Abstract
Purpose
In a prospective prenatal cohort study, we examined associations of second trimester and cord plasma 25-hydroxyvitamin D (25[OH]D) with small-for-gestational age (SGA), and the extent to which vitamin D might explain black/white differences in SGA.
Methods
We studied 1067 white and 236 black mother-infant pairs recruited from 8 obstetrical offices early in pregnancy in Massachusetts. We analyzed 25(OH)D levels using an immunoassay and performed multivariable logistic models to estimate the odds of SGA by category of 25(OH)D level.
Results
Mean (standard deviation [SD]) second trimester 25(OH)D level was 60 nmol/L (21) and was lower for black (46 nmol/L [22]) than white (62 nmol/L [20]) women. 59 infants were SGA (4.5%) and more black than white infants were SGA (8.5% vs. 3.7%). The odds of SGA were higher with maternal 25(OH)D levels <25 vs. ≥25 nmol/L (adjusted odds ratio [OR] 3.17; 95% confidence interval [CI]:1.16, 8.63). The increased odds of SGA among black vs. white participants decreased from an OR of 2.04(1.04, 4.04) to 1.68(0.82, 3.46) after adjusting for 25(OH)D.
Conclusions
Second trimester 25(OH)D levels <25 nmol/L were associated with higher odds of SGA. Our data raise the possibility that Vitamin D status may contribute to racial disparities in SGA.
MeSH: Vitamin D, Infant, Small for Gestational Age, African Continental Ancestry Group, Health Status Disparities, Pregnancy
INTRODUCTION
Poor fetal growth precedes many perinatal(1) and childhood morbidities(2), as well as chronic adult conditions including insulin resistance and hypertension(3–6). Maternal vitamin D status may affect fetal growth due to its role in adequate placental development(7), but existing studies are conflicting or suffer from key limitations. Higher maternal vitamin D dietary intake has been associated with higher birthweight (8–11), but these studies are limited by the lack of sunlight exposure data or blood 25(OH)D level, which provides a measure of vitamin D status that integrates both dietary and endogenous sources of vitamin D. Interventional trials of vitamin D supplementation in pregnancy have had mixed results with respect to birthweight(12–16). The few observational studies of 25(OH)D levels and birthweight have similarly conflicting results and have been limited by the inability to consider dietary confounders (17), and by exclusive enrollment of white mothers(18, 19).
African American women are 2–3 times more likely than white women to deliver small-for-gestational age (SGA) infants(20). It is unknown whether maternal vitamin D status contributes to the racial disparity in risk of SGA(21). In the US, pregnant black women have lower plasma levels of the optimal marker of vitamin D status(22), 25-hydroxyvitamin D (25[OH]D), than pregnant white women(23, 24). Among 928 pregnant participants in the National Health and Nutrition Examination Survey in 2001–2006, mean 25(OH)D levels were 77 nmol/L and 39 nmol/L in white and black women, respectively(25). While dietary intake affects vitamin D status(22), the contribution of cutaneous synthesis from sunlight exceeds dietary sources(26). Darker pigmented skin synthesizes vitamin D less efficiently than lighter pigmented skin(27), leading to lower vitamin D levels among black compared to white subjects.
To clarify the relationship between vitamin D status during pregnancy and fetal growth, we analyzed data from a prospective prenatal cohort study of pregnant black and white women and their offspring. We evaluated associations of second trimester maternal and umbilical cord plasma 25(OH)D levels at delivery with birthweight-for-gestational age. We then sought to understand whether vitamin D status contributes to black/white differences in this measure of fetal growth. We hypothesized that lower maternal second trimester 25(OH)D levels and lower infant cord blood 25(OH)D levels would be associated with lower birthweight-for-gestational age (a measure of fetal growth). We hypothesized that this association would be present for both black and white participants and that the lower 25(OH)D levels among black women and infants would account, at least in part, for their increased odds of SGA.
METHODS
We studied participants from Project Viva, a prospective cohort study of gestational factors and offspring health(28). We recruited women attending their initial prenatal visit at 8 obstetrical offices of a multi-specialty group practice in Massachusetts. Eligibility criteria included fluency in English, gestational age <22 weeks, and singleton pregnancy. Details of recruitment and retention procedures are available elsewhere(28, 29). All participants provided written informed consent. Institutional review boards of participating institutions approved the study.
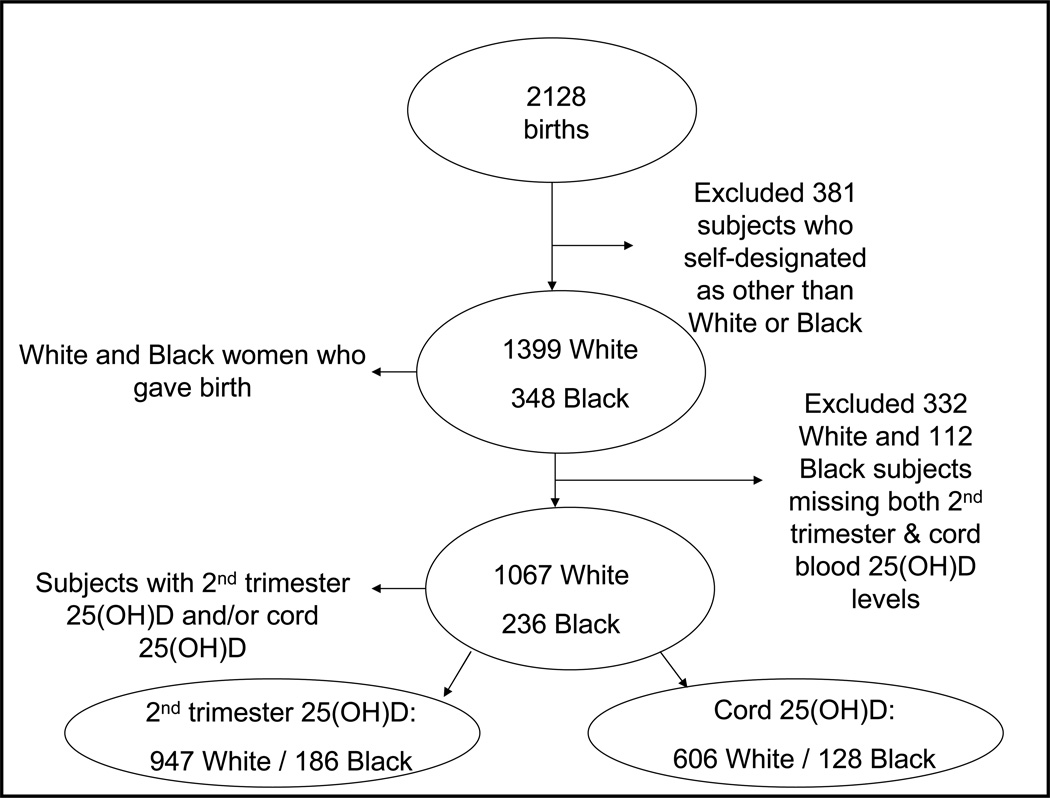
Of the 2128 participants who gave birth, 1399 were white and 348 were black. We chose to analyze data from only black and white mother-infant pairs because of our interest in the potential role vitamin D status might play in black-white fetal growth disparities. We excluded 332 white and 112 black women who were missing both second trimester maternal plasma and cord plasma, leaving 1067 white and 236 black women in the analysis cohort with either second trimester maternal plasma (947 white and 186 black) and/or cord plasma (606 white and 128 black) (Figure 1). All participants had complete outcome data. Compared with the 444 white and black participants who were not included in this analysis, mothers in the present study were slightly more likely to be married or cohabitating (93% vs. 90%), but did not differ by age, annual household income, maternal education status, pre-pregnancy BMI, or gestational weight gain (data not shown). To address missing covariate data, for unadjusted analyses we performed the analyses two ways, including participants with missing covariate data and then only complete cases. We found no material differences. Thus, in multivariable models, we included participants only with complete covariate data.
Figure 1.
Flow of Participation, Project Viva
Measurement of vitamin D status
We measured 25[OH]D levels in second trimester maternal blood and in umbilical cord blood at delivery. We collected maternal blood during the routine non-fasting mid-pregnancy clinical blood draw between 26–28 weeks’ gestation. We collected umbilical venous blood following delivery from infants who were delivered at one of the two study hospital sites and recorded the season of blood draw for each 25(OH)D sample collected. We refrigerated blood samples, separated the plasma and stored aliquots in liquid nitrogen at −80°C. We measured 25(OH)D level for each specimen twice, first with an automated chemiluminescence immunoassay (CLIA)(30) and then with a manual radioimmunoassay (RIA)(31). For quality control, the laboratory used US National Institute of Standards and Technology (NIST) level 1. Because the singlicate 25(OH)D result from each assay slightly differed (maternal r=0.81, cord plasma r=0.88), we averaged the two available values for each specimen to obtain a more stable estimate of 25(OH)D level. In general, analyses using either the CLIA data only or RIA data only yielded similar results but with slightly wider confidence intervals (data not shown).
Ascertainment of birth data
We obtained infant birthweight from the hospital medical record. We calculated gestational age in weeks by subtracting the date of the last menstrual period from the date of delivery. Eighty-six percent of the participants had ultrasound data available at 16–20 weeks. For the approximately 12% of ultrasound gestational age estimates that differed by >10 days from the LMP pregnancy dating, we used the ultrasound dating. We determined birthweight-for-gestational age by using as a reference a combined 1999–2000 US natality set(32). We categorized infants as small-for-gestational age (SGA) if their birthweights were <10th percentile for their gestational ages(33).
Assessment of covariates
Using a combination of interviews, study questionnaires and medical record reviews, we collected information on maternal age, race/ethnicity, parity, smoking habits, education, marital status, household income, infant gender, gestational diabetes and preeclampsia. Mothers self-designated race/ethnicity. For this analysis we included mother-infant pairs in whom mothers self-designated as Non-Hispanic black or Non-Hispanic white. We calculated pre-pregnancy body mass index (BMI) based on self-reported pre-pregnancy height and weight. We calculated gestational weight gain by subtracting the pre-pregnancy weight from the last clinically reported weight prior to delivery. We obtained dietary intake data (fish and micronutrient intake including calcium, folate and iron) with a previously validated, semi-quantitative food frequency questionnaire administered in the first and second trimesters of pregnancy (34, 35). We adjusted micronutrient intake for energy intake using a residuals model(36).
Statistical analysis
We first performed bivariate analyses to determine maternal and infant characteristics associated with previously described clinical categories of vitamin D status(25, 37–39): severe deficiency (<25 nmol/L), deficiency (25 to <50 nmol/L), insufficiency (50 to <75 nmol/L), and sufficiency (≥75 nmol/L). In preliminary analyses examining 25(OH)D levels and birthweight-for-gestational age z scores, we observed an apparent threshold at 25 nmol/L and subsequently dichotomized 25(OH)D at this point (<25 nmol/L vs. ≥25 nmol/L). We then ran unadjusted and multivariable-adjusted linear regression models of 25(OH)D and birthweight-for-gestational age including covariates associated with fetal and vitamin D levels in the literature and in our data. We similarly ran multivariable-adjusted logistic regression models of 25(OH)D and SGA. We excluded candidate variables that did not alter the relationship between vitamin D and fetal growth or SGA from final models, including fish, calcium, folate and iron intakes, and maternal parity, education, income, and gestational weight gain. We also examined the association between race (black vs. white) and SGA, and the extent to which the association might be explained by vitamin D status by running multivariable logistic regression models including both black and white participants before and after adjusting for 25(OH)D level. All analyses were performed using SAS version 9.2.
RESULTS
Mean second trimester 25(OH)D level was 60 nmol/L (SD 21), and levels were lower among black vs. white mothers: 46 nmol/L (SD 22) vs. 62 nmol/L (SD 20), respectively. Mean cord 25(OH)D level was 47 nmol/L (SD 19), and was lower among black vs. white infants; 31 nmol/L (16) vs. 51 nmol/L (SD 18), respectively. Among women with available second trimester 25(OH)D levels, there were 53 SGA infants (4.7%); 17 were black (9.1%) and 36 white (3.8%). Among infants with cord plasma available, there were 25 SGA infants (3.4%); 10 were black (7.8%) and 15 white (2.5%).
Bivariate, descriptive analysis (Table 1) revealed higher 25(OH)D levels in women who were white, older, married or co-habitating, more highly educated, with higher household incomes, non-smokers, and with lower pre-pregnancy body mass indices. Preterm birth was not the focus of this study, but we note that gestational age did not substantially differ across categories of second trimester or cord plasma 25(OH)D levels.
Table 1.
Characteristics of 236 Black and 1067 White Mother/Infant Pairs Enrolled in Project Viva According to Category of Second Trimester and Cord plasma 25-hydroxyvitamin D Concentration
| Second trimester plasma 25(OH)D (n=1113) | ||||
|---|---|---|---|---|
| Severe Deficiency <25 nmol/L (n=47) |
Deficiency 25-<50 nmol/L (n=314) |
Insufficiency 50-<75 nmol/L (n=543) |
Sufficiency ≥75 nmol/L (n=229) |
|
| Mean (SD) | ||||
| Maternal age at enrollment (years) | 30.1 (6.7) | 31.9 (5.3) | 32.7 (4.5) | 33.1 (4.5) |
| Pre-pregnancy BMI (kg/m2) | 29.4 (7.4) | 26.2 (6.1) | 24.1 (4.5) | 23.7 (4.6) |
| Gestational weight gain (kg) | 13.1 (6.9) | 15.5 (6.0) | 16.1 (5.0) | 15.1 (5.5) |
| Second trimester 25(OH)D (nmol/L) | 20 (4) | 39 (7) | 62 (7) | 90 (15) |
| Cord plasma 25(OH)D (nmol/L) | 22 (11) | 38 (14) | 51 (16) | 64 (17) |
| Gestational age at delivery (weeks) | 39.4 (1.6) | 39.7 (1.5) | 39.6 (1.6) | 39.6 (1.6) |
| Birthweight (kg) | 3.46 (0.68) | 3.55 (0.52) | 3.53 (0.51) | 3.51 (0.52) |
| Birthweight-for-gestational age (z-score) | 0.07 (1.17) | 0.28 (0.98) | 0.26 (0.93) | 0.24 (0.89) |
| n (column %) | ||||
| Race | ||||
| Black | 34 (72.3) | 78 (24.8) | 55 (10.1) | 19 (8.3) |
| White | 13 (27.6) | 236 (75.2) | 488 (89.9) | 210 (91.7) |
| College graduate | 19 (40.4) | 197 (62.7) | 395 (72.7) | 182 (79.5) |
| Married or cohabitating | 40 (85.1) | 284 (90.4) | 516 (95.2) | 222 (96.9) |
| Smoking status | ||||
| Never | 33 (78.6) | 196 (64.1) | 353 (66.7) | 156 (69.3) |
| Former | 4 (9.5) | 64 (20.9) | 115 (21.7) | 51 (22.7) |
| During pregnancy | 5 (11.9) | 46 (15.0) | 61 (11.5) | 18 (8.0) |
| Household income >$70,000/year | 3 (27.3) | 153 (66.8) | 340 (72.8) | 148 (74.0) |
| Primiparous | 15 (31.9) | 149 (47.5) | 267 (49.2) | 109 (47.6) |
| Female infant | 19 (40.4) | 149 (47.5) | 261 (48.1) | 118 (51.5) |
| Category of birthweight-for-gestational age | ||||
| Small-for-gestational age (<10th %ile) | 7 (14.9) | 18 (5.7) | 22 (4.1) | 6 (2.6) |
| Appropriate-for-gestational age | 34 (72.3) | 245 (78.0) | 438 (80.7) | 195 (85.2) |
| Large-for-gestational age (>90th %ile) | 6 (12.8) | 51 (16.2) | 83 (15.3) | 28 (12.2) |
| Category of cord plasma 25(OH)D (n=734) | ||||
|
<25 nmol/L (n=84) |
25-<50 nmol/L (n=337) |
50-<75nmol/L (n=261) |
≥75 nmol/L (n=52) |
|
| Mean (SD) | ||||
| Cord plasma 25(OH)D (nmol/L) | 17 (4) | 39 (7) | 60 (7) | 87 (12) |
| Gestational age at delivery (weeks) | 39.5 (1.6) | 39.8 (1.6) | 39.6 (1.7) | 39.5 (1.3) |
| Birthweight-for-gestational age (z score) | 0.00 (1.05) | 0.31 (0.93) | 0.41 (0.84) | 0.23 (0.92) |
| n (Column %) | ||||
| Category of birthweight-for-gestational age | ||||
| Small-for-gestational age (<10th %ile) | 9 (10.7) | 9 (2.7) | 6 (2.3) | 1 (1.9) |
| Appropriate-for-gestational age | 64 (76.2) | 276 (81.9) | 218 (83.5) | 43 (82.7) |
| Large-for-gestational age (>90th %ile ) | 11 (13.1) | 52 (15.4) | 37 (14.2) | 8 (15.4) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index
Participants with second trimester 25(OH)D data had missing data for the following covariates: pre-pregnancy BMI n=3, pregnancy weight gain n= 11, marital status n=1, smoking n=31 and income n= 76.
Fetal growth as a continuous variable
We did not detect a statistically significant association between second trimester 25(OH)D levels <25 vs. ≥25 nmol/L and a continuous measure of birthweight-for-gestational age (z score) in unadjusted analyses (β −0.19, 95% confidence interval [CI] −0.46, 0.09). Multivariable linear regression models adjusting for season of blood draw, maternal age, pre-pregnancy body mass index and race attenuated this estimate (adjusted β −0.13 [95%CI: −0.42, 0.17]). Cord plasma 25(OH)D levels <25 vs. ≥25 nmol/L were associated with lower birthweight-for-gestational age in both unadjusted (β −0.34 [95%CI: −0.54, −0.13]) and adjusted (β −0.25 [95%CI: −0.49, −0.01]) models.
Small-for-gestational age
Mothers with second trimester 25(OH)D levels <25 vs. ≥25 nmol/L had increased odds of delivering an SGA infant in unadjusted analyses (OR 3.93 [95%CI: 1.65, 9.34]) (Table 2). Multivariable logistic regression models adjusting for season of blood draw, maternal age, pre-pregnancy BMI and race slightly attenuated this association (OR 3.17 [95%CI: 1.16, 8.63]). Infants with cord plasma 25(OH)D levels <25 vs. ≥25 nmol/L had higher odds of SGA in unadjusted (OR 4.72 [95%CI: 2.00, 11.12]) and adjusted analyses (OR 4.64 [95%CI: 1.61, 13.36]). This association did not differ by race; P values for interaction were 0.75 and 0.47 for second trimester and cord plasma respectively.
Table 2.
Associations of Categories of 25-hydroxyvitamin D Concentration With Small-for-Gestational Age (vs. Appropriate-for-Gestational Age), Data From 1067 White and 236 Black Mother/Infant Pairs Enrolled in Project Viva.
| Category of Second Trimester Plasma 25(OH)D (nmol/L) n=1113 |
n(SGA)/n | Model 1 (unadjusted) OR [95% CI] |
Model 2a OR [95% CI] |
Model 3b OR [95% CI] |
|---|---|---|---|---|
| <25 | 7/47 | 4.10 [1.63, 10.28] | 4.42 [1.60, 12.23] | 3.63 [1.24, 10.60] |
| 25-<50 | 18/314 | 1.46 [0.77, 2.78] | 1.51 [0.78, 2.92] | 1.43 [0.73, 2.79] |
| 50-<75 | 22/543 | 1.0 [reference] | 1.0 [reference] | 1.0 [reference] |
| ≥75 | 6/229 | 0.61 [0.24, 1.53] | 0.61 [0.24, 1.55] | 0.62 [0.24, 1.56] |
| <25 vs. ≥25 | 3.93 [1.65, 9.34] | 3.94 [1.51, 10.29] | 3.17 [1.16, 8.63] | |
|
Category of Cord Plasma 25(OH)D (nmol/L) n=734 |
n(SGA)/n |
Model 1 [unadjusted] OR [95% CI] |
Model 2a OR [95% CI] |
Model 3b OR [95% CI] |
| <25 | 9/84 | 5.11 [1.75, 14.90] | 6.19 [1.82, 21.07] | 4.84 [1.33, 17.66] |
| 25-<50 | 9/337 | 1.18 [0.42, 3.38] | 1.16 [0.40, 3.38] | 1.07 [0.36, 3.16] |
| 50-<75 | 6/261 | 1.0 [reference] | 1.0 [reference] | 1.0 [reference] |
| ≥75 | 1/52 | 0.84 [0.10, 7.20] | 0.85 [0.10, 7.38] | 0.87 [0.10, 7.61] |
| <25 vs. ≥25 | 4.72 [2.00, 11.12] | 5.68 [2.07, 15.56] | 4.64 [1.61, 13.36] | |
Abbreviations: 25(OH)D, 25-nydroxyvitamin D; OR, odds ratio; CI, confidence interval; SGA, small-tor-gestational age
Adjusted for season of blood draw, maternal age and pre-pregnancy BMI;
Model 2 + adjustment for race
In multivariable logistic regression models examining the associations of race with SGA, black infants had higher odds of SGA than white infants (OR 2.04 [95%CI: 1.04, 4.04]) (Table 3). When we incorporated second trimester 25(OH)D status into the model, the black/white odds ratio for SGA was attenuated by 18% (OR 1.68 [95%CI: 0.82, 3.46]). In the subset of infants with available cord plasma 25(OH)D, the odds ratio for SGA among black vs. white infants was 2.72 [95%CI: 1.09, 6.77]) before adding 25(OH)D to the model and 1.85 [95%CI: 0.70, 4.91] afterwards, suggesting possible mediation effect.
Table 3.
Associations of Small-for-Gestational Age (vs. Appropriate-for-Gestational Age) in Black vs. White Subjects Before and After Adjusting for 25-hydroxyvitamin D Levels (<25 vs. ≥25 nmol/L). Data From 1067 White and 236 Black Mother/Infant Pairs Enrolled in Project Viva.
| Model 1a | Model 2b | |
|---|---|---|
| OR [95% CI] |
||
|
Second trimester 25(OH)D (n=1091) Black vs. white |
2.04 [1.04, 4.04] | 1.68 [0.82, 3.46] |
|
Cord plasma 25(OH)D (n=691) Black vs. white |
2.72 [1.09, 6.77] | 1.85 [0.70, 4.91] |
Abbreviations: SGA, Small-for-gestational age; AGA, appropriate-for-gestational age; 25(OH)D, 25 hydroxyvitamin D
Model 1. Adjusted for season of blood draw, maternal age, pre-pregnancy body mass index
Model 2. Model 1 + 25(OH)D level
We found that adding pre-pregnancy body mass index altered the effect estimates of the association between 25(OH)D levels and SGA. In analyses of second trimester plasma 25(OH)D levels, incorporating pre-pregnancy BMI changed partially adjusted odds ratios for SGA from 3.06 (95%CI: 1.24, 7.55) to 3.94 (95%CI 1.51, 10.29) (Table 2, Model 2).
DISCUSSION
We found that second trimester 25(OH)D levels <25 vs. 25 ≥nmol/L were associated with higher odds of SGA in black and white infants. We had hypothesized that lower levels of 25(OH)D in black vs. white women and their infants might account for the difference in fetal growth between the two groups. When we incorporated 25(OH)D status into combined logistic regression models, the increased odds of SGA among black vs. white infants was attenuated. However, because of small numbers the confidence intervals were wide. Additionally, using such a method to examine potential mediation assumes no unmeasured confounding and no effect modification by race. We did not find evidence of effect modification by race (second trimester interaction P= 0.75, cord P=0.47). While 25(OH)D status may account for part of difference in SGA between black and white infants, because of the small number of cases and thus wide confidence intervals, our findings should be considered hypothesis generating, and confirmation with future studies is needed. If confirmed, our data suggest the need for a randomized controlled trial to determine if vitamin D supplementation early in pregnancy reduces disparities in fetal growth.
A review by Specker of the effect of vitamin D in pregnancy conducted suggests that the role of vitamin D status and fetal growth remains inconclusive(12). However, three out of four trials are consistent with our results, showing increased birthweight among infants born to mothers supplemented with vitamin D(13, 14, 16). The trial that did not reveal a difference in birthweights did not achieve 25(OH)D levels greater than 25 nmol/L, the threshold noted in our study, nor was any adjustment made for gestational age(15).
In three observational studies in which 25(OH)D levels during pregnancy were measured, the associations between vitamin D status and fetal growth/gestational age have also been inconsistent(17, 18, 40). In an analysis of 559 mother-infant pairs in South India, Farrant and colleagues found no association between vitamin D insufficiency at 30 weeks’ gestation and newborn size; mean birthweights (adjusted to 40 weeks’ gestation) were 2.9 kg for infants born to mothers with 25(OH)D levels both above (34%) and below 50nmol/L (66%)(40). The authors reported birthweight in kilograms using only one decimal place, possibly hiding smaller differences. They did not adjust for covariates other than gestational age, nor did they report birth outcome results for the subset of women (31%) who had 25(OH)D levels <28 nmol/L or mention the proportion of infants born SGA.
In a study of 374 predominantly white (>99%) Australian women and infants, Morley et al(18) found that birthweights were 157 grams(95%CI −47, 361) lower among infants born to mothers with 25OHD <28 nmol/L vs. ≥28 nmol/L measured at 28–32 weeks’ gestation, but this finding may have been explained by gestations that were 0.7 weeks shorter rather than reduced fetal growth. Subsequent analyses of these same data suggested that vitamin D receptor genotype might modify the relationship between 25(OH)D and birthweight; among infants with the more functional VDR FokI genotype, maternal vitamin D deficiency was associated with a 288 gram (95%CI 65, 512) lower birthweight(19), but again the investigators did not adjust for gestational age.
In a nested case-control study, Bodnar et al reported that white women with low and high second trimester 25(OH)D levels (vs. the reference group with levels of 37.5 to 75 nmol/L) had increased odds of delivering an SGA infant; adjusted odds ratios were 7.5 (95%CI 1.8–31.9) and 2.1 (95%CI 1.2–3.8) for low and high 25(OH)D levels, respectively (17). The investigators defined SGA as birthweight-for-gestational age <10th percentile based on a local reference of 50,000 births at one hospital. They did not find clear associations among black infants, with adjusted odds ratios of 1.5 (95%CI 0.6–3.5) and 2.2 (95%CI 0.5–9.0) for low and high levels, respectively. We did not observe the same U-shaped distribution that they found among their white subjects. This difference in results may have been a function of a different vitamin D distribution; 47% of white women in their study had 25(OH)D levels >75 nmol/L compared to just 22% of white women in our study.
It is unclear why we observed an association between second trimester severe 25(OH)D deficiency and the dichotomous SGA but not with the continuous measure of fetal growth. It is possible that above a minimal level of 25(OH)D the risk of SGA disappears, i.e., a threshold effect. We speculate that by encouraging adequate implantation and placentation through its anti-inflammatory effects(41), vitamin D may promote fetal growth through normal placental vascular development. However, it is possible that when placentation occurs normally, vitamin D may not play a role in fetal growth in the normal range of fetal size, explaining the lack of an association between second trimester 25(OH)D and birthweight-for-gestational age on a continuum.
We found that adding pre-pregnancy body mass index strengthened the association between 25(OH)D levels and SGA. This finding may be because it is widely known that pregnant women with higher BMI’s have lower 25(OH)D levels than thinner subjects(42, 43). In addition, larger women tend to have larger infants. Thus, controlling for maternal BMI strengthens the association between maternal vitamin D status and the risk of SGA.
Our study had several strengths, including prospective data collection, 25(OH)D measurement in mothers and infants, use of a national population reference for SGA, and detailed data on possible dietary confounding variables including fish, calcium, folate and iron, which did not affect our findings. Nevertheless, the study has potential limitations. Residual confounding by unmeasured factors such as serum parathyroid hormone, calcium and phosphate levels could also partially explain our findings. The relatively high socioeconomic status of our cohort may limit the generalizability of our findings, although the prevalence of vitamin D deficiency among our subjects is consistent with US national data(44). We used maternal self-designated race not ancestry markers in our assignment of race. However, we propose that this represents a strength of our study as self-designated race, a social designation, can have a powerful impact on birth outcomes(45). Selection bias could have affected our findings, given that we were able to analyze only 1303 of the potential 1747 white or black mother-infant pairs in our cohort. However, we speculate that missing these data has probably biased our results toward the null hypothesis since sicker women or earlier deliveries would be more likely not to have blood available for analysis, to have lower 25(OH)D levels and to have higher rates of SGA. Low 25(OH)D levels can be associated with disease states that affect fetal growth such as gestational diabetes(46) and preeclampsia(47). However, possibly because of low incidences in our cohort, controlling for each in our analyses did not alter our findings (data not shown); 7.8 % of black mothers and 4.2% of white mothers developed gestational diabetes and 2.6% of black mothers and 1.1 % of white mothers developed preeclampsia. Lastly, we acknowledge continued uncertainty about the optimal method of measuring 25(OH)D levels (48, 49). To address this issue we measured 25(OH)D using two established assays (CLIA(30)and RIA(31)) and used the average of the two values to assign the 25(OH)D levels of each participant for analysis.
In conclusion, we found that second trimester 25(OH)D levels <25 vs. ≥25nmol/L were associated with higher odds of SGA in black and white infants. Our findings also raise the possibility that maternal 25(OH)D status might partially explain racial disparities in SGA. Providing vitamin D supplements to pregnant women may improve birth outcomes and mitigate disparities in fetal growth. Randomized, controlled trials are needed to prove this assertion.
Acknowledgements
This work was supported by the US National Institutes of Health (HD 034568, HL 068041, HL 064925), Harvard Medical School, the Harvard Pilgrim Health Care Foundation, and the Klarman Scholars Program at Beth Israel Deaconess Medical Center.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- AGA
appropriate-for-gestational age
- BMI
body mass index
- CI
confidence interval
- LGA
large-for-gestational age
- NIST
National Institute of Standards and Technology
- OR
odds ratio
- RIA
radioimmunoassay
- SGA
small-for-gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thureen P, Anderson M, Hay W. The Small -for-Gestational Age Infant. NeoReviews. 2001;2:e139–e149. [Google Scholar]
- 2.Hack M. Effects of intrauterine growth retardation on mental performance and behavior, outcomes during adolescence and adulthood. Eur J Clin Nutr. 1998;52(Suppl 1):S65–S70. discussion S70-61. [PubMed] [Google Scholar]
- 3.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2009 doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 5.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 6.Yajnik CS. Nutrient-mediated teratogenesis and fuel-mediated teratogenesis: two pathways of intrauterine programming of diabetes. Int J Gynaecol Obstet. 2009;104(Suppl 1):S27–S31. doi: 10.1016/j.ijgo.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–1034. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85:231–234. doi: 10.1016/j.earlhumdev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Sabour H, Hossein-Nezhad A, Maghbooli Z, Madani F, Mir E, Larijani B. Relationship between pregnancy outcomes and maternal vitamin D and calcium intake: A cross-sectional study. Gynecol Endocrinol. 2006;22:585–589. doi: 10.1080/09513590601005409. [DOI] [PubMed] [Google Scholar]
- 10.McGrath JJ, Keeping D, Saha S, Chant DC, Lieberman DE, O'Callaghan MJ. Seasonal fluctuations in birth weight and neonatal limb length; does prenatal vitamin D influence neonatal size and shape? Early Hum Dev. 2005;81:609–618. doi: 10.1016/j.earlhumdev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174:1273–1277. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Specker B. Vitamin D requirements during pregnancy. Am J Clin Nutr. 2004;80:1740S–1747S. doi: 10.1093/ajcn/80.6.1740S. [DOI] [PubMed] [Google Scholar]
- 13.Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 14.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488–492. [PubMed] [Google Scholar]
- 15.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal Serum 25-Hydroxyvitamin D Concentrations Are Associated with Small-for-Gestational Age Births in White Women. J Nutr. 2010 doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 19.Morley R, Carlin JB, Pasco JA, Wark JD, Ponsonby AL. Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. Eur J Clin Nutr. 2009;63:802–804. doi: 10.1038/ejcn.2008.55. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 21.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv. 2010;65:273–284. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 25.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202:436, e431–e438. doi: 10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed Proc. 1987;46:1876–1882. [PubMed] [Google Scholar]
- 28.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 29.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58, e51–e58. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 32.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004;19:307–319. doi: 10.1177/0885066604269663. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 35.Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20:35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willett W. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 37.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 38.Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr. 2009;102:876–881. doi: 10.1017/S0007114509297236. [DOI] [PubMed] [Google Scholar]
- 39.van den Ouweland JM, Beijers AM, Demacker PN, van Daal H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1163–1168. doi: 10.1016/j.jchromb.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 42.Bodnar LM, Catov JM, Roberts JM, Simhan HN. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J Nutr. 2007;137:2437–2442. doi: 10.1093/jn/137.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perampalam S, Ganda K, Chow KA, Opie N, Hickman PE, Shadbolt B, et al. Vitamin D status and its predictive factors in pregnancy in 2 Australian populations. Aust N Z J Obstet Gynaecol. 2011;51:353–359. doi: 10.1111/j.1479-828X.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- 44.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David R, Collins J., Jr Disparities in infant mortality: what's genetics got to do with it? Am J Public Health. 2007;97:1191–1197. doi: 10.2105/AJPH.2005.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross AC Institute of Medicine (U.S.) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 49.de la Hunty A, Wallace AM, Gibson S, Viljakainen H, Lamberg-Allardt C, Ashwell M. UK Food Standards Agency Workshop Consensus Report: the choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br J Nutr. 2010;104:612–619. doi: 10.1017/S000711451000214X. [DOI] [PubMed] [Google Scholar]