinor-iPF2α-III, iPF2α-VI, and 8,12-iso-iPF2α-VI) were quantified by liquid chromatography (LC) with tandem mass spectrometry (MS) detection (LC-MS/MS) on a Shimadzu 20A series LC and Applied Biosystems API 4000 QTrap MS/MS instruments, as previously described (24). Calibration of the instrument for sample collection was performed by adding pure F2-IsoPs into pooled human urine and injected into the machine before and after the IRAS participants’ samples, covering the entire expected range of physiological F2-IsoP concentrations. Lower limits of quantification (>80% accuracy) were 0.007, 0.34, 0.25, and 0.12 mg/mL for iPF2α-III; 2,3-dinor-iPF2α-III, iPF2α-VI, and 8,12-iso-iPF2)-VI, respectively. Urinary levels of F2-IsoPs were adjusted by creatinine to take into account differences in urine diluteness. Creatinine was assayed by a fast electrospray ionization–tandem MS method, as described previously (30).
Other Covariates
History of previous polyps was obtained through self-report at 2002–2004. The participants were asked whether they were ever told that they had colorectal polyps and about the date of diagnosis. Demographic data (age and gender), measurements of glucose tolerance, and data for other covariates were collected during baseline visits in 1992–1994. Race/ethnicity was self-reported. To insure valid measurements of glucose tolerance, all IRAS participants fasted for 12 hours and refrained from heavy exercise, smoking, and alcohol consumption for 24 hours before the visit. Glucose tolerance was measured precisely at each examination using an oral glucose tolerance test and the World Health Organization criteria. A 75-gram glucose load (Orange-dex; Custom Laboratories, Baltimore, MD) was administered over a period of <10 minutes. Blood was collected at 0 and 2 hours. Normal glucose tolerance (NGT) was defined as fasting glucose and 2-hour glucose < 140 mg/dl. Impaired glucose tolerance (IGT) was defined as fasting glucose < 140 mg/dl and 2-hour glucose 140 and <200 mg/dl.
Height was measured to the nearest 0.5 cm and weight was measured to the nearest 0.1 kg. These measurements have been conducted in duplicate following a standardized protocol, and averages were used in the analysis. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Smoking status was assessed by self-report. Alcohol consumption was assessed as part of the 114-item food frequency questionnaire (31, 32), which was modified for the IRAS to incorporate regional and ethnic food habits and supplements.
Statistical Analysis
Student’s t-test and χ2-test were used to assess differences in the distribution of demographic and baseline variables by adenoma versus no-adenoma status. Crude association between F2-IsoPs and study characteristics were examined using Student’s t-test, ANOVA, and Spearman corelation coefficient. Adjusted odds ratios (ORs) for the associations between each of the F2-IsoPs and colorectal adenoma, along with their corresponding 95% confidence intervals (CIs), were calculated from logistic regression models. Model 1, the minimally adjusted model, included the demographic variables (age, gender, and race/ethnicity) and previous polyps as covariates. Model 2, the fully adjusted model, included additional adjustments for baseline glucose tolerance status (IGT or NGT), BMI, alcohol use and smoking history. All statistical analyses utilized two-sided tests with the threshold for statistical significance established as p = 0.05.
Results
Among the examined baseline characteristics, age and previous adenomatous polyps showed crude association with occurrence of adenoma in 2002–2004 (Table 1). Consistent with previous studies (8), females had higher levels of urinary F2-IsoPs relative to male. As was shown previously in this cohort (29), race/ethnicity categories were associated with F2-IsoP levels, with African Americans having the lowest levels of F2-IsoPs (Table 2). Glucose tolerance was not associated with F2-IsoP levels. Other characteristics, such as age, BMI, smoking history, and previous polyps were associated with some but not all F2-IsoPs measured (Table 2).
Table 1.
Occurrence of colorectal adenoma by demographic characteristics and risk factors among 425 participants in the IRAS cohort study.
| Characteristics | Adenomas | NO adenomasa | |
|---|---|---|---|
| N (%) | N (%) | ||
| Gender | Males | 46 (48.9) | 134 (40.5) |
| Females | 48 (51.1) | 197 (59.5) | |
| p-value b | 0.14 | ||
| Agec | 40–49 | 21 (22.3) | 136 (41.1) |
| 50–59 | 39 (41.5) | 127 (38.4) | |
| 60–69 | 34 (36.2) | 68 (20.5) | |
| p-value | < 0.01 | ||
| Race/Ethnicity | Black | 30 (31.9) | 81 (24.5) |
| Non-Hispanic White | 34 (36.2) | 136 (41.1) | |
| Hispanic | 30 (31.9) | 114 (34.4) | |
| p-value | 0.34 | ||
| Glucose tolerance c | NGT | 69 (73.40) | 241 (72.8) |
| IGT | 25 (26.60) | 90 (27.2) | |
| p-value | 0.91 | ||
| BMIc | Normal (<25) | 21 (22.3) | 101 (30.6) |
| Overweight (25–30) | 46 (48.9) | 145 (43.9) | |
| Obese (>30) | 27 (28.7) | 84 (25.5) | |
| p-value | 0.30 | ||
| Smoking status d | Never | 39 (41.5) | 159 (48.0) |
| Former | 44 (46.8) | 129 (39.0) | |
| Current | 11 (11.7) | 43 (13.0) | |
| p-value | 0.39 | ||
| Previous polyps c | No | 74 (78.7) | 305 (92.2) |
| Yes | 20 (21.3) | 26 (7.9) | |
| p-value | < 0.01 | ||
| Alcohol intake c | Never | 36 (38.3) | 135 (40.8) |
| Ever | 58 (61.7) | 196 (59.2) | |
| p-value | 0.66 | ||
No adenoma category include participants without polyps and with hyperplastic polyps;
χ2 test;
Data collected from 1992–1994;
Data collected in 2002–2004
Table 2.
Baseline levels of urinary F2-Isoprotsanes (ng/mg creatinine) in subgroups with different characteristics.
| Characteristics | iPF2α-III | 2,3-dinor-iPF2α-III | iPF2α-VI | 8,12-iso-iPF2α-VI | |
|---|---|---|---|---|---|
| A. Categorical demographic and baseline characteristics, Mean (SD) | |||||
| Case-control status | No-Adenoma, Controls (n=331) a | 0.25 (0.17) | 4.24 (2.68) | 6.41 (3.38) | 4.10 (2.60) |
| Adenoma, Cases (n=94) | 0.25 (0.19) | 3.91 (2.10) | 6.28 (3.95) | 4.19 (3.09) | |
| p-value b | 0.7 | 0.3 | 0.8 | 0.8 | |
| Gender | Females (n=245) | 0.29 (0.19) | 4.92 (2.72) | 7.44 (3.95) | 4.46 (2.90) |
| Males (n=180) | 0.18 (0.12) | 3.13 (1.90) | 4.94 (3.20) | 3.66 (2.36) | |
| p-value b | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| Race/Ethnicity | African American (n=111) | 0.19 (0.15) | 3.47 (2.01) | 5.30 (3.08) | 3.34 (1.91) |
| Non-Hispanic white (n=170) | 0.24 (0.14) | 3.97 (2.08) | 6.20 (3.68) | 4.09 (2.62) | |
| Hispanic (n=144) | 0.30 (0.21) | 4.93 (3.20) | 7.43 (4.33) | 4.77 (3.16) | |
| p-value c | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| Glucose Tolerance | NGT (n= 310) | 0.25 (0.18) | 4.04 (2.41) | 6.32 (3.75) | 4.15 (2.83) |
| IGT (n= 115) | 0.24 (0.15) | 4.49 (2.93) | 6.54 (4.07) | 4.05 (2.36) | |
| p-value b | 0.47 | 0.11 | 0.60 | 0.74 | |
| Smoking | Never (n= 198) | 0.23 (0.17) | 4.10 (2.60) | 6.49 (3.96) | 4.09 (2.78) |
| Former (n= 173) | 0.23 (0.15) | 3.96 (2.12) | 6.08 (3.85) | 4.12 (2.76) | |
| Current (n= 54) | 0.34 (0.21) | 5.05 (3.45) | 6.97 (3.46) | 4.23 (2.30) | |
| p-value c | < 0.01 | 0.02 | 0.30 | 0.95 | |
| Previous polyps | No (n=379) | 0.24 (0.17) | 4.11 (2.63) | 6.36 (3.90) | 4.08 (2.63) |
| Yes (n=46) | 0.30 (0.20) | 4.58 (1.94) | 6.59 (3.53) | 4.45 (3.29) | |
| p-value b | 0.04 | 0.25 | 0.70 | 0.39 | |
| B. Continuous demographic and baseline characteristics | |||||
| Spearman Correlation Coefficients (p-value) | |||||
| Age | −0.02 (0.66) | −0.03 (0.50) | 0.02 (0.64) | −0.19 (< 0.01) | |
| BMI | 0.02 (0.68) | 0.21 (< 0.01) | 0.04 (0.46) | 0.15 (0.002) | |
| Alcohol intake (g per day) | −0.14 (< 0.01) | −0.06 (0.19) | −0.13 (< 0.01) | −0.003 (0.95) | |
SD = Standard deviation
No adenoma category include participants without polyps and with hyperplastic polyps;
Student t-test;
ANOVA F-test with p-value
The minimally and fully adjusted models (Model 1 and Model 2) showed similar results (Figure 1). In both models, the estimates of the associations between F2-IsoPs and adenomatous polyps varied around the null (Figure 1). The fully adjusted model yielded the following ORs (95% CIs) for occurrence of colorectal adenomatous polyps scaled to 1 SD of F2-IsoP distribution: 1.16 (0.88–1.50), 0.88 (0.63–1.17), 1.04 (0.80–1.34), and 1.16 (0.90–1.48) for iPF2α-III, iPF2α-VI, 8,12-iso-iPF2α-VI, and 2,3-dinor-iPF2α-III, respectively. Similarly, we did not find an association between four F2-IsoPs and advanced adenomatous polyps (n = 24); the ORs for the fully adjusted model with 95% CIs were 1.08 (0.79–1.44), 0.81 (0.55–1.13), 0.96 (0.70–1.28), and 1.12 (0.84–1.47) for iPF2α-III, 2,3-dinor-iPF2α-III, iPF2α-IV and 8,12-iso-iPF2α-VI, respectively.
Figure 1.
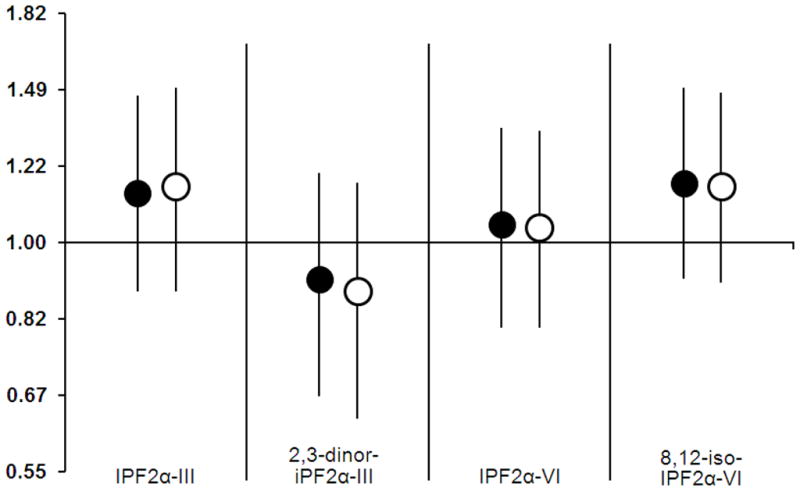
Prospective association between urinary F2-Isoprotsanes (ng/mg creatinine) and colorectal adenoma polyps. Model 1 (black circles) is a minimally adjusted model and includes age, gender, race/ethnicity and previous polyps as covariates. Model 2 (white circles) is a fully adjusted model with the additional covariates, IGT-status, BMI, alcohol use, and smoking. Both models showed similar results.
We could not exclude the possibility that adenomas could be present at the time of urine collection; this was likely to be the case among the participants reporting previous polyps. To address this point, a sensitivity analysis was conducted. Namely, the individuals that reported previous polyps before 2002–2004 were excluded from the analysis. This exclusion did not influence the results; the ORs for the fully adjusted model, with 95% CIs, were 1.20 (0.89–1.59), 0.89 (0.62–1.23), 1.01 (0.74–1.34), and 1.11 (0.84–1.47) for iPF2α-III, 2,3-dinor-iPF2α-III, iPF2α-IV and 8,12-iso-iPF2α-VI, respectively.
Using the minimally adjusted model, we also examined associations with other risk factors for colorectal adenoma. Similar to previously published findings (33–35), occurrence of adenoma was associated with male gender, age, BMI, and smoking status (past, but not current). The associations with gender and smoking status were weak and not statistically significant (data not shown), as previously reported in other cohort studies (33–35).
Discussion
In this prospective study, we examined the potential association between the F2-IsoPs and adenomatous polyps, a precursor to CRC. Our main finding is that urinary F2-IsoPs are not associated with occurrence of adenomatous polyps during a 10 year period of follow-up. This suggests that higher oxidative status, as measured by lipid peroxidation, does not promote the development of adenomatous polyps. Furthermore, these findings imply that systemic oxidative status, assessed as oxidation damage to lipids, may not be a risk factor for CRC, although external oxidative exposures are established as risk factors for colorectal cancer. This controversy may be reconciled by considering the differential effects of extraneous exposures on local versus systemic oxidative status. It is possible that the external oxidative exposures promote local oxidative stress within colorectal mucosa and that such local redox shift is not reflected at the systemic level, because systemic oxidative status presents an integrative index of the redox balance of all tissues. It is also possible that oxidative status is more tightly regulated at the systemic level and less balanced at the tissue level. Our data from a previously published clinical model of oxidative stress supports this concept by demonstrating that the systemic oxidative stress induced by chemotherapy is balanced within 24 hours (30). However, tissue-specific side effects of chemotherapy are observed in the most metabolically active tissues (e.g. neurotoxicity and cardiotoxicity) (24). These data suggest that specific tissue-related markers of oxidative status might be more informative, compared to the systemic oxidative status measures, in determining whether internal oxidative stress is a risk factor for a specific cancer.
Previous studies have investigated the association of F2-IsoPs with cancer, mainly focusing on iPF2α-III (also known as 15-F2t-isoprostane). In men, the risk of lung cancer was increased at higher levels of urinary iPF2α-III, whereas no association was found among women (8). In a case-control study, nested within a multiethnic cohort, no association was found between serum iPF2α-III and the risk of prostate cancer or risk of advanced prostate cancer (9). For breast cancer, the results were even more puzzling; urinary iPF2α-III and 2,3-dinor-iPF2α-III were measured and these markers were associated with breast cancer risk among women with BMI ≥29, whereas an inverse association was observed among women with low BMI (≤23) (10). Despite the differences in the results and the examined outcomes, the unifying theme in these findings is that none of the cancer types showed an overall association with different measures of systemic F2-IsoP levels. Even the associations found within sub-groups showed different directions. The convincingly null associations between four urinary F2-IsoPs (two of which were measured in previous studies) with colorectal adenomatous polyps add to the argument that there is no overall association between these oxidative status markers and certain cancer types. In addition, studies that showed positive associations (with lung and breast cancer) reported shorter follow-up periods (8, 10). For example, in the lung cancer study, the reported median time between specimen collection and diagnosis was one year (10). The studies with shorter follow-up periods could not rule out that clinically undetected malignancies may lead to an increase in urinary F2-isoP levels. Therefore, it is possible that these putative positive associations could actually represent a consequence and not a cause of cancer.
The strengths of our study lies in the measurement of multiple F2-IsoPs and in the fact that these F2-IsoPs have been previously validated in a clinical model of oxidative stress (26). The study also appears to have good external validity, due to the fact that the positive associations with age, smoking status, BMI, and gender were similar to the values previously reported in the literature (33–35). The weakness of our study is lack of colonoscopy at the baseline, suggesting that some of the participants may have had adenoma polyps at the time of urine collection. Because colorectal polyps are often asymptomatic, a portion of the polyps discovered in 2002–2004 could have been present at baseline. The concern is that adenomatous polyps present at baseline could influence the baseline levels of F2-IsoPs and therefore the results. However, this concern is applicable only to the findings of positive associations. Our null findings imply two possible scenarios if adenomatous polyps were present at the baseline: (1) F2-IsoP levels remained unchanged during the development of adenomatous polyps or (2) developing these polyps decreased F2-IsoP levels, which seems to be unlikely as there is no plausible biological hypothesis suggesting such change. To further address this issue, a sensitivity analysis was performed, where those who reported previous polyps were excluded from the analysis, a sub-group with the highest possibility of polyps present at baseline (n=46). This manipulation did not change the final results, suggesting that a potential source of weakness, applicable to this study, is unlikely to be a significant source of distortion in the final results.
In summary, our results do not support the hypothesis that internal systemic oxidative status increases the risk of precursors to CRC. We suggest that the next steps in this research should include specific tissue-related markers of oxidative status.
Acknowledgments
This study was supported by National Institutes of Health grants R01DK081028, R25CA126938, and R01CA88007.
Selected Abbreviations and Acronyms
- BMI
Body mass index
- CI
Confidence Interval
- CRC
Colorectal cancer
- F2-IsoP
F2-isoprostanes
- IGT
Impaired glucose tolerance
- IRAS
Insulin Resistance Atherosclerosis Study
- LC
Liquid chromatography
- MS
Mass spectrometry
- NGT
Normal glucose tolerance
- OR
Odds Ratio
Footnotes
Author Contributions
Sharareh Siamakpour-Reihani, Peter Scarbrough, and Dora Il’yasova wrote the manuscript, Frances Wang and Ralph D’Agostino conducted the statistical analysis and participated in the discussions of the results, Ivan Spasojevic and Karel Base measured F2-Isoprostanes, Rebecca Sedjo participated in the discussion of the results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674:36–4. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Thaiparambil JT, Vadhanam MV, Srinivasan C, Gairola CG, Gupta RC. Time-dependent formation of 8-oxo-deoxyguanosine in the lungs of mice exposed to cigarette smoke. Chem Res Toxicol. 2007;20:1737–1740. doi: 10.1021/tx700289g. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Equchi H, Arihiro K, Ito R, Koyama K, Soda M, et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007;46:242–248. doi: 10.1002/mc.20277. [DOI] [PubMed] [Google Scholar]
- 5.Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruitvegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Cai H, Xiang YB, Cai Q, Yang G, Liu D, et al. Intra-person variation of urinary biomarkers of oxidative stress and inflammation. Cancer epidemiol Biomarkers Prev. 2010;19:947–952. doi: 10.1158/1055-9965.EPI-10-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, et al. Oxidants in mitochondria: from physiology to diseases. Biochem Biophys Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- 8.Epplein M, Franke AA, Cooney RV, Morris JS, Wilkens LR, Goodman MT, et al. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:1962–1970. doi: 10.1158/1055-9965.EPI-09-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill JK, Franke AA, Steven Morris J, Cooney RV, Wilkens LR, Le Marchand L, et al. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control. 2009;20:1161–1171. doi: 10.1007/s10552-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s Health Study. J Clin Oncol. 2009;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fam SS, Morrow JD. The isorpostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem. 2003;10:1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- 12.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 13.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1952–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 15.Kolligs FT, Crispin A, Munte A, Wagner A, Mansmann U, Goke B. Risk of advanced colorectal neoplasia according to age and gender. PLoS One. 2011;6:e20076. doi: 10.1371/journal.pone.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellad ZF, Weiss DG, Ahnen DJ, Lieberman DA, Jackson GL, Provensale D. Colonoscopy withdrawl time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105:1746–1752. doi: 10.1038/ajg.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 18.Tiemersma EW, Wark PA, Ocké MC, Bunschoten A, Otten MH, Kok FJ, et al. Alcohol consumption, alcohol dehydrogenase 3 polymorphism, and colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2003;12:419–425. [PubMed] [Google Scholar]
- 19.Martinez ME, McPherson RS, Annegers JF, Levin B. Cigarette smoking and alcohol consumption as risk factors for colorectral adenomatous polyps. J Natl Cancer Inst. 1995;87:274–279. doi: 10.1093/jnci/87.4.274. [DOI] [PubMed] [Google Scholar]
- 20.Shin A, Hong CW, Sohn DK, Chang Kim B, Han KS, Chang HJ, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am J Epidemiol. 2011;174:552–562. doi: 10.1093/aje/kwr098. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Il’yasova D, Sztaray J, Young SP, Wang F, Millington DS. Quantification of the oxidative damage biomarker 2,3-dinor-8-isoprostaglandin-F(2alphpa) in human urine using liquid chromatography-tandem mass spectrometry. 2010;399:302–304. doi: 10.1016/j.ab.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 25.Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J Lipid Res. 2007;48:1607–1617. doi: 10.1194/jlr.M700097-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, et al. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid Redox Signal. 2011;14:2453–2460. doi: 10.1089/ars.2010.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB, Jr, Wagenknecht LE. Urinary f(2)-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.292. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 29.Sedjo RL, Byers T, Levin TR, Haffner SM, Saad MF, Tooze JA, et al. Change in body size and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2007;16:526–531. doi: 10.1158/1055-9965.EPI-06-0229. [DOI] [PubMed] [Google Scholar]
- 30.Il’yasova D, Spasojevic I, Wang F, Tolun AA, Base K, Young SP, et al. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer Epidemiol Biomarkers Prev. 2010;19:1506–1510. doi: 10.1158/1055-9965.EPI-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 32.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 34.Reid ME, Marshall JR, Roe D, Lebowitz M, Alberts D, Mattacharyya AK, et al. Smoking exposure as a risk factor for prevalent and recurrent colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2003;12:1006–1011. [PubMed] [Google Scholar]
- 35.Lee GE, Park HS, Yun KE, Jun SH, Kim HK, Cho SIe. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring) 2008;16:1343–1439. doi: 10.1038/oby.2008.216. [DOI] [PubMed] [Google Scholar]


