Abstract
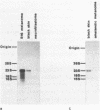
We have isolated a pigment cell-specific cDNA clone from a B16 mouse melanoma cDNA library by differential hybridization. The mRNA of isolated cDNA is highly expressed in B16 melanoma cells and in black mouse (C57BL/6) skin, but is not detectable in mouse neuroblastoma cells nor in K1735 mouse amelanotic melanoma cells. The protein sequence deduced from the nucleotide sequence of the cloned cDNA shows significant similarity to the entire region of Neurospora tyrosinase. To know the identity of cDNA, we transfected K1735 amelanotic melanoma and COS-7 cells with the cDNA carried in a simian virus 40 vector (pKCRH2). We confirmed that the isolated cDNA encodes mouse tyrosinase by immunofluorescence staining of transfected cells using two different anti-T4-tyrosinase monoclonal antibodies. Tyrosinase is composed of 513 amino acids with a molecular weight of 57,872 excluding a hydrophobic signal peptide of 24 amino acids.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett J. B. The tyrosinases of mouse melanoma. Isolation and molecular properties. J Biol Chem. 1971 May 25;246(10):3079–3091. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Putnam F. W. Internal duplication and evolution of human ceruloplasmin. Proc Natl Acad Sci U S A. 1981 May;78(5):2805–2809. doi: 10.1073/pnas.78.5.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Gruys E., Cifone M. A., Barnes Z., Bucana C. Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. J Natl Cancer Inst. 1981 Oct;67(4):947–956. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HOCHSTEIN P., COHEN G. The cytotoxicity of melanin precursors. Ann N Y Acad Sci. 1963 Feb 15;100:876–886. [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M., Montague P. M. Mammalian tyrosinase: isozymic forms of the enzyme. Int J Biochem. 1981;13(1):99–103. doi: 10.1016/0020-711x(81)90141-5. [DOI] [PubMed] [Google Scholar]
- Herrmann W. P., Uhlenbruck G. Serological studies on the carbohydrate moiety of human tyrosinase. Arch Dermatol Res. 1975 Dec 31;254(3):275–280. doi: 10.1007/BF00557969. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke M. L. Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst. 1979 Sep;63(3):541–548. doi: 10.1093/jnci/63.3.541. [DOI] [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- Körner A., Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982 Sep 17;217(4565):1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., FITZPATRICK T. B. Biochemistry of melanin formation. Physiol Rev. 1950 Jan;30(1):91–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., FITZPATRICK T. B., CALKINS E., SUMMERSON W. H. Mammalian tyrosinase; the relationship of copper to enzymatic activity. J Biol Chem. 1950 Dec;187(2):793–802. [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: molecular weight, copper content and spectral properties. FEBS Lett. 1976 Oct 15;69(1):157–160. doi: 10.1016/0014-5793(76)80675-8. [DOI] [PubMed] [Google Scholar]
- Lerch K. Primary structure of tyrosinase from Neurospora crassa. II. Complete amino acid sequence and chemical structure of a tripeptide containing an unusual thioether. J Biol Chem. 1982 Jun 10;257(11):6414–6419. [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984 Feb 16;307(5952):604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Otaki N. Tyrosinase as glycoprotein. Arch Dermatol Forsch. 1975;252(3):211–216. doi: 10.1007/BF00557921. [DOI] [PubMed] [Google Scholar]
- Nishioka K. Particulate tyrosinase of human malignant melanoma. Solubilization, purification following trypsin treatment, and characterization. Eur J Biochem. 1978 Apr;85(1):137–146. doi: 10.1111/j.1432-1033.1978.tb12221.x. [DOI] [PubMed] [Google Scholar]
- Ohkura T., Yamashita K., Mishima Y., Kobata A. Purification of hamster melanoma tyrosinases and structural studies of their asparagine-linked sugar chains. Arch Biochem Biophys. 1984 Nov 15;235(1):63–77. doi: 10.1016/0003-9861(84)90255-8. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J. M. Factors regulating growth and pigmentation of melanoma cells. J Invest Dermatol. 1976 Apr;66(4):201–209. doi: 10.1111/1523-1747.ep12482134. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M., Lerner A. B. 5,6-Dihydroxyindole is a melanin precursor showing potent cytotoxicity. Nature. 1978 Dec 7;276(5688):626–628. doi: 10.1038/276627a0. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Körner A., Bergstrom A., Bologna J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature. 1980 Aug 7;286(5773):617–619. doi: 10.1038/286617a0. [DOI] [PubMed] [Google Scholar]
- Pfiffner E., Lerch K. Histidine at the active site of Neurospora tyrosinase. Biochemistry. 1981 Oct 13;20(21):6029–6035. doi: 10.1021/bi00524a017. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- SEIJI M., SHIMAO K., BIRBECK M. S., FITZPATRICK T. B. Subcellular localization of melanin biosynthesis. Ann N Y Acad Sci. 1963 Feb 15;100:497–533. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Hariu A., Kato C., Seiji M. Transfer of tyrosinase to melanosomes in Harding-Passey mouse melanoma. Arch Biochem Biophys. 1983 Aug;225(1):75–85. doi: 10.1016/0003-9861(83)90008-5. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Montague P. M., Hearing V. J. Anti-T4-tyrosinase monoclonal antibodies--specific markers for pigmented melanocytes. J Invest Dermatol. 1985 Nov;85(5):426–430. doi: 10.1111/1523-1747.ep12277121. [DOI] [PubMed] [Google Scholar]
- Wick M. M. An experimental approach to the chemotherapy of melanoma. J Invest Dermatol. 1980 Feb;74(2):63–65. doi: 10.1111/1523-1747.ep12519812. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]