Summary
Organ transplantation is the treatment of choice for patients with end-stage organ dysfunction. In spite of advances in understanding of donor and recipient physiology, organ preservation, operative techniques, and immunosuppression long term graft survival still remains a major problem primarily due to chronic rejection. Alloimmune responses to mismatched major histocompatibility antigens have been implicated as an important factor leading to rejection. However, there is increasing evidence pointing towards cross talk between the alloimmune and autoimmune responses creating a local inflammatory milieu which eventually leads to fibrosis and occlusion of the lumen in the transplanted organ i.e., chronic rejection. In this review, we will discuss recent studies and emerging concepts for the interdependence of alloimmune and autoimmune responses in the immunopathogenesis of chronic allograft rejection. The role of autoimmunity in the development of chronic rejection is an intriguing and exciting area of research in the field of solid-organ transplantation with significant potential to develop novel therapeutic strategies towards preventing chronic allograft rejection.
Keywords: chronic rejection, TH17, regulatory T-cells, autoimmunity
Introduction
Solid organ transplantation remains the treatment option of end stage organ dysfunction. Enhanced understanding of post-operative care, prevention of ischemia-reperfusion injury, reduction in surgical complications, advances in immunosuppressive therapy are all critical factors in achieving marked success following organ transplantation with significant decrease in acute rejection episodes (Fishman, 2007;Hauptman and O'Connor, 1997). However, recent studies have shown that despite a reduction in acute rejection episodes, the long-term graft survival remains relatively unchanged during the last decade (Meier-Kriesche, et al., 2004). For example, the 5-year survival of lung allograft is just 30–50% and this further reaches a nadir of 10% by 9 years following lung transplant (LTx) (Hachem, 2009). Although slightly better, the rates for other organs including heart (Hornick and Rose, 2006), islets (Robertson, 2004), and kidney (El-Zoghby, et al., 2009), remain unsatisfactory (Ojo, et al., 2003). Chronic allograft rejection continues to be the most important cause of long-term graft failure and current immunosuppressive regimens have not significantly reduced its incidence.
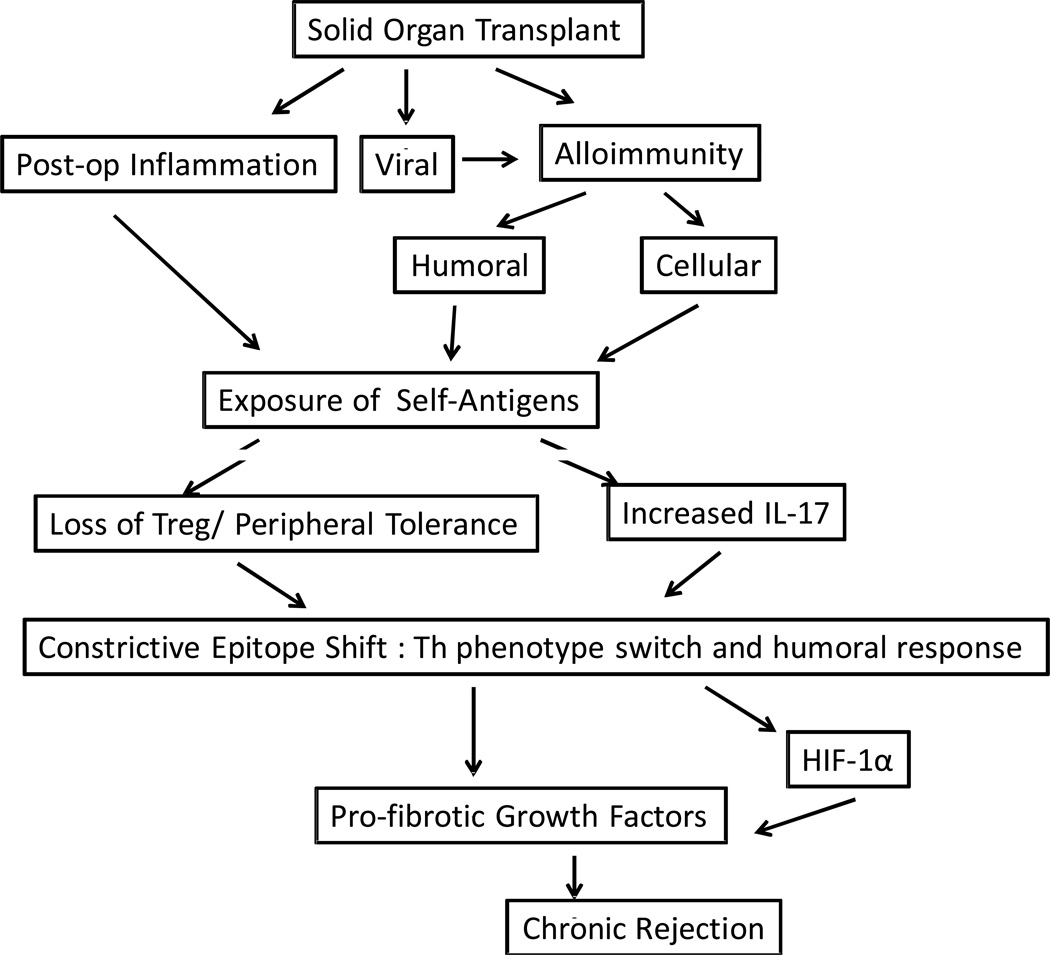
Chronic rejection is characterized by decreased physiological function of the organ and histologically characterized by fibrosis primarily affecting the blood vessels and tubular structures in the allograft. This manifests as chronic allograft nephropathy in kidney, cardiac allograft vasculopathy in heart and bronchiolitis obliterans syndrome (BOS) in lung allografts. Both immune (antigen-dependent) and non-immune (antigen-independent) factors lead to fibroproliferative changes that cause occlusion of tubular structures in the allograft (Mertens, et al., 2010;Millington and Madsen, 2010;Paraskeva, et al., 2011;Tiriveedhi, et al., 2010). Recent studies have shown that allorecognition of mismatched donor HLA is a critical event that initiates chronic rejection. Donor antigen recognition can occur either by direct or indirect or both pathways (Game and Lechler, 2002) and recent evidences have shown that indirect antigen presentation as the major player involved in the pathogenesis of chronic rejection (Benichou, et al., 1999;Heeger, 2003). The indirect pathway involves presentation of processed donor antigens by recipient APCs to recipient T-cells triggering an alloimmune response including activation of B cells to produce donor specific antibodies (Abs) (Heeger, 2003). Further, experimental models have suggested that the alloimmune response, in particular the isotype switch from IgM to IgG, is dependent on theCD4+ T helper mediated indirect recognition pathway, which is tightly regulated by both T-cell costimulatory signals and recognition of cognate antigen (Bestard, et al., 2008;Lietz, et al., 2005;Steele, et al., 1996). In this review, we will discuss the role of alloimmune responses that facilitate the development of a local inflammatory milieu resulting in the development of immune response to self-antigens which we believe plays an important role in the development of fibrotic changes and thus ultimately leading to chronic allograft rejection.
Role of alloimmune responses in chronic rejection
Several reports have demonstrated that development of Abs to mismatched HLA class I and II is associated with the development of chronic rejection, clinically diagnosed as BOS after human LTx (Girnita, et al., 2007;Jaramillo, et al., 1999;Palmer, et al., 2002;Reznik, et al., 2001;Rizzo, et al., 1997;Rose and Smith, 2009). Based on this body of evidence it is reasonable to postulate that the presence of ‘shed’ donor HLA antigens in the bronchoalveolar lavage fluids following LTx, are processed and presented to T helper cells. Such T helper cells, engaged in indirect recognition pathway, can produce lymphokines required for the growth and maturation of antigen specific B cells resulting in Abs to mismatched HLA antigens. Furthermore, it has been demonstrated that anti-HLA can activate human airway epithelial cells (AEC) resulting in the production of several growth factors including fibrinogenic growth factors. (Jaramillo, et al., 1999;Reznik, et al., 2001;Yamakuchi, et al., 2007). The CD4+T-helper cells recognize processed forms of soluble HLA in the context of self-major histocompatibility antigens (MHC) and these T-cells are likely involved in development of humoral immune responses to donor HLA. The presence of circulating cytotoxic anti-HLA have been strongly associated with chronic allograft rejection of heart (Ciancio, et al., 2005;Jindra, et al., 2008;Nakajima, et al., 1999) and renal transplants (Morris, et al., 2010). Therefore, it is reasonable to propose that development of Abs to mismatched HLA during the post-transplant period may indeed be involved in the pathogenesis of chronic rejection.
Chronic rejection of cardiac allografts is manifested by cardiac allograft vasculopathy (CAV), characterized by occlusion of coronary vessels. The 5-year incidence of CAV is 30–40% and, the development of donor-specific HLA Abs has been correlated with chronic rejection (Kaczmarek, et al., 2008). Chronic rejection termed chronic allograft nephropathy (CAN) is the leading cause of renal function deterioration and accounts for nearly 40% of the graft loss at 10 years (Hertig, et al., 2008). Increased levels of pre-transplant anti-HLA and de novo post-transplant donor specific Abs, as well as CD4+ alloreactive T-cells responding to donor derived peptides have all been correlated with CAN (Birnbaum, et al., 2009). However, the de novo development of Abs directed to donor HLA are not always detectable in the circulation of patients undergoing chronic rejection. Though, this challenges the unequivocal role of alloimmunity in the pathogenesis of chronic rejection, it is likely that monitoring for the Abs done at certain intervals may have missed transient development of Abs which activated the immune processes culminating in chronic rejection.
Role of immune responses to self-antigens in chronic rejection
Several recent studies suggested an important role for autoimmunity in the pathogenesis of allograft rejection (Burke, et al., 2011;Kalache, et al., 2011;Shilling and Wilkes, 2009). Studies from our laboratories in human LTx recipients have shown a strong correlation between the development of Abs to a self protein, K-α1 tubulin (KAT), and development of BOS following human LTx (Goers, et al., 2008). Reports by Wilkes and Burlingham have also provided compelling evidence for autoimmunity to Collagen V (ColV), a sequestrated yet immunologic self protein present in the lung tissue, for the development of chronic lung allograft rejection (Benichou, et al., 1999;Burlingham, et al., 2007;Haque, et al., 2002;Iwata, et al., 2008;Mizobuchi, et al., 2003;Sumpter and Wilkes, 2004;Yoshida, et al., 2006). Tissue remodeling following transplantation can expose cryptic self-antigens or antigenic determinants that can trigger Th-cellular immune response (Tiriveedhi, et al., 2012). Further, lung allografts are uniquely susceptible to injuries from a variety of both endogenous and exogenous agents due to their direct communication with the environment resulting in increased inflammation and tissue repair. Therefore, the findings by Wilkes and Burlingham that autoimmunity to ColV plays an important role in the pathogenesis of chronic lung allograft rejection is significant (Haque, et al., 2002;Yoshida, et al., 2006). Studies have also shown ColV reactive T-cells in rat lung allograft undergoing rejection (Haque, et al., 2002). More important is that ColV specific T-cells derived from rat lung allografts can cause rejection of isografts when adoptively transferred without affecting native lung (Haque, et al., 2002). Our studies have shown high frequency of ColV reactive T-cells in human lung allograft recipients (Bharat, et al., 2006) and BOS was associated with expansion of IFN-γ producing ColV and KAT specific Th-1 cells with a concomitant reduction in IL-10 secreting T-cells (Bharat, et al., 2006;Saini, et al., 2011).
Though there is a compelling role for alloimmune responses in the pathogenesis of chronic rejection, a proportion of the transplant recipients undergoing chronic rejection may not have any detectable HLA Abs (Grossman and Shilling, 2009;Hachem, 2009). In many such cases, Abs against non-HLA antigens has been implicated in the development of chronic rejection. Studies with sera from LTx recipients with BOS where there were no demonstrable Abs to donor HLA lead us to identify Abs against self-antigens, KAT, an airway epithelial surface antigen (Goers, et al., 2008). In addition Abs to ColV, an extracellular matrix protein have also been demonstrated (Iwata, et al., 2008;Saini, et al., 2011). Also significant is our finding that about 50% of BOS+ patients with detectable anti-HLA also developed Abs against KAT (Saini, et al., 2011). The development of Abs to both donor HLA as well as to KAT preceded the clinical diagnosis of BOS (Saini, et al., 2011).
Recently, we demonstrated that binding of anti-KAT to AEC activates a PKC-driven calcium maintenance pathway that is regulated by heat shock proteins (HSP) 27 and 90, culminating in increased growth factor production, cellular mitosis and proliferation (Goers, et al., 2008). Exposure of AECs to sera from BOS+ LTx recipients also resulted in an lipid raft mediated up-regulation in pro-fibrotic growth factors HB-EGF, TGF-β, and VEGF (Tiriveedhi, et al., 2010). Furthermore, using AECs in culture in vitro under normoxic conditions following ligation of cell surface tubulins by its specific Abs caused upregulation of the transcription factor hypoxia inducible factor (HIF-1α) a known mediator of fibrotic cascade (Tiriveedhi et al Cell Immunol 2011-In press). Collectively, these results strongly suggest that binding of anti-KAT to AECs results in up-regulation of proinflammatory response genes and activation of fibro proliferation cascades.
Higher frequency of T-cells specific for KAT as well as ColV have been noted in LTx undergoing chronic rejection (Fukami, et al., 2009;Hachem, 2009). Longitudinal study in LTx patients also demonstrated an association between ColV specific IL-17 responses with onset of BOS (Burlingham, et al., 2007). ColV-specific responses in BOS patients were found to be dependent on both CD4+ T-cells and monocytes and required IL-17, TNF-α and IL-1β. Further, adoptive transfer of lymph node cells expressing high levels of IL-17 and IL-23 gene transcripts from ColV sensitized mice have been shown to induce obliterative lesions in the lung isograft (Burlingham, et al., 2007). These results demonstrate that cell mediated immunity to self-antigens can also lead to chronic rejection.
Experiments using syngenic heart transplants provided initial evidence that chronic rejection can be induced even in the absence of an alloimmune response (Atz and Reed, 2008;Jindra, et al., 2008). T cell mediated autoreactivity against cardiac myosin have been shown to develop and persist in the absence of an alloimmune responses, indicating that response to cardiac myosin, a self-antigen, is associated with the pathogenesis of CAV (Kaczmarek, et al., 2008;Rose and Smith, 2009). Additionally, pre-transplant sensitization against cardiac myosin can result in accelerated rejection of both allo and syngenic cardiac grafts (Rose and Smith, 2009). Studies have also indicated that Abs against vimentin, a cytoskeleton protein, is an independent risk factor for coronary atherosclerosis following cardiac transplantation and may contribute to the accelerated onset of transplant associated CAV (Leong, et al., 2008;Rose and Smith, 2009). Previous studies from our laboratory, demonstrated that CAV patients developed Abs to self-antigens including myosin, vimentin, ColV and KAT (Nath, et al., 2010;Nath, et al., 2011). Furthermore, increased frequency of IL-17 secreting CD4+ T-cells and decreased frequency of IL-10 secreting CD4+ T-cells specific to self-antigens were noted (Nath, et al., 2010). Thus, a role for Th17 mediated immune responses to self-antigens resulting in the breakdown of peripheral tolerance play a central role in the development of CAV following heart transplantation.
Similarly, in kidney allografts, transplant glomerulopathy has an incidence of 20% by 5 years post-transplant (Fotheringham, et al., 2009). A recent multi-center trial involving refractory vascular allograft rejection in the absence of detectable anti-HLA demonstrated the presence of Abs directed at two epitopes of the second extracellular loop of the angiotensin II type 1 (AT1) receptor. Further, it has been suggested that detection of anti-AT1 receptor might be useful to identify those at risk for refractory allograft rejection (Fotheringham, et al., 2009;Li and Yang, 2009). Other Ab targets for kidney allograft rejection in animal and human studies have demonstrated mesangial cell and glomerular basement membrane specific self proteins, Decorin, Agrin, type IV Col and Vimentin in the development of chronic allograft nephropathy (Fotheringham, et al., 2009;Joosten, et al., 2005;Li and Yang, 2009;Paul, et al., 1998).
Do alloimmune responses lead to de novo autoimmune responses?
As discussed above, there is convincing evidence for both alloimmunity as well as autoimmunity to self-antigens in the development of chronic rejection. We will now discuss the possible role of an alloimmunity in the development and perpetuation of autoimmune responses post-transplant which we propose to be an important mediator of chronic rejection of the allograft.
When AECs were stimulated with anti-MHC class I, they underwent proliferation with the secretion of pro-fibrogenic growth factors (Jaramillo, et al., 2003). In addition, we also demonstrated that administration of anti-MHC class I in a murine model of heterotopic tracheal transplantation in immune deficient RAG knockout mice, there was increases in growth factors and pro-apoptotic genes as well as pro-inflammatory cytokines resulting in OAD (Maruyama, et al., 2005). This led to the hypothesis that such a pro-inflammatory cytokine milieu may facilitate the development of immune responses to self-antigens by exposure of cryptic antigens or their determinants as well as lowering the threshold for the activation of T-cells by modulating T regulatory cell populations resulting in the proliferation of self-reactive T-cells.
Using the murine model of anti-MHC class I induced OAD, we were able to establish the role of alloimmunity in the development of autoimmunity (Fukami, et al., 2009). In this model, following administration of anti-MHC class I, there was development of autoimmunity that lead to cellular infiltration, epithelial hyperplasia as well deposition of collagen and fibro-proliferation in the bronchioles and vessels. The mice that were given isotype control Abs, non-specific MHC Abs or anti-keratin did not demonstrate any lesions. More importantly, anti-MHC induced the development of Abs to ColV and KAT. These humoral responses were concomitant with a cellular response to ColV and KAT that secreted IFN-γ, IL4 and IL-17. A critical role of IL-17 in this autoimmune response was also evident since administration of neutralizing Abs to IL-17 not only abrogated obstructive airway lesions, but also significantly reduced both the humoral as well as cellular immune responses to self-antigens.
In a more recent study, we analyzed LTx recipients and monitored them for the development of donor specific antibodies (DSA), Abs to self-antigens (ColV, KAT) and the development of BOS (Saini, et al., 2011). It was demonstrated that in patients who develop BOS, DSA was detectable on an average 3 months following transplantation, which was followed by the development of Abs to self-antigens (about 60 days) (Saini, et al., 2011). It was also demonstrated that Abs to self-antigens continued to persist even when DSA was undetectable in the circulation.
Fedoseyeva et al have also provided evidence for alloimmunity being essential to the development of autoimmunity in a murine heart transplant model (Benichou and Fedoseyeva, 1996). They found that mice that received an allograft across MHC barrier developed T cell responses to cardiac myosin, however, mice that received a syngeneic cardiac graft or those that were recipients of an allogeneic skin graft, did not demonstrate such an immune response to myosin. In this model, sensitization to myosin also leads to the rejection of cardiac allografts. In a recent study on heart transplant recipients we demonstrated that DSA developed within 2.8 months of cardiac transplantation which was followed by the development of Abs to myosin and vimentin at 4–6 months post-transplant (Nath, et al., 2010). These immune responses preceded the diagnosis of chronic rejection suggesting that the immune responses to self-antigens may be pathogenic towards development of chronic rejection.
All of these reports strongly support that alloimmune responses precede autoimmune responses which leads to the pathogenesis of chronic rejection. They also suggest that monitoring for the development of DSA as well as immunity to self-antigens can be important biomarkers to predict the development of chronic rejection following transplantation. Preemptive treatment of patients who developed DSA with IVIG and rituximab have shown lower incidence of BOS (Hachem, et al., 2010). Furthermore, we found that those who cleared DSA as well as Abs to self-antigens following Ab directed treatment with rituximab and intravenous immunoglobulin demonstrated greater freedom from development of BOS as compared to those that did not clear Abs (unpublished results).
Reciprocal role of CD4+ regulatory T-cells (Treg) and Th17 in the autoimmunity
Regulatory T-cells characterized by CD4+ CD25+Foxp3+ expression have been shown to play an important role in maintaining tolerance toward self-antigens, and it has been suggested that a reduction in either their number or function can potentiate autoimmune responses (Sakaguchi and Sakaguchi, 2005). Several reports including from our laboratory have shown that loss of Tregs is associated with the development of chronic rejection (Bharat, et al., 2010;Bhorade, et al., 2011;Cypel, et al., 2009). These Tregs have also been shown to promote the development of IL-10 secreting T-cells that were protective against both development of autoimmunity as well as the development of chronic rejection (Bharat, et al., 2006;Bharat, et al., 2006).
In the post-transplant period, immunosuppression can significantly affect T-cell kinetics. Current immunosuppressive drugs such as cyclosporine and tacrolimus can have profound effect in T-regulatory cell population by its effect on IL2 synthesis and secretion. This although is beneficial in preventing acute rejection can result in the decrease in Treg and its functions (Sakaguchi and Sakaguchi, 2005). Thus, these immunosuppressive drugs albeit prevent acute rejection, may potentially lead to development of autoimmunity due to suppression of Tregs which will lead to chronic rejection.
Viral infections represent another major risk factor for the development of chronic rejection following organ transplantation (Bharat, et al., 2010;Li and Yang, 2009;Potena and Valantine, 2007). We demonstrated that respiratory viral infections following LTx were a significant risk factor for a decline in Treg numbers in the post-transplant period (Bharat, et al., 2010). This viral induced decline in Tregs resulted in an increased prevalence of Abs to ColV (7% vs. 0%) and KAT (31% vs. 12%) when compared to those without viral infection and Treg decline (Bharat, et al., 2010). In a murine model, we demonstrated that murine respiratory virus (Sendai virus) infected AECs had increased expression of Fas-ligand (FasL) (Bharat, et al., 2010). Co-culturing Tregs with these FasL+ epithelial cells resulted in increased apoptosis of T-cells compared to those co-cultured with normal epithelial cells, thus demonstrating that viral infection can lead to apoptosis of Tregs. These results strongly suggest that a decrease in Tregs brought about by immunosuppression and/or viral infections in the post-transplant period may result in the development of an autoimmune response due to the loss of peripheral tolerance to self-antigens.
As discussed above, cellular immune responses to self-antigens are key players in the development of BOS. These responses are predominantly mediated by IL-17 secreting Th17 cells (Burlingham, et al., 2007). Th17 cells that are involved in mucosal immunity contribute to the development of autoimmunity in both animal models and humans. IL-17 is a pro-inflammatory and pro-fibrotic cytokine and thus can facilitate the process of chronic rejection. Experiments discussed above demonstrated that anti-MHC administration lead to immune responses to self-antigens and neutralization of IL-17 can abrogate the development of obliterative airway disease (OAD) (Fukami, et al., 2009). Furthermore, depletion of Treg in this model using FoxP3/DTR transgenic mice can also lead to accentuated OAD lesions and immune responses to self-antigens (Tiriveedhi et al unpublished data). Therefore, Treg mediated suppression of IL17 may play an important role in controlling immune responses to self-antigens and breakdown of this balance can result in the development of immune responses to self-antigens leading to chronic rejection.
Cytokines that have been postulated to influence the differentiation of T-cells into Th17 cells include IL-1β, IL-6, IL-23, TGF-β and IL-21. These cytokines, especially IL-1β, IL-6 and TGF-β, have been shown to be increased in LTx recipients. IL-6 in particular can also direct Tregs to express IL-17 in an antigen specific manner, thus linking the adaptive and the innate immune response to self-antigens. Thus, neutralization of IL-17 as well as IL-6 is an important direction towards future therapy in the prevention of chronic rejection.
Conclusions and future implications
Evidence presented above offer important insights toward development of newer therapeutic strategies to prevent and/or treat chronic rejection which is a major clinical problem following solid organ transplantation. The role for allo- and auto-Abs has been strongly suggested since depletion of these Abs by plasmapheresis both pre- and post-transplant have been shown to significantly decrease the incidence of chronic rejection. Similarly, administration of IVIG that has been successfully used in treating various autoimmune diseases has been shown to decrease the incidence of Ab mediated rejection and may have an effect in reducing the incidence of chronic rejection. Viral infections represent the most preventable risk factor for reducing the incidence of chronic rejection. Therefore, aggressive surveillance for infection along with prophylactic medication in the post-transplant period may help to prevent Treg suppression by viruses that can lead to chronic rejection. Besides this, Treg sparing immunosuppressive agents such as Rapamycin may also be of value in preventing the development of immune responses to self-antigens and thus preventing chronic rejection. Furthermore, agents which will suppress IL-17 and pro-fibrogenic HIF-1α specific chemical inhibitors including YC-1 (3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole) (Yasui, et al., 2008;Yeo, et al., 2003) which is already in anti-cancer therapy, should be tested to determine their value in attenuating autoimmune responses and reducing chronic rejection.
With significant improvements in the survival of allografts, chronic rejection is increasingly recognized as the problem in organ transplantation. Alloimmunity and resulting immune responses to self-antigens during the post-transplant period is emerging to be one of the most important risk factor for the development of chronic allograft rejection. Both these immune responses seem to perpetuate each other with alloimmune responses triggering autoimmune responses which further enhance alloimmune responses, resulting in tissue inflammation and repair processes that eventually results in a pro-inflammatory and pro-fibrogenic state with deposition of fibrous tissue that is hallmark of the chronic rejection. Besides this there is emerging evidence for the role of Th17 cells that contributes to allo and autoimmunity as well as fibrosis. Understanding the role of Tregs in the development of de novo autoimmunity and chronic rejection may also assist in developing new strategies to prevent Treg decline, thereby preserving their function in order to prevent development of immune responses to self-antigens and chronic rejection.
Figure 1.
Acknowledgements
This work was supported by NIH/NHLBI-ARRA Award HL056643, and NIH/NHBLI/NIAID HL092514, and the BJC Foundation (TM). We thank Ms. Billie Glasscock for her support in preparing this manuscript.
Footnotes
Conflicts of interest: None of the authors have any conflicts of interest.
REFERENCES
- Atz ME, Reed EF. Role of anti-MHC class I antibody in facilitating transplant accommodation. Crit Rev Immunol. 2008;28:485. doi: 10.1615/critrevimmunol.v28.i6.20. [DOI] [PubMed] [Google Scholar]
- Benichou G, Fedoseyeva EV. The contribution of peptides to T cell allorecognition and allograft rejection. Int Rev Immunol. 1996;13:231. doi: 10.3109/08830189609061750. [DOI] [PubMed] [Google Scholar]
- Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352. [PubMed] [Google Scholar]
- Bestard O, Nickel P, Cruzado JM, Schoenemann C, Boenisch O, Sefrin A, Grinyo JM, Volk HD, Reinke P. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. 2008;19:1419. doi: 10.1681/ASN.2007050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. Journal of immunology. 2006;177:5631. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90:1637. doi: 10.1016/j.athoracsur.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhorade S, Ahya VN, Baz MA, Valentine VG, Arcasoy SM, Love RB, Seethamraju H, Alex CG, Bag R, Deoliveira NC, Husain A, Vigneswaran WT, Charbeneau J, Krishnan JA, Durazo-Arvizu R, Norwick L, Garrity E. Comparison of sirolimus with azathioprine in a tacrolimus-based immunosuppressive regimen in lung transplantation. Am J Respir Crit Care Med. 2011;183:379. doi: 10.1164/rccm.201005-0775OC. [DOI] [PubMed] [Google Scholar]
- Birnbaum LM, Lipman M, Paraskevas S, Chaudhury P, Tchervenkov J, Baran D, Herrera-Gayol A, Cantarovich M. Management of chronic allograft nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:860. doi: 10.2215/CJN.05271008. [DOI] [PubMed] [Google Scholar]
- Burke GW, 3rd, Vendrame F, Pileggi A, Ciancio G, Reijonen H, Pugliese A. Recurrence of autoimmunity following pancreas transplantation. Curr Diab Rep. 2011;11:413. doi: 10.1007/s11892-011-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. The Journal of clinical investigation. 2007;117:3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, Mattiazzi A, Cordovilla T, Roth D, Kupin W, Rosen A, Esquenazi V, Tzakis AG, Miller J. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80:457. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, Sato M, Medin J, Davidson BL, de Perrot M, Waddell TK, Slutsky AS, Keshavjee S. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1 doi: 10.1126/scitranslmed.3000266. 4ra9. [DOI] [PubMed] [Google Scholar]
- El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- Fotheringham J, Angel CA, McKane W. Transplant glomerulopathy: morphology, associations and mechanism. Nephron Clin Pract. 2009;113:c1. doi: 10.1159/000228069. [DOI] [PubMed] [Google Scholar]
- Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. Journal of immunology. 2009;182:309. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Girnita AL, McCurry KR, Zeevi A. Increased lung allograft failure in patients with HLA-specific antibody. Clin Transpl. 2007:231. [PubMed] [Google Scholar]
- Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad the future. Transl Res. 2009;153:153. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hachem RR. Lung allograft rejection: diagnosis and management. Curr Opin Organ Transplant. 2009;14:477. doi: 10.1097/MOT.0b013e32832fb981. [DOI] [PubMed] [Google Scholar]
- Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- Hauptman PJ, O'Connor KJ. Procurement and allocation of solid organs for transplantation. N Engl J Med. 1997;336:422. doi: 10.1056/NEJM199702063360607. [DOI] [PubMed] [Google Scholar]
- Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3:525. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol. 2008;19:1584. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick P, Rose M. Chronic rejection in the heart. Methods Mol Biol. 2006;333:131. doi: 10.1385/1-59745-049-9:131. [DOI] [PubMed] [Google Scholar]
- Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr., Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, Gopalakrishnan B, Greenspan DS, Christie JD, Wilkes DS. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Human immunology. 2003;64:521. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, Cooper JD, Patterson GA, Mohanakumar T. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, Dang J, Mischel PS, Baldwin WM, 3rd, Fishbein MC, Kupiec-Weglinski JW, Reed EF. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten SA, Sijpkens YW, van Ham V, Trouw LA, van der Vlag J, van den Heuvel B, van Kooten C, Paul LC. Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant. 2005;5:383. doi: 10.1111/j.1600-6143.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, Ueberfuhr P. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6:229. [PubMed] [Google Scholar]
- Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187:1023. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Mahesh BM, Day JR, Smith JD, McCormack AD, Ghimire G, Podor TJ, Rose ML. Vimentin autoantibodies induce platelet activation and formation of platelet-leukocyte conjugates via platelet-activating factor. J Leukoc Biol. 2008;83:263. doi: 10.1189/jlb.0607339. [DOI] [PubMed] [Google Scholar]
- Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nature reviews. Nephrology. 2009;5:513. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- Lietz K, John R, Burke E, Schuster M, Rogers TB, Suciu-Foca N, Mancini D, Itescu S. Immunoglobulin M-to-immunoglobulin G anti-human leukocyte antigen class II antibody switching in cardiac transplant recipients is associated with an increased risk of cellular rejection and coronary artery disease. Circulation. 2005;112:2468. doi: 10.1161/CIRCULATIONAHA.104.485003. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:2126. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:378. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- Mertens V, Dupont L, Sifrim D. Relevance of GERD in lung transplant patients. Curr Gastroenterol Rep. 2010;12:160. doi: 10.1007/s11894-010-0106-3. [DOI] [PubMed] [Google Scholar]
- Millington TM, Madsen JC. Innate immunity and cardiac allograft rejection. Kidney Int Suppl. 2010:S18. doi: 10.1038/ki.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, Jr., Cummings OW, Fujisawa T, Blum JS, Wilkes DS. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171:1140. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: a 30-month analysis in living donor kidney transplantation. Hum Immunol. 2010;71:268. doi: 10.1016/j.humimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Nakajima J, Poindexter NJ, Hillemeyer PB, Trulock EP, Cooper JD, Patterson GA, Mohanakumar T, Sundaresan RS. Cytotoxic T lymphocytes directed against donor HLA class I antigens on airway epithelial cells are present in bronchoalveolar lavage fluid from lung transplant recipients during acute rejection. J Thorac Cardiovasc Surg. 1999;117:565. doi: 10.1016/s0022-5223(99)70336-3. [DOI] [PubMed] [Google Scholar]
- Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29:1277. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010;29:1277. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath DS, Tiriveedhi V, Basha HI, Phelan D, Moazami N, Ewald GA, Mohanakumar T. A role for antibodies to human leukocyte antigens, collagen-V, and K-alpha1-Tubulin in antibody-mediated rejection and cardiac allograft vasculopathy. Transplantation. 2011;91:1036. doi: 10.1097/TP.0b013e318211d2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, Savik K, Reinsmoen NL. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, Snell G, Westall G. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11:2190. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- Paul LC, Muralidharan J, Muzaffar SA, Manting EH, Valentin JF, de Heer E, Kashgarian M. Antibodies against mesangial cells and their secretory products in chronic renal allograft rejection in the rat. Am J Pathol. 1998;152:1209. [PMC free article] [PubMed] [Google Scholar]
- Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Current opinion in infectious diseases. 2007;20:425. doi: 10.1097/QCO.0b013e328259c33b. [DOI] [PubMed] [Google Scholar]
- Reznik SI, Jaramillo A, SivaSai KS, Womer KL, Sayegh MH, Trulock EP, Patterson GA, Mohanakumar T. Indirect allorecognition of mismatched donor HLA class II peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Am J Transplant. 2001;1:228. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]
- Reznik SI, Jaramillo A, SivaSai KSR, Womer KL, Sayegh MH, Trulock EP, Patterson GA, Mohanakumar T. Indirect allorecognition of mismatched donor HLA class II peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Am J Transpl. 2001;1:228. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Sundaresan S, Lynch J, Trulock EP, Cooper J, Patterson GA, Mohanakumar T. Increased concentration of soluble human leukocyte antigen class I levels in the bronchoalveolar lavage of human pulmonary allografts. J Heart Lung Transplant. 1997;16:1135. [PubMed] [Google Scholar]
- Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- Rose ML, Smith JD. Clinical relevance of complement-fixing antibodies in cardiac transplantation. Hum Immunol. 2009;70:605. doi: 10.1016/j.humimm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, Meyers B, Patterson GA, Mohanakumar T. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol. 2005;24:211. doi: 10.1080/08830180590934976. [DOI] [PubMed] [Google Scholar]
- Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele DJ, Laufer TM, Smiley ST, Ando Y, Grusby MJ, Glimcher LH, Auchincloss H., Jr. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- Tiriveedhi V, Angaswamy N, Brand D, Weber J, Gelman AG, Hachem R, Trulock EP, Meyers B, Patterson G, Mohanakumar T. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol. 2012;167:158. doi: 10.1111/j.1365-2249.2011.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochem Biophys Res Commun. 2010;399:251. doi: 10.1016/j.bbrc.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriveedhi V, Weber J, Seetharam A, Mohanakumar T. Cross-talk of alloimmune response and autoimmunity: role in pathogenesis of chronic rejection. Discov Med. 2010;9:229. [PubMed] [Google Scholar]
- Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, Wasowska BA, Baldwin WM, 3rd, Pober JS, Lowenstein CJ. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007;104:1301. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Ogura A, Asanuma T, Matsuda A, Kashiwakura I, Kuwabara M, Inanami O. Inhibition of HIF-1alpha by the anticancer drug TAS106 enhances X-ray-induced apoptosis in vitro and in vivo. Br J Cancer. 2008;99:1442. doi: 10.1038/sj.bjc.6604720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]