Abstract
Hemoglobin (Hb) variants are associated with reduced risk of life-threatening Plasmodium falciparum malaria syndromes, including cerebral malaria and severe malarial anemia. Despite decades of research, the mechanisms by which common Hb variants – sickle HbS, HbC, α-thalassemia, fetal HbF – protect African children against severe and fatal malaria have not been fully elucidated. In vitro experimental and epidemiological data have long suggested that Hb variants do not confer malaria protection by restricting the growth of parasites in red blood cells (RBCs). Recently, four Hb variants were found to impair cytoadherence, the binding of P. falciparum-infected RBCs (PfRBCs) to microvascular endothelial cells (MVECs), a centrally important event in both parasite survival and malaria pathogenesis in humans. Impaired cytoadherence is associated with abnormal display of P. falciparum erythrocyte membrane protein 1 (PfEMP1), the parasite’s major cytoadherence ligand and virulence factor, on the surface of host RBCs. We propose a model in which Hb variants allow parasites to display relatively low levels of PfEMP1, sufficient for sequestering PfRBCs in microvessels and avoiding their clearance from the bloodstream by the spleen. By preventing the display of high levels of PfEMP1, Hb variants may weaken the binding of PfRBCs to MVECs, compromising their ability to activate endothelium and initiate the downstream microvascular events that drive the pathogenesis of malaria.
Keywords: Malaria, Plasmodium falciparum, PfEMP1, cytoadherence, hemoglobinopathy, sickle, pathogenesis
1. Introduction
The morbidity and mortality of malaria is astounding [1]. In sub-Saharan Africa alone, Plasmodium falciparum parasites cause an estimated 600 million episodes of malaria and kill more than 1 million children each year [2]. P. falciparum most commonly causes ‘uncomplicated’ malaria, characterized by fever, additional symptoms including headache, body aches and malaise, as well as acute and chronic anemia. Only 1–2% of children develop the complications of ‘severe’ malaria, which require parenteral antimalarial therapy and aggressive clinical management to prevent death [1]. These include cerebral malaria (impaired consciousness, convulsions), severe malarial anemia (Hb concentration < 5 g/dl) and respiratory distress (acidotic deep breathing). To understand the pathogenesis of malaria, it is critical to appreciate that not all children with bloodstream P. falciparum infections develop malaria. In areas of sub-Saharan Africa where the intensity of P. falciparum transmission is high, children acquire ‘disease-controlling’ immunity to malaria. This immunity enables children to tolerate parasitemias without developing the life-threatening complications and, eventually, even the symptoms of malaria. The result of this immunity is that a large fraction of parasitized African children are healthy at any given time. Such children are said to have ‘asymptomatic parasitemia’ but not malaria. How African children tolerate their parasite densities – which in some cases can be quite high – has not been adequately explained.
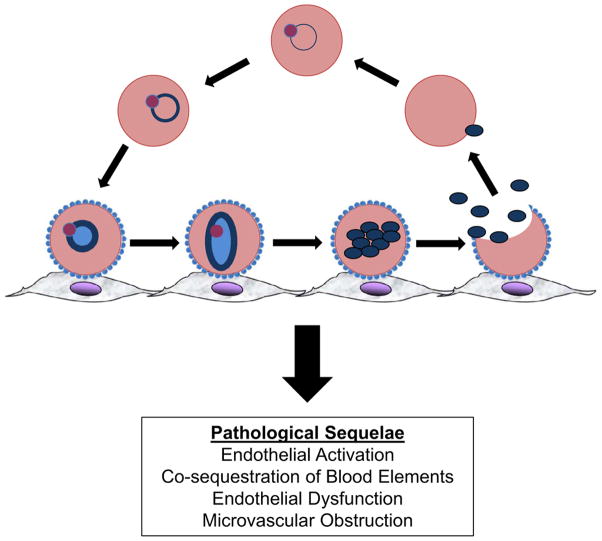
The blood stages of P. falciparum parasites are associated with the symptoms and complications of malaria. While circulating in the bloodstream, parasites undergo 48-hour cycles of invasion, growth and division in red blood cells (RBCs). This exponential expansion of parasite densities is made possible by the binding of P. falciparum-infected RBCs (PfRBCs) to microvascular endothelial cells (MVECs) (Figure 1). This cytoadherence phenotype – which is observed after the late ring-stage of trophozoite development – enables ‘mature’ parasites to avoid clearing mechanisms in the spleen by sequestering in post-capillary venules (‘microvessels’) of the brain and other vital organs. While sequestration is critical for parasite survival, it also induces pathological sequelae in microvessels that are associated with the symptoms and severe manifestations of malaria (Figure 1). If parasites are not eradicated with effective antimalarial drugs, bloodstream parasitemias can persist for months, during which time a small fraction of parasites continuously leave the asexual replication cycle and differentiate into male and female gametocytes. After these sexual stages are taken up by a female anopheline mosquito ingesting a blood meal, they mate and infect the mosquito’s midgut. Further differentiation and expansion of parasites generates thousands of progeny sporozoites that travel to the mosquito’s salivary glands. Sporozoites inoculated into another human host continue their life-cycle by migrating through the dermis and eventually infecting hepatocytes. In these cells, single parasites develop and divide into tens of thousands of merozoites that eventually egress into the bloodstream, invade RBCs and sequester en masse.
Figure 1. The asexual life-cycle of blood-stage Plasmodium falciparum parasites.
As early ring-stage parasites (top center) mature, they traffic PfEMP1 and other knob-associated proteins to the surface of their host RBCs, enabling their late ring-stage forms to adhere to microvascular endothelial cells. While sequestered in microvessels, parasites continue to mature into trophozoite and segmented schizonts. Merozoites that egress from schizonts infect RBCs, producing a new brood of ring-stage parasites in the bloodstream. The sequestration of parasitized RBCs leads to multiple deleterious effects in humans. These are initiated by endothelial activation and include the co-sequestration of blood elements (RBCs, leukocytes and platelets), endothelial dysfunction and microvascular obstruction.
2. How does P. falciparum cause severe and uncomplicated malaria?
To understand how parasitized children develop malaria, it is helpful to keep in mind that parasite densities usually – but not always – correlate with the severity of P. falciparum infections [3]. Thus, children with severe malaria generally have higher parasite densities than those with uncomplicated malaria, and children with uncomplicated malaria generally have higher parasite densities than those with asymptomatic parasitemia. However, there are important exceptions to these observations. For example, children living continuously in endemic areas can tolerate relatively high parasite densities without developing malaria symptoms. Indeed, we have observed P. falciparum densities as high as 330,000/μl of whole blood in healthy Malian children. On the other hand, individuals from non-endemic areas (e.g., tourists, migrant workers) who have little or no history of exposure to P. falciparum may develop severe and even fatal malaria at relatively low parasite densities.
High-density P. falciparum parasitemias are believed to cause the symptoms and signs of malaria through several mechanisms. First, blood-stage parasites consume large amounts of plasma glucose as they replicate, causing hypoglycemia-related impaired consciousness and convulsions. Second, parasites metabolize this glucose into large amounts of lactic acid, causing deep breathing states that lead to respiratory distress and failure. Third, parasites produce large amounts of reactive oxygen species, causing anemia by oxidatively damaging nonparasitized RBCs – a process that accelerates the senescence and removal of RBCs in the spleen. Finally, parasites are believed to release pro-inflammatory factors that produce fever and impair erythropoiesis. One recently discovered example is uric acid, formed after schizont rupture from accumulated hypoxanthine [4] or found as precipitates in the parasite cytosol (A. Rodriguez, et al., unpublished), which induces the production of IL (interleukin)-1, IL-6 and tumor necrosis factor (TNF) from human peripheral blood mononuclear cells (PBMCs) and activates dendritic cells. Through these and other mechanisms, high-density parasitemias are believed to have greater propensities than low-density parasitemias for producing the symptoms and severe manifestations of malaria.
Since sequestration enables parasites to survive and reach high densities in the blood, this event is critical to the pathogenesis of severe malaria. However, parasite sequestration also occurs in individuals with uncomplicated malaria and asymptomatic parasitemia. While the biomass of sequestered PfRBCs correlates positively with malaria severity [3], some individuals are nevertheless able to tolerate large numbers of sequestered parasites without developing symptoms. This indicates that the mere presence of PfRBCs in microvessels may not be sufficient to cause malaria, but that host responses to sequestered PfRBCs contribute significantly to pathology. Indeed, cytoadherence activates MVECs, initiating a complex series of downstream events that are centrally involved in malaria pathogenesis. These include the obstruction, inflammation and dysfunction of microvessels in the brain [5] and virtually every other organ and tissue (e.g., brain, lung, kidney, heart, liver, intestine, muscle and dermis) examined at autopsy. Microvascular obstruction has also been observed in the retina [6] and rectal mucosa [7] of live patients with malaria. These studies reveal microvessels packed not only with PfRBCs in intimate contact with MVECs, but also with ‘co-sequestered’ nonparasitized RBCs, leukocytes and platelets. Known binding interactions between PfRBCs and nonparasitized RBCs (i.e., ‘rosetting’), leukocytes and platelets [8] stabilize a complex of blood elements that impairs blood flow, leading to tissue hypoxia and other inducers of microvascular inflammation.
Evidence for endothelial activation has been obtained from autopsies and dermal biopsies from live patients with malaria. In cases of fatal cerebral malaria, for example, sequestered PfRBCs were found to co-localize with the expression of ICAM-1, VCAM-1 and E-selectin on MVECs [9, 10]. The levels of soluble forms of these adhesion molecules (sICAM-1, sVCAM-1 and sE-selectin) were also found to be elevated in the plasma of malaria patients and correlated with their disease severity [11]. Increased expression of these adhesion molecules on MVECs likely promotes the retention of leukocytes and enhances the sequestration of subsequent broods of blood-stage parasites. The sequestration of PfRBCs also induces the exposure of tissue factor on MVECs, where it may activate coagulation and promote thrombocytopenia. In this model of malaria pathogenesis [12], coagulation factors induce the secretion of pro-inflammatory cytokines (TNF, IL-1 and IL-6), which interact with leukocytes to further induce tissue factor expression in MVECs. Taken together, these and other data from malaria patients indicate that the widespread activation of MVECs by PfRBCs initiates successive cycles of systemic microvascular inflammation, obstruction and coagulation.
Endothelial dysfunction – an imbalance of the vasodilating and vasoconstricting substances produced by (or acting on) the endothelium – occurs in a variety of diseases and is largely attributed to reduced bioavailability of the vasodilator nitric oxide (NO). Only recently has in vivo evidence of endothelial dysfunction been obtained in patients with malaria. In one study, endothelial dysfunction was associated with increased parasite biomass and various measures of hemolysis (increased plasma levels of cell-free Hb, lactate dehydrogenase and arginase) [13]. Patients with severe malaria had significantly low levels of NO and arginine, a precursor for NO synthesis. In another study, cell-free Hb and arginase levels were elevated in patients with severe malaria, compared to those with moderately severe malaria or those who were healthy [14]. These perturbations were also associated with endothelium activation, elevated pro-inflammatory cytokinemia and impaired tissue perfusion. These and other data support a model of malaria pathogenesis in which the lysis of PfRBCs and nonparasitized RBCs releases cell-free Hb and arginase into the plasma. Hb and parasite-induced superoxides quench NO bioactivity, while arginase destroys arginine. The combined effects of decreased NO bioavailability and production then exacerbate microvascular obstruction and inflammation. This is because NO relaxes microvascular tone and maintains normal endothelial function by exerting anti-inflammatory effects on MVECs (e.g., reduces tissue factor expression) and leukocytes (e.g., reduces TNF expression).
3. PfEMP1, an important P. falciparum cytoadherence ligand and virulence factor
P. falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of parasite-encoded, antigenically-variant proteins that mediate cytoadherence and thus act as important virulence factors. PfEMP1 proteins are trafficked out of the parasite, through the RBC cytoplasm and to the external surface of the RBC membrane, where they are incorporated into electron-dense protrusions termed ‘knobs’ (Figure 2). In microvessels, PfEMP1 mediates the binding of PfRBCs not only to MVECs, but also to nonparasitized RBCs, monocytes and platelets. In each parasite genome, a diverse family of approximately 60 var genes encodes PfEMP1 variants [15–17]. By allelic exclusion of its var genes, each parasite expresses a single PfEMP1 variant on the surface of its individual host RBC. Homologous recombination between var genes during meiosis in the mosquito has produced a seemingly limitless diversity of PfEMP1 proteins, especially in high-transmission settings where individuals simultaneously infected with several parasite clones are bitten by mosquitoes.
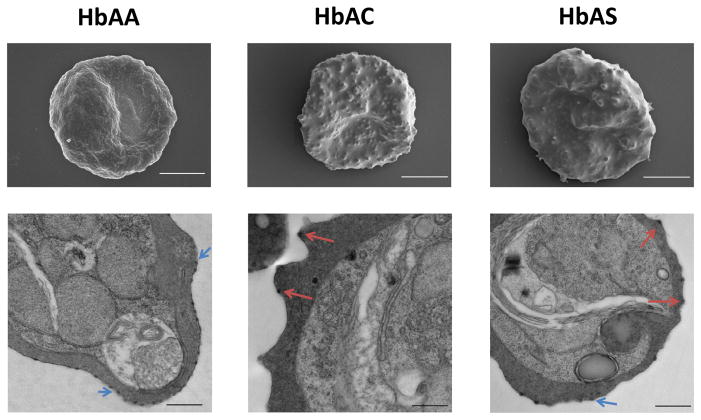
Figure 2. Hemoglobin C and sickle hemoglobin S are associated with abnormal PfEMP1/knob display.
Scanning (top row) and transmission (bottom row) electron micrographs of P. falciparum-infected red blood cells (RBCs) that are homozygous for normal hemoglobin (Hb) A, or heterozygous for the Hb variants HbC and HbS (sickle-cell trait). Parasitized RBCs (young-ring stage) were obtained from HbAA, HbAC and HbAS Malian children with malaria, immediately cultured ex vivo to the trophozoite stage expressing PfEMP1 and knobs, and then processed for imaging. Blue arrows indicate the normally-shaped, uniformly-distributed knobs characteristic of HbAA RBCs. Red arrows indicate the abnormally-shaped, heterogeneously-distributed knobs observed on significant fractions of HbAC and HbAS RBCs. Scale bars represent 2 μm (top row) and 0.5 μm (bottom row).
Each PfEMP1 protein contains multiple segments, classified as either DBL (Duffy binding-like) or CIDR (cysteine-rich interdomain region) domains, which are expressed on the surface of PfRBCs. Many PfEMP1 variants contain only four of these domains (‘short’ variants), whereas others contain five to nine domains (‘long’ variants). The various domains are believed to bind different host receptors, only some of which have been identified. For example, the CIDR1α and DBL2β domains of PfEMP1 proteins bind to CD36 and ICAM-1, respectively, and mediate cytoadherence. The CIDR1α1 domain of PfEMP1 proteins binds to CR1 on nonparasitized RBCs and mediates rosetting. The importance of these host-pathogen interactions to P. falciparum survival is evident in the considerable intra- and inter-clonal diversity of DBL and CIDR domains in parasite populations [18–20]. This diversity enables parasites to survive by evading the PfEMP1 variant-specific IgG responses that impair cytoadherence and rosetting, as well as mediate opsonization, complement activation and antibody-dependent cytotoxicity.
4. Genetic resistance against severe falciparum malaria – the case of hemoglobin variants
The mortality associated with P. falciparum malaria syndromes has exerted significant evolutionary pressure on the human genome. In 1949, J. B. S. Haldane proposed that β-thalassemia mutations – which diminish the production of β-globin chains – exist as a balanced polymorphism [21]. Specifically, he suggested that the reduced fitness of β-thalassemia homozygotes due to severe anemia was balanced by the increased fitness of β-thalassemia heterozygotes due to protection against P. falciparum malaria. While this hypothesis has never been adequately tested in epidemiological studies, it was extended in 1954 by A. S. Allison to include sickle Hb (HbS) [22]. In Africa, HbSS homozygotes almost invariably die from sickle-cell anemia in the first few years of life, while HbAS heterozygotes are highly protected against severe P. falciparum malaria and death. To date, several Hb variants have been investigated for their possible protection against P. falciparum malaria [23]. For some of these Hb variants (e.g., HbE, β-thalassemia), robust epidemiological data associating them with protection against P. falciparum malaria are lacking [24]. Epidemiological data from Africa strongly indicate that malaria protection is afforded by HbS, HbC and α-thalassemia; however, these data have not been adequately reconciled with candidate mechanisms of protection suggested from in vitro experiments. How fetal HbF, which all children carry in the first few months of life, might protect African infants against malaria has been difficult to study due to the presence of maternally-acquired immune IgG. In this section, we review recent evidence that HbC, HbS, α-thalassemia and HbF impair the cytoadherence of PfRBCs to MVECs and blood elements by causing ‘abnormal PfEMP1/knob display’. We suggest that these Hb variants may attenuate parasite virulence by weakening host-pathogen interactions, preventing many African children with uncomplicated malaria from developing severe disease.
4.1 Hemoglobin C
Normal adult Hb (HbA) is a tetramer of two α-globin and two β-globin chains. The HbC variant is produced by a single lysine-for-glutamate substitution in the β-globin chain. This polymorphism arose on a single haplotype in present-day northern Ghana, where up to 35% of children carry HbC, and is presently confined to West Africa. While HbAC heterozygotes are healthy, HbCC homozygotes may have a mild, chronic hemolytic anemia and splenomegaly. Since these conditions have no clinical consequences or apparent fitness costs, it is believed that HbC could become genetically fixed in human populations if it were under net-positive selection. Only recently has HbC been associated with reduced risk of severe P. falciparum malaria, specifically in Mali, Burkina Faso and Ghana [25–28]. A recent meta-analysis found no robust evidence that HbC reduces the incidence of uncomplicated malaria or asymptomatic parasitemia [24]. Most studies have not found significant differences in parasite densities between HbAA, HbAC and HbCC children with malaria. In some cases, HbAC and HbCC children have been found to harbor parasite densities as high as 200,000/μl whole blood [25]. These observations suggest that P. falciparum is capable of invading and developing normally in HbAC and HbCC RBCs in vivo. In support of this proposal, parasites were shown to invade and develop normally in HbAC and HbCC RBCs in vitro [29, 30]. These in vivo and in vitro findings initially suggested to us that HbC does not protect against severe malaria by preventing the development of high-density parasitemias.
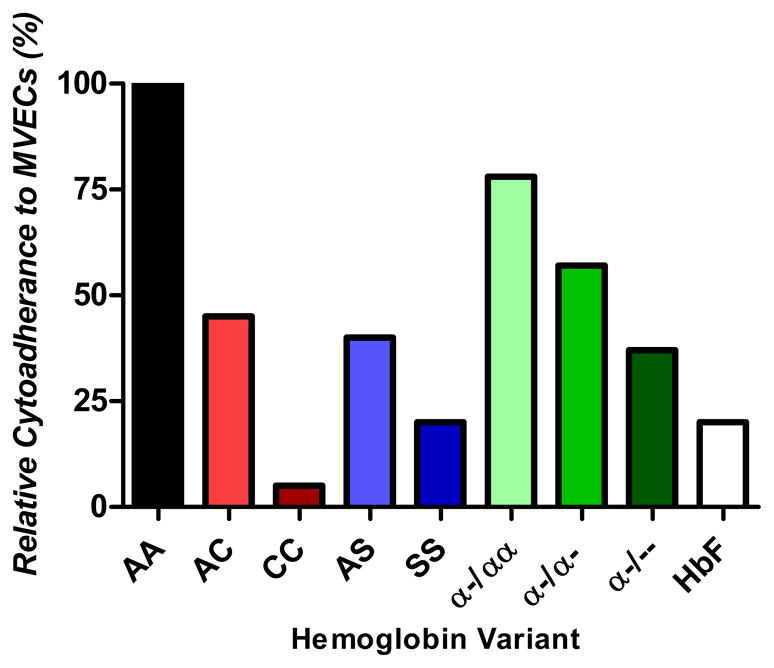
How might HbC protect against severe P. falciparum malaria without keeping parasite densities low? We hypothesized that HbC weakens the host-pathogen interactions that are critical to the pathogenesis of severe malaria. This mechanism would enable parasites to sequester and achieve normal densities in the blood (which they require to survive and transmit their gametocyte forms to other hosts), but prevent them from efficiently activating endothelium and binding circulating blood elements. In support of this hypothesis, we found that HbAC impairs the adherence of PfRBCs to MVECs and nonparasitized RBCs by 30–50% and 45%, respectively (Figure 3) [30, 31]. HbCC completely abrogates the binding of PfRBCs to these host cells, demonstrating an HbC dose-effect on these host-pathogen interactions. Compared to HbAA PfRBCs, HbAC and HbCC PfRBCs showed increasing degrees of ‘abnormal PfEMP1/knob display’ defined as the combination of (i) reduced PfEMP1 levels, (ii) reduced knob densities, (iii) abnormal distributions of both PfEMP1 and knobs on the surface of PfRBCs, and (iv) aberrant – wider and more protuberant – knob morphologies (Figure 2) [30, 32]. Reductions in PfEMP1 levels are most simply implicated in weakening cytoadherence interactions. Whether the abnormal knob distributions and aberrant knob morphologies, on the other hand, are also involved in impairing cytoadherence has not yet been specifically investigated, although several possibilities exist. Abnormal knob distributions, for example, may not provide a uniform display of PfEMP1 proteins to engage a similarly uniform array of host cytoadherence receptors. Also, abnormal knob morphologies may not present PfEMP1 in optimum orientations for binding to host receptors.
Figure 3. Relative cytoadherence of P. falciparum-infected red blood cells to microvascular endothelial cells.
Freshly-drawn red blood cells (RBCs) containing the indicated hemoglobin (Hb) variant were inoculated with laboratory-adapted P. falciparum clones or P. falciparum isolates from Malian children with malaria. After one cycle of invasion and development to the trophozoite stage expressing PfEMP1 and knobs, the cytoadherence of these samples was compared to parasitized HbAA samples processed in parallel. In each experiment, the cytoadherence of HbAA samples is normalized to 100%. These summary data are derived from experiments conducted in [30, 31, 54, 58].
Our data suggest a candidate mechanism by which HbC confers protection against severe malaria (Figure 4). In this model, HbC PfRBCs bind strongly enough to MVECs to efficiently sequester from the spleen, but not so strongly that they maximally activate host endothelium. This phenotype may enable parasites to multiply to densities in HbAC and HbCC children that are similar to those achieved in HbAA children. However, in HbAC and HbCC children these parasites induce sufficient endothelial activation to cause uncomplicated, but not severe, malaria. Abnormal PfEMP1/knob display may also prevent sequestered HbC PfRBCs from efficiently binding various blood elements (non-parasitized RBCs, monocytes and activated platelets) that circulate past them (Figure 4). Thus, this mechanism of protection may also lessen the degree of microvascular obstruction caused by co-sequestered blood elements. By weakening the binding of PfRBCs to platelet ‘bridges’, HbC may prevent the efficient sequestration of PfRBCs to MVECs in the brain, which do not express significant levels of the major cytoadherence receptor CD36 [33].
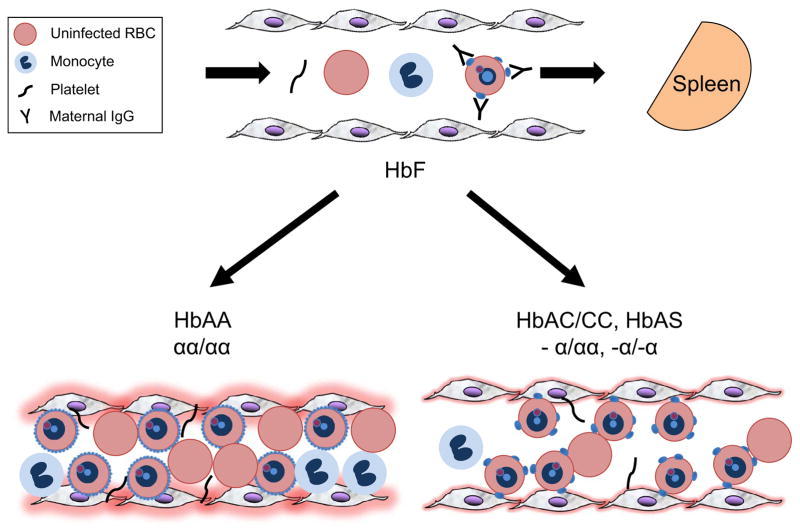
Figure 4. A model to explain how hemoglobin variants protect young African children from the severe manifestations of Plasmodium falciparum malaria.
In the young infant, fetal hemoglobin (Hb) F works cooperatively with maternally-acquired PfEMP1-specific IgG to effectively prevent the sequestration of P. falciparum-infected red blood cells (PfRBCs), resulting in their clearance from the bloodstream by the spleen. This results in a quiescent endothelium. As the child ages, maternal IgG is catabolized and HbF is quantitatively replaced with normal adult HbAA or one or more Hb variant. In a child with no Hb variants (i.e., HbAA, αα/αα), knobs cover the entire surface of PfRBCs and enable their strong binding to microvascular endothelial cells (MVECs). Endothelial cell activation ensues, characterized in part by increased expression of ICAM-1 and tissue factor. Along with endothelial activation, the co-sequestration of blood elements (RBCs, monocytes and platelets) and activation of the coagulation cascade causes endothelial dysfunction (i.e., vasoconstriction) – shown by narrowing of the microvessel lumen – and microvascular obstruction. This results in a high level of microvascular inflammation, shown by an intense reddening of the endothelial cells. In a child with one or more Hb variant (i.e., HbC, sickle HbS and α-thalassemia), knobs are fewer and mal-distributed on the surface of PfRBCs and thus the number of ligand-receptor interactions is markedly diminished. The weaker binding of PfRBCs to host cells leads to reduced endothelial activation, less co-sequestration of blood elements and preservation of endothelial function. This results in a slightly inflamed endothelium, with sufficient microvascular obstruction to cause uncomplicated malaria, but not enough to cause severe, life-threatening malaria.
4.2 Hemoglobin S
The sickle HbS variant is produced by a single valine-for-glutamate substitution in the β-globin chain. The HbS polymorphism arose independently on several haplotypes in sub-Saharan Africa, suggesting that it achieved a high prevalence by natural selection for increased fitness rather than by gene flow between African populations [34, 35]. HbS represents a classic example of a balanced polymorphism [36]. HbSS children have a severe, chronic hemolytic anemia that usually results in early death, reviewed in [37]. HbAS children, on the other hand, are healthy and experience fewer episodes of both uncomplicated and severe falciparum malaria than HbAA children [38–42]. In some, but not all, studies, parasite densities are lower in HbAS than in HbAA children during episodes of asymptomatic parasitemia, uncomplicated malaria and severe malaria [28, 40, 43]. These findings have been predominantly interpreted to suggest that HbAS confers malaria protection through mechanisms that reduce parasite multiplication in vivo. Results from in vitro experiments have suggested a variety of potential mechanisms that may keep parasite densities low in vivo, including (i) impaired merozoite invasion of HbAS RBCs [44], (ii) poor growth of parasites in HbAS RBCs in a low oxygen tension environment that recapitulates that of post-capillary venules [45], and (iii) enhanced phagocytosis of HbAS PfRBCs at the immature ring-stage of parasite development [46].
However, some investigators have found that laboratory-adapted and field-isolated parasites grow normally in HbAS RBCs even under conditions of reduced oxygen tension [31, 46–48]. Further, appreciably high parasitemias are readily found in HbAS as well as HbAA children who experience episodes of malaria or are found to be asymptomatically parasitemic (unpublished observations) [49]. A recent meta-analysis found that HbAS children have parasite densities that are lower, the same or higher than those in HbAA children [24]. These findings suggest that HbAS children are better able to tolerate their parasitemias without developing malaria symptoms. Since HbS is ‘unstable’ like HbC [50], we hypothesized that HbS protects against severe malaria by a mechanism similar to HbC. In support of this, we found that HbAS and HbSS cause 40–55% and 80% reductions in the binding of PfRBCs to MVECs (Figure 3) [31]. Commensurate reductions were also observed in the binding of PfRBCs to monocytes. As for HbC, impaired cytoadherence of HbS PfRBCs is associated with abnormal PfEMP1/knob display. These data, combined with other data showing that HbS impairs the binding of PfRBCs to nonparasitized RBCs (i.e., ‘rosetting’) [48] suggest that HbS and HbC confer malaria protection by a common mechanism (Figure 4).
Impaired cytoadherence as a mechanism of malaria protection may help to explain a variety of puzzling observations in epidemiological studies of HbAS and HbAA children. For example, some studies have found that parasite densities are lower in HbAS than in HbAA children with uncomplicated and severe malaria [24]. To explain this finding, we propose that in areas where immunity is rapidly acquired and sustained (i.e., areas with intense, year-round transmission), high-titer PfEMP1-specific IgG responses may greatly impair the binding of PfRBCs to MVECs. By preventing parasite sequestration and opsonizing PfRBCs for phagocytosis in the spleen, these IgG responses would have the effect of keeping parasite densities low in both HbAS and HbAA children. However, in the face of any given PfEMP1-specific IgG repertoire, sequestration would be more impaired in HbAS children (due to abnormal PfEMP1/knob display) than in their HbAA counterparts. If cytoadherence is weakened sufficiently enough, the combination of HbAS and PfEMP1-specific IgG may altogether prevent the sequestration of some fraction of PfRBCs, leading to lower parasite densities in some HbAS children with malaria.
Paradoxically, the association of HbAS with lower parasite densities in some settings suggests that it takes fewer parasites to cause malaria symptoms in HbAS compared to HbAA children. In other words, the parasites causing uncomplicated or severe malaria in HbAS children seem to be more intrinsically virulent than those causing the same illnesses in HbAA children. One possible explanation for this finding is that HbAS selects for broods of parasites that express PfEMP1 variants capable of overcoming the malaria-protective effect of abnormal PfEMP1/knob display. Such PfEMP1 variants may mediate exceptionally strong binding to MVECs and blood elements in several ways. For example, HbAS may select for PfEMP1 variants containing CIDR1α domains with particularly high affinity for CD36, the major cytoadherence receptor expressed on MVECs, monocytes and platelets. HbAS may also select for PfEMP1 variants that that are capable of binding to multiple cytoadherence receptors on the same MVEC. For example, a PfEMP1 variant containing CIDR1α, DBL-2β and an as-yet-unidentified domain, may bind simultaneously to CD36, ICAM-1 and gC1qR [42] receptors expressed on the same MVEC. Although expressed at lower levels (due to abnormal PfEMP1/knob display), such PfEMP1 variants would nevertheless mediate ‘high-avidity’ interactions between PfRBCs and host cells. Testing whether the expressed var gene repertoire differs between P. falciparum isolates obtained from HbAA and HbAS children will be particularly challenging. This is because children with malaria often harbor multiple broods of parasite clones, each of which may express multiple var genes. Sequencing expressed var gene subdomains, which tend to be extremely sequence-diverse between parasite clones, and assembling these subdomains into full-length var genes represent additional challenges.
In each parasitized African child, the strength of cytoadherence at various host-pathogen interfaces clearly involves a complex relationship between PfEMP1 variant, Hb variant, the titer and affinity of the PfEMP1-specific IgG repertoire and even host receptor (CD36, ICAM-1) polymorphisms. Since both HbAS and PfEMP1-specific IgG may both exert their protective effects at the level of PfEMP1, we and others have proposed that HbAS may influence the acquisition of protective IgG responses [51];[52]. In Kenya, Williams et al. found that the degree of malaria protection in HbAS children increases with age, a surrogate of disease-controlling immunity in endemic areas [43]. While these and other investigators [51] have proposed that HbAS enhances the natural acquisition of immunity to malaria, the mechanism of this putative effect has not been elucidated. Observations that purified IgG from malaria-immune adults can clear high-density parasitemias in Gambian children with malaria strongly indicate that P. falciparum-specific IgG is a central effector of protection [53]. This interpretation is also suggested by work showing that immune IgG abrogates the adherence of PfRBCs to MVECs in vitro [54]. The proportion of IgG responses that are directed at PfEMP1 and other parasite antigens expressed on the RBC surface is not known.
Using a protein microarray representing 491 P. falciparum proteins expressed during exoerythrocytic and erythrocytic stages of the parasite, Tan et al. addressed the hypothesis that P. falciparum-specific IgG responses are enhanced in HbAS children compared to HbAA children [55]. In this study, plasma samples from Malian children aged 2–10 years before and after a 6-month malaria season were probed against the microarray, which represents 25% of the P. falciparum proteome. While the magnitude and breadth of P. falciparum-specific IgG responses increased with age, and from before to after the malaria season, they did not differ between HbAS and HbAA children. While these data do not support the hypothesis that HbAS protects against malaria by generally enhancing P. falciparum-specific IgG responses, it remains possible that HbAS protects against malaria by enhancing IgG responses to antigens not adequately tested in this study. For example, the microarray used in this study was largely devoid of PfEMP1 (as well as other variant surface antigens), leaving open the possibility that HbAS enhances the acquisition of PfEMP1-specific IgG responses. Future studies are needed to determine whether the magnitude and breadth of PfEMP1-specific IgG responses are greater in HbAS compared to HbAA children. However, even if these IgG responses are found to be equivalent in these groups of children, the IgG repertoire of HbAS children may be more effective at impairing cytoadherence because of abnormal PfEMP1/knob display.
4.3 α-thalassemia
α-thalassemia is an inherited disorder in which reduced production of α-globin chains leads to decreased amounts of normal α2β2 tetramers and increased amounts of unpaired β-globin chains. In sub-Saharan Africa, α-thalassemia is produced by a 3.7-kb deletion that leaves one functional copy of duplicated α-globin genes on chromosome 16. Heterozygotes (α–/αα) have an essentially normal phenotype while homozygotes (α–/α–) have mild microcytic anemia of little clinical consequence. A recent meta-analysis found virtually no evidence that either α-thalassemia genotype reduces the incidence of uncomplicated malaria or asymptomatic parasitemia [24]. Only very recently have epidemiological studies associated α-thalassemia with reduced risk of severe P. falciparum malaria, particularly severe malarial anemia, in sub-Saharan Africa [42, 56, 57]. While the mechanism of this protection has not been established, we believe that candidate mechanisms should be consistent with well-established observations in a variety of epidemiological settings: specifically, α-thalassemia is not associated with lower parasite densities in children with asymptomatic parasitemia, uncomplicated malaria and severe malaria; and very high parasite densities are routinely observed in α-thalassemic children. These observations indicate that α-thalassemia does not protect against severe malaria by impairing the ability of parasites to invade or develop within RBCs, or by promoting the removal of PfRBCs from the bloodstream.
To explain how α-thalassemia reduces the incidence of severe malaria without affecting parasite density in vivo, we hypothesized that this trait also weakens cytoadherence. Such a mechanism seemed plausible as α-thalassemic RBCs contain excess unpaired β-globin chains, which form β4 tetramers that are ‘unstable’ like HbS and HbC. To test this hypothesis, we obtained P. falciparum isolates directly from Malian children with malaria and inoculated them into αα/αα (normal), –α/αα (heterozygous) and –α/–α (homozygous) RBCs. To further test the dose effect of α-thalassemia on cytoadherence, we also inoculated laboratory-adapted parasite clones into – α /–– RBCs from two individuals of Mediterranean descent with HbH disease. Following a single cycle of parasite invasion and maturation to the trophozoite stage, we found that –α/αα, –α/–α and –– /–α PfRBCs showed 22%, 43% and 63% reduced binding to MVECs compared to αα/αα PfRBCs (Figure 3) [58]. We also found commensurate reductions in the binding of PfRBCs to monocytes. α-thalassemia was associated with abnormal PfEMP1/knob display reminiscent of HbAC and HbAS PfRBCs. This suggests that α-thalassemia protects against severe malaria not by keeping parasite densities low, but by attenuating parasite virulence (Figure 4). In Papua New Guinea, Fowkes et al. have suggested that homozygous α-thalassemia protects specifically against severe malarial anemia without reducing parasite densities [59]. According to their model, increases in absolute numbers of RBCs (due to microcytosis) protect homozygous α-thalassemic children against severe anemia by reducing the amount of Hb loss at any given parasitemia.
4.4 Hemoglobin F
In Africa, infant susceptibility to P. falciparum malaria increases substantially as fetal HbF and maternal immune IgG disappear from the circulation. During the first few months of life, however, resistance to malaria is evidenced by extremely low parasitemias, absence of fever and lack of severe malaria. This resistance is often attributed in part to poor parasite growth in HbF-containing RBCs. While several studies showed that P. falciparum readily invades cord blood (CB) RBCs, the presence of HbF in three RBC types (CB, infant, and adult hereditary persistence of fetal Hb) has been associated with parasite growth restriction [60, 61]. Biochemical explanations for these findings were provided by studies concluding that the ability of HbF RBCs to handle the oxidative stress imposed by developing parasites is impaired, or that HbF is inefficiently digested by parasite hemoglobinases [62]. Efforts to study HbF-mediated malaria protection in infants have been complicated by the presence of transplacentally-acquired immune IgG that effectively protects the mother from uncomplicated and severe malaria. Since passive transfer of adult immune IgG to African children can rapidly clear parasitemias [53], and high-titer maternal immune IgG is present in young infants, we sought to develop an alternative model to explain the dramatic protection of infants from malaria. Specifically, we hypothesized that HbF – an ‘unstable’ Hb [63] – and maternal immune IgG impair cytoadherence by causing abnormal PfEMP1/knob display and blocking PfEMP1 interactions with host receptors, respectively, in infants.
To test this hypothesis, we first established that P. falciparum clones invade and develop normally in fetal (cord blood, CB) RBCs containing up to 95% HbF. Using these samples, we found that HbF is associated with 80%, 30% and 25% reductions, respectively, in the binding of PfRBCs to MVECs (Figure 3), monocytes and nonparasitized RBCs [54]. Abnormalities in PfEMP1/knob display on CB PfRBCs correlated with these findings and are reminiscent of those on HbC, HbS and α-thalassemic PfRBCs. IgGs purified from the plasma of immune Malian adults nearly abolished the cytoadherence of CB PfRBCs to MVECs [54]. These data suggest a model of malaria protection in which HbF and maternal IgG act cooperatively at the level of PfEMP1 to prevent the sequestration and rosetting of PfRBCs in the first several months of life (Figure 4). In highly malarious areas of Africa, an infant’s contemporaneous expression of HbC, HbS or α-thalassemia and development of an immune IgG repertoire may effectively reconstitute the waning protective effects of HbF and maternal immune IgG. These processes would have the effect of extending malaria resistance from infancy into early childhood.
5. How do hemoglobin variants cause abnormal PfEMP1/knob display?
It is not known how HbC, HbS, α-thalassemia and HbF cause abnormal PfEMP1/knob display. One explanation for reduced PfEMP1 levels and knob densities is that Hb variants impair the trafficking of PfEMP1 and other knob components to the surface of PfRBCs. We have proposed one candidate mechanism involving the oxidative instability of HbC, HbS, β4 tetramers (present in α-thalassemic RBCs) and HbF. Accelerated oxidation of these Hb variants compared to normal HbA leads to increased levels of hemichromes, which bind the cytoplasmic tail of band 3 and plaster the inner leaflet of the RBC membrane [30]. There, hemichromes may impair the docking of Maurer’s clefts (vesicles that transport PfEMP1 through the RBC cytoplasm) at the inner leaflet of the RBC membrane or block the translocation of PfEMP1 and other knob components to the external surface of the RBC. Another possibility is that hemichrome-associated ferric iron oxidizes RBC membrane proteins and lipids, restricting their lateral mobility and compromising the parasite’s ability to remodel its host RBC membrane. Yet another possibility is that parasites are relatively less able to acquire the lipids, cholesterol and other components of human plasma it requires for the biogenesis of its vesicular trafficking pathways.
Recent work by Cyrclaff et al. [64] has begun to investigate how HbS and HbC might interfere with the trafficking of PfEMP1 to the RBC membrane. They show by cryo-electron tomography that P. falciparum hijacks and remodels the RBC’s cytoskeletal actin into a network of ‘bridges’, which it uses to traffic PfEMP1 and perhaps other virulence proteins to the inner leaflet of the RBC membrane. In normal HbAA PfRBCs, these networks appeared to be extensively developed and to tether Maurer’s clefts to individual knobs. In HbCC and HbSC PfRBCs, however, they observed significant abnormalities in the remodeled actin networks, such as shorter bridges and heterogeneous actin branching patterns. These data suggest that these particular Hb variants may interfere with the construction of actin bridges that connect Maurer’s clefts and vesicles to knobs. A direct role for Hb variants was investigated in experiments showing that hemolysates of HbCC and HbSC RBCs impaired the polymerization of actin in vitro. HbCC and HbSC PfRBCs showed aberrant Maurer’s cleft morphologies, but how these Hb variants might produce these effects is less clear. One possibility is that parasite-remodeled RBC actin is also used to shape and maintain the morphology of Maurer’s clefts. Whether HbAS and HbAC PfRBCs show similar abnormalities in actin networks or Maurer’s clefts has not been investigated, as well as the effects of HbAS and HbAC RBC hemolysates on actin polymerization. Future work to identify compounds that produce these phenotypes in HbAA PfRBCs may also be useful in studying the mechanism of abnormal PfEMP1/knob display.
6. Working model: Hemoglobin variants and PfEMP1-specific IgG work cooperatively to confer malaria protection by weakening host-pathogen interactions
We propose the following model to explain a variety of epidemiological and clinical observations made in children who inhabit malaria-endemic areas of sub-Saharan Africa. In the first few months of life, a child is almost completely protected against both uncomplicated and severe malaria by the combined effects of HbF and a maternally-acquired repertoire of PfEMP1-specific IgG (Figure 4). This IgG repertoire is at high titer and is broadly-reactive against the vast majority of PfEMP1 variants expressed in parasites that circulate locally in the child’s community. Initially, this IgG repertoire protects the child from malaria in the same way it protects the mother: by preventing the adherence of PfRBCs to MVECs and blood elements and thereby keeping parasitemias and inflammation very low. As the maternal IgG repertoire is catabolized over the first few months of life, the PfRBCs in the infant’s bloodstream become increasingly able to sequester and rosette. These processes have the effects of increasing parasite density and enhancing the ability of PfRBCs to cause endothelial inflammation, obstruction and dysfunction. Once sufficiently high densities of parasites and levels of inflammation are achieved, the older infant presents with its first episode of malaria.
In children who lack Hb variants, the disappearance of maternal IgG renders them completely unprotected against the PfEMP1-mediated phenomena associated with uncomplicated and severe malaria (Figure 4). Thus, these children develop high densities of PfRBCs, which bind strongly to MVECs and blood elements and cause malaria. Should the parasite isolate express a PfEMP1 variant that mediates especially strong binding to host cell surfaces, the child with uncomplicated malaria develops severe disease. In considering how Hb variants confer protection from severe malaria, our model assumes that the levels of PfEMP1-specific, maternal IgG decay at similar rates in children with and without Hb variants. At any given titer, however, the effectiveness of the maternally-acquired IgG repertoire is greater in children with Hb variants. This is because there are less PfEMP1 ligands that the IgG repertoire needs to block in order to weaken cytoadherence interactions. Since HbAS, HbC and α-thalassemia all protect against severe malaria, we hypothesize that each of these variants cause enough abnormal PfEMP1/knob display in vivo to impair cytoadherence interactions sufficiently well to reduce the risk of severe malaria.
To explain how HbAS generally confers greater protection against severe malaria than HbC and α-thalassemia, we have not yet considered it necessary to invoke additional mechanisms of protection. Instead, we hypothesize that HbAS simply impairs the cytoadherence of PfRBCs to a greater degree than HbC and α-thalassemia in vivo. One candidate mechanism for this possibility is that P. falciparum induces sickling of PfRBCs in the low-oxygen tension of post-capillary venules [65], and that sickled PfRBCs do not interact as efficiently with MVECs and blood elements under conditions of physiological flow. This possibility may explain also how HbAS confers protection against uncomplicated malaria, while HbC and α-thalassemia do not. If HbAS PfRBCs express greater degrees of abnormal PfEMP1/knob display than other Hb variants in vivo, this may explain how HbAS delays the time to first episode of malaria during a transmission season [66]. In this case, parasites infecting HbAS children may require considerably longer periods of time to switch to a relatively rare set of PfEMP1 variants that are able to overcome the malaria-protective effects of abnormal PfEMP1/knob display.
Our model also explains other clinical and epidemiological findings. In areas of sub-Saharan Africa where immunity is consistently and rapidly acquired, the incidences of severe malaria, uncomplicated malaria and asymptomatic parasitemia eventually decrease with age irrespective of Hb type. Indeed, children who survive beyond 5 years of age generally do not develop severe malaria, older teenagers no longer present with uncomplicated malaria, and adults are frequently found to have asymptomatic parasitemias below the level of detection by microscopy. These progressively increasing levels of protection may be conferred by IgG responses that become higher in titer and more broadly-reactive with age. Once it achieves a certain magnitude and breadth, the PfEMP1-specific IgG repertoire of all children ‘swamps out’ any observable protective effect of their Hb variant. This may explain the lack of uncomplicated malaria and low-level parasitemias in all adults irrespective of their Hb type.
An additional clinical finding is that non-immune individuals with Hb variants are not protected from malaria syndromes. A recent report from Chicago is particularly illuminating. In this report [67], the authors presented five cases of P. falciparum malaria in siblings of a family who had traveled to Africa without taking chemoprophylaxis. Two of the children had sickle cell anemia (HbSS), one of which developed life-threatening malaria and hemolysis. The other three children had HbAS, two of whom developed severe malaria. To date, such observations have been confined to case reports. One way to evaluate the protective effects of Hb variants in the absence of immunity is to work in areas where HbAS, HbC or α-thalassemia are present at high prevalence, but where the inhabitants are non-or semi-immune. One possible study population is the semi-immune Khmer people of Western Cambodia, who acquire malaria occupationally in the forested areas along the Cambodia-Thailand border and carry a high prevalence (25%) of α-thalassemia. Further, our model may explain how non-immune individuals without Hb variants (i.e., travelers) can develop life-threatening malaria syndromes at very low parasite densities. Without the effects of PfEMP1-specific IgG and abnormal PfEMP1/knob display, such individuals have absolutely no protection against PfEMP1-mediated phenomena. Parasites expressing virtually any PfEMP1 variant would efficiently sequester, binding avidly to endothelium and blood elements. In this setting, the rapid parasite multiplication rates and significant degrees of microvascular inflammation and obstruction may produce the symptoms of uncomplicated and severe malaria at lower parasite densities than in individuals with Hb variants, PfEMP1-specific IgG, or both.
Finally, our model suggests that HbE, an ‘unstable’ Hb variant at high prevalence in Cambodia [68] and neighboring regions, may also confer protection against malaria by causing abnormal PfEMP1/knob display. The effects of β-thalassemia on the incidence and severity of malaria are difficult to study because of malaria elimination in the Mediterranean and surrounding areas where β-thalassemia is at high prevalence. Nevertheless, our model predicts that the unpaired α globin chains in β-thalassemic RBCs may also cause abnormal PfEMP1/knob display through the formation of ‘unstable’ α4 tetramers. Our model predicts the same effect for highly-prevalent G6PD deficiency states (the ‘A-’ allele in Africa, the ‘Viangchan’ allele in Cambodia, the ‘Mahidol’ allele in Thailand), in which normal HbAA becomes ‘unstable’ in the setting of antioxidant depletion. Additional studies are needed to determine whether and to what degree HbE, β-thalassemia and G6PD deficiency confer malaria protection and whether these polymorphisms cause abnormal PfEMP1/knob display.
7. Concluding remarks
A simple, well-known observation continues to inspire our research program: in malaria-endemic areas of Africa where immunity is rapidly acquired, HbS almost completely protects against death from P. falciparum malaria. The mechanisms by which this and other Hb variants protect against severe malaria remain obscure. Recently, attention has shifted away from investigating how Hb variants may restrict parasite growth within RBCs to how they enable African children to tolerate high parasite densities without succumbing to malaria. We have begun to elucidate what may be a common mechanism by which Hb variants mitigate disease severity. The processes of PfRBC sequestration and rosetting are centrally involved in malaria pathogenesis as they enable PfRBCs to avoid clearance by the spleen and cause microvascular inflammation, obstruction and dysfunction. We have found that HbC, HbS, α-thalassemia and HbF impair the adherence of PfRBCs to MVECs; we and others have also found evidence that these Hb variants impair rosetting. These phenotypes are associated with abnormal display of PfEMP1 – the parasite’s main virulence factor and cytoadherence ligand – expressed on ‘knobs’ at the surface of PfRBCs.
Our work may indicate that diverse Hb variants were naturally selected by malaria for their common phenotype of abnormal PfEMP1/knob display. If true, this finding strongly suggests that ‘high-avidity’ PfEMP1-mediated interactions are responsible for killing African children, and that ‘death-preventing’ immunity weakens the strength of these interactions. In malaria-endemic areas where immunity is rapidly acquired, we propose that PfEMP1-specific IgG and Hb variants work cooperatively to prevent and weaken cytoadherence, thereby attenuating the virulence of P. falciparum. Acting together, these factors may progressively drive down the incidence of severe malaria, uncomplicated malaria and asymptomatic parasitemia – in that order. Further work to investigate the viability of this mechanism – or other unrelated mechanisms [69] – should improve our understanding of how genetic resistance and acquired immunity prevent the life-threatening manifestations and symptoms of malaria, and eventually reduce parasite densities to very low levels. Ultimately, these efforts may identify mechanisms of parasite virulence and host inflammation that are amenable to therapeutics or vaccines.
The findings that diverse Hb variants (HbC, HbS, α-thalassemia and HbF) cause abnormal PfEMP1/knob display – and the possibility that HbE, β-thalassemia and G6PD deficiency do as well – provide fresh insights into both malaria pathogenesis and protection. Importantly, they provide additional support for the hypothesis that PfEMP1-mediated phenomena are proximately responsible for causing the fatal complications of malaria. Further, our findings suggest that it is the strength of cytoadherence and rosetting interactions that drive the pathogenesis of both uncomplicated and severe malaria syndromes. Host factors that reduce the strength of PfEMP1-mediated binding interactions can mitigate the downstream pathological effects of parasite sequestration. These factors include Hb variants as well as PfEMP1-specific IgG, which children acquire over time while they are naturally exposed to numerous parasite isolates and the myriad PfEMP1 variants they express.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO, Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NP. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orengo JM, Leliwa-Sytek A, Evans JE, Evans B, van de Hoef D, Nyako M, Day K, Rodriguez A. Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response. PLoS One. 2009;4:e5194. doi: 10.1371/journal.pone.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 6.White VA, Lewallen S, Beare NA, Molyneux ME, Taylor TE. Retinal pathology of pediatric cerebral malaria in Malawi. PLoS One. 2009;4:e4317. doi: 10.1371/journal.pone.0004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, Rahman MR, Hasan M, Bin Yunus E, Ghose A, Ruangveerayut R, Limmathurotsakul D, Mathura K, White NJ, Day NP. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 8.Faille D, El-Assaad F, Alessi MC, Fusai T, Combes V, Grau GE. Platelet-endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb Haemost. 2009;102:1093–1102. doi: 10.1160/TH09-05-0337. [DOI] [PubMed] [Google Scholar]
- 9.Armah H, Dodoo AK, Wiredu EK, Stiles JK, Adjei AA, Gyasi RK, Tettey Y. High-level cerebellar expression of cytokines and adhesion molecules in fatal, paediatric, cerebral malaria. Ann Trop Med Parasitol. 2005;99:629–647. doi: 10.1179/136485905X51508. [DOI] [PubMed] [Google Scholar]
- 10.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 11.Turner GD, Ly VC, Nguyen TH, Tran TH, Nguyen HP, Bethell D, Wyllie S, Louwrier K, Fox SB, Gatter KC, Day NP, White NJ, Berendt AR. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152:1477–1487. [PMC free article] [PubMed] [Google Scholar]
- 12.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–1529. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 16.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 17.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kyes SA, Kraemer SM, Smith JD. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot Cell. 2007;6:1511–1520. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraemer SM, Kyes SA, Aggarwal G, Springer AL, Nelson SO, Christodoulou Z, Smith LM, Wang W, Levin E, Newbold CI, Myler PJ, Smith JD. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics. 2007;8:45. doi: 10.1186/1471-2164-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldane J. The rate of mutation of human genes. Hereditas. 1949;35:267–273. [Google Scholar]
- 22.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams TN. Red blood cell defects and malaria. Mol Biochem Parasitol. 2006;149:121–127. doi: 10.1016/j.molbiopara.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012 doi: 10.1016/S1473-3099(12)70055-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, Kone A, Kayentao K, Djimde A, Plowe CV, Doumbo O, Wellems TE, Diallo D. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 26.Modiano D, Luoni G, Sirima BS, Simpore J, Verra F, Konate A, Rastrelli E, Olivieri A, Calissano C, Paganotti GM, D’Urbano L, Sanou I, Sawadogo A, Modiano G, Coluzzi M. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 27.Mockenhaupt FP, Ehrhardt S, Cramer JP, Otchwemah RN, Anemana SD, Goltz K, Mylius F, Dietz E, Eggelte TA, Bienzle U. Hemoglobin C and resistance to severe malaria in Ghanaian children. J Infect Dis. 2004;190:1006–1009. doi: 10.1086/422847. [DOI] [PubMed] [Google Scholar]
- 28.May J, Evans JA, Timmann C, Ehmen C, Busch W, Thye T, Agbenyega T, Horstmann RD. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007;297:2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 29.Fairhurst RM, Fujioka H, Hayton K, Collins KF, Wellems TE. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood. 2003;101:3309–3315. doi: 10.1182/blood-2002-10-3105. [DOI] [PubMed] [Google Scholar]
- 30.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, Hoang HL, Hayton K, Guindo A, Makobongo MO, Schwartz OM, Tounkara A, Doumbo OK, Diallo DA, Fujioka H, Ho M, Wellems TE. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 31.Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakite SA, Arie T, Krause MA, Guindo A, Tubman A, Fujioka H, Diallo DA, Doumbo OK, Ho M, Wellems TE, Fairhurst RM. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. ProcNatl Acad Sci USA. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arie T, Fairhurst RM, Brittain NJ, Wellems TE, Dvorak JA. Hemoglobin C modulates the surface topography of Plasmodium falciparum-infected erythrocytes. J Struct Biol. 2005;150:163–169. doi: 10.1016/j.jsb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Wassmer SC, Lepolard C, Traore B, Pouvelle B, Gysin J, Grau GE. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis. 2004;189:180–189. doi: 10.1086/380761. [DOI] [PubMed] [Google Scholar]
- 34.Nagel RL, Fleming AF. Genetic epidemiology of the beta s gene. Baillieres Clin Haematol. 1992;5:331–365. doi: 10.1016/s0950-3536(11)80023-5. [DOI] [PubMed] [Google Scholar]
- 35.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, Weatherall DJ, Hay SI. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison AC. Polymorphism and Natural Selection in Human Populations. Cold Spring Harb Symp Quant Biol. 1964;29:137–149. doi: 10.1101/sqb.1964.029.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Xia Y. Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect. 2012;14 doi: 10.1016/j.micinf.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 39.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 40.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, Marsh K. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams TN, Mwangi TW, Wambua S, Peto TE, Weatherall DJ, Gupta S, Recker M, Penman BS, Uyoga S, Macharia A, Mwacharo JK, Snow RW, Marsh K. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswas AK, Hafiz A, Banerjee B, Kim KS, Datta K, Chitnis CE. Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PLoS Pathog. 2007;3:1271–1280. doi: 10.1371/journal.ppat.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, Wambua S, Kortok M, Snow RW, Marsh K. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–703. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 45.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA. 1978;75:1994–1997. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 47.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol. 2004;34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Carlson J, Nash GB, Gabutti V, al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 49.Beaudry JT, Fairhurst RM. Microvascular sequestration of Plasmodium falciparum. Blood. 2011;117:6410. doi: 10.1182/blood-2010-09-305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannan R, Labotka R, Low PS. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. J Biol Chem. 1988;263:13766–13773. [PubMed] [Google Scholar]
- 51.Bayoumi RA. The sickle-cell trait modifies the intensity and specificity of the immune response against P. falciparum malaria and leads to acquired protective immunity. Med hypotheses. 1987;22:287–298. doi: 10.1016/0306-9877(87)90193-9. [DOI] [PubMed] [Google Scholar]
- 52.Verra F, Simpore J, Warimwe GM, Tetteh KK, Howard T, Osier FH, Bancone G, Avellino P, Blot I, Fegan G, Bull PC, Williams TN, Conway DJ, Marsh K, Modiano D. Haemoglobin C and S role in acquired immunity against Plasmodium falciparum malaria. PLoS One. 2007;2:e978. doi: 10.1371/journal.pone.0000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 54.Amaratunga C, Lopera-Mesa TM, Brittain NJ, Cholera R, Arie T, Fujioka H, Keefer JR, Fairhurst RM. A role for fetal hemoglobin and maternal immune IgG in infant resistance to Plasmodium falciparum malaria. PLoS One. 2011;6:e14798. doi: 10.1371/journal.pone.0014798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan X, Traore B, Kayentao K, Ongoiba A, Doumbo S, Waisberg M, Doumbo OK, Felgner PL, Fairhurst RM, Crompton PD. Hemoglobin S and C heterozygosity enhances neither the magnitude nor breadth of antibody responses to a diverse array of Plasmodium falciparum antigens. J Infect Dis. 2011;204:1750–1761. doi: 10.1093/infdis/jir638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mockenhaupt FP, Ehrhardt S, Gellert S, Otchwemah RN, Dietz E, Anemana SD, Bienzle U. Alpha(+)-thalassemia protects African children from severe malaria. Blood. 2004;104:2003–2006. doi: 10.1182/blood-2003-11-4090. [DOI] [PubMed] [Google Scholar]
- 57.Williams TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CR, Maitland K. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 58.Krause M, Diakite S, Lopera-Mesa T, Amaratunga T, Traore A, Doumbia S, Konate D, Keefer J, Diakite M, Fairhurst R. Alpha-thalassemia impairs the cytoadherence of Plasmodium falciparum infected erythrocytes. PLoS One. 2012 doi: 10.1371/journal.pone.0037214. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowkes FJ, Allen SJ, Allen A, Alpers MP, Weatherall DJ, Day KP. Increased microerythrocyte count in homozygous alpha(+)-thalassaemia contributes to protection against severe malarial anaemia. PLoS Med. 2008;5:e56. doi: 10.1371/journal.pmed.0050056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasvol G, Weatherall DJ, Wilson RJ, Smith DH, Gilles HM. Fetal haemoglobin and malaria. Lancet. 1976;1:1269–1272. doi: 10.1016/s0140-6736(76)91738-4. [DOI] [PubMed] [Google Scholar]
- 61.Pasvol G, Weatherall DJ, Wilson RJ. Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977;270:171–173. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]
- 62.Shear HL, Grinberg L, Gilman J, Fabry ME, Stamatoyannopoulos G, Goldberg DE, Nagel RL. Transgenic mice expressing human fetal globin are protected from malaria by a novel mechanism. Blood. 1998;92:2520–2526. [PubMed] [Google Scholar]
- 63.Advani R, Mentzer W, Andrews D, Schrier S. Oxidation of hemoglobin F is associated with the aging process of neonatal red blood cells. Pediatr Res. 1992;32:165–168. doi: 10.1203/00006450-199208000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, Lanzer M. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science. 2011;334:1283–1286. doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 65.Luzzatto L, Nwachuku-Jarrett ES, Reddy S. Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet. 1970;1:319–321. doi: 10.1016/s0140-6736(70)90700-2. [DOI] [PubMed] [Google Scholar]
- 66.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glikman D, Nguyen-Dinh P, Roberts JM, Montgomery CP, Daum RS, Marcinak JF. Clinical malaria and sickle cell disease among multiple family members in Chicago, Illinois. Pediatrics. 2007;120:e745–748. doi: 10.1542/peds.2007-0041. [DOI] [PubMed] [Google Scholar]
- 68.Sanguansermsri T, Flatz G, Flatz SD. Distribution of hemoglobin E and beta-thalassemia in Kampuchea (Cambodia) Hemoglobin. 1987;11:481–486. doi: 10.3109/03630268708998008. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, Rebelo S, Henri A, Beuzard Y, Soares MP. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]