Abstract
Objectives
To examine the association between baseline beta-blocker (BB) dose and outcomes in the HF-ACTION trial.
Background
BBs reduce morbidity and mortality in chronic heart failure (HF) patients with reduced ejection fraction, but it is unclear whether titrating to higher BB doses improves outcomes in this setting.
Methods
HF-ACTION was a randomized, multicenter trial enrolling 2331 ambulatory HF patients with systolic dysfunction (New York Heart Association class II–IV, left ventricular ejection fraction <0.35) randomized to exercise training vs. usual care, with median follow-up of 2.5 years. BB dose at baseline was standardized using carvedilol equivalents and analyzed as a continuous variable and by discrete dose groups. The relationship between BB dose and the primary endpoint of all-cause mortality or all-cause hospitalization, and other cardiovascular secondary endpoints, was determined before and after adjustment for variables significantly associated with outcomes in the HF-ACTION cohort.
Results
95% of patients were on a BB. There was a significant inverse relationship between BB dose and all-cause death or hospitalization but not other cardiovascular endpoints after adjustment for other predictors of outcome, with a linear benefit up to the 50 mg daily dose. There was a significant inverse association between BB dose and change in peak VO2 at 3 months. There was no increase in bradycardia with higher doses of BB.
Conclusion
There was a significant inverse relationship between BB dose and the endpoint of all-cause death or all-cause hospitalization in this well-treated HF cohort with systolic dysfunction, supporting recommendations that titrating doses up to 50 mg per day may confer a benefit in such patients.
Clinical Trial Registration
(HF-ACTION) ClinicalTrials.gov, identifier NCT00047437
Keywords: beta-blockers, heart failure, dose, mortality, exercise
Introduction
Beta-blockers (BBs) are an important pharmacologic therapy and reduce morbidity/mortality in patients with heart failure (HF) due to a reduced left ventricular ejection fraction (LVEF) (1). Guidelines recommend using BB therapy to treat outpatients with HF at doses consistent with those studied in randomized, controlled trials. There is little evidence, however, that clinical trial BB doses are being used in clinical practice (2). Furthermore, it is unclear whether there is a dose-response relationship between BBs and outcomes. In the only study prospectively designed to test dose-response relationships with the BB carvedilol in patients with systolic HF, Bristow et al reported dose-related improvements in LVEF and survival (3). However, this study was limited by a small sample size and a low number of deaths, making it difficult to interpret the association with survival. In a post-hoc subgroup analysis of the MERIT-HF trial, no dose-response relationship with mortality was observed for metoprolol CR/XL in the overall cohort, but a wide variation in dose-response existed between patients (4). Heart rate reduction was similar across 3 dose groups, indicating the degree of beta-blockade may have been equivalent and thus limiting the ability to test a true association between dose and mortality benefit, since previous studies have demonstrated that the degree of heart rate reduction may be related to outcome (5–7).
The Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial was the largest trial to date to test the effects of exercise training vs. usual care in HF patients with moderate-to-severe left ventricular (LV) systolic dysfunction. In HF-ACTION, approximately 95% of the 2331 patients received a BB, providing a large, well-treated contemporary HF population in which to explore relationships between BB dose and outcomes. We aimed to examine the relationship between baseline BB dose and outcomes in the HF-ACTION study population, hypothesizing that patients on higher doses may experience improved outcomes. In addition, we aimed to examine whether higher doses of BBs were associated with an increase in bradycardia and a decrease in other adverse cardiovascular events such as stroke and myocardial infarction (MI).
Methods
The HF-ACTION trial design and outcomes have been described (8,9). Briefly, the study was a multicenter, randomized, controlled trial testing the long-term safety and efficacy of aerobic exercise training plus evidence-based medical therapy vs. evidence-based medical therapy alone in medically stable outpatients with LV systolic dysfunction (LVEF <35%) and New York Heart Association (NYHA) class II–IV HF. Adult patients receiving angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers and beta-adrenergic blockade for ≥6 weeks (unless there was a documented rationale for variation) were eligible. Investigators were provided with the following instructions in the operations manual regarding use of evidence-based BB therapy and dose titration: patients must be on optimal heart failure therapy according to American Heart Association/American College of Cardiology and Heart Failure Society of America guidelines, or have documented rationale for variation, and be on stable doses for 6 weeks prior to enrollment. There was no specific forced titration strategy used, but there was education and reinforcement to achieve evidence-based levels of target doses based on clinical trials. The primary endpoint was the composite of all-cause death or all-cause hospitalization. Patients were randomly assigned to usual care alone or usual care plus exercise training, consisting of a prescription of supervised aerobic exercise training at 60%–70% of heart rate reserve 3 times per week, followed by home-based training at the same intensity 5 times per week, totaling 36 sessions. Randomization was stratified by center and HF etiology. Participants were followed for a median of 2.5 years.
Data Considerations and Outcome Measures
Patient characteristics, health statuses, laboratory values, and physiologic parameters at rest and during a cardiopulmonary exercise (CPX) test were collected on standardized forms at baseline and at several points throughout the study (laboratory values only at baseline). Beta-blocker dose at baseline was standardized using carvedilol equivalents and analyzed as a continuous variable and by discrete dose groups (0, 1–13, 14–25, 26–50, 51–200 mg daily). Dosing groups were selected based on the common titration schedule for carvedilol (i.e., doubling of the dose every 2–4 weeks up to target doses recommended by guidelines) (1).
The composite primary endpoint of all-cause mortality and all-cause hospitalization, and an endpoint of mortality alone, were determined and adjusted using variables found to be significantly associated with outcomes (10). Other pre-specified secondary endpoints included cardiovascular mortality or cardiovascular hospitalization, cardiovascular mortality alone, and cardiovascular mortality or HF hospitalization. Adverse cardiovascular events were collected throughout the study and included fatal or non-fatal HF hospitalization, MI, unstable angina pectoris, arrhythmia, bradycardia, stroke, or transient ischemic attack. Bradycardia was defined as symptomatic bradycardia with a heart rate <50 beats per minute. All events were adjudicated by a blinded clinical events committee. Exercise and functional parameters of a 6-minute walk test, exercise time on a CPX test, peak oxygen uptake (VO2), and heart rate at peak exercise were also examined.
Statistical Methods
Baseline characteristics were summarized by counts and percentages for categorical variables and by medians with interquartile ranges for continuous variables. The unadjusted relationship between BB dose at baseline and the primary endpoint was explored using piecewise regression models. The linear and piecewise linear models were compared with the null model using likelihood ratio tests. For the primary endpoint (all-cause death or hospitalization) and secondary endpoint (all-cause death), predictive models were developed using a broad range of candidate variables, including demographics, medical history, laboratory values, exercise test values, and quality of life indices (Kansas City Cardiomyopathy Questionnaire). These models provide a useful tool for estimating the risk of the given endpoint for specific patients and were used for adjustment in this analysis. Cox proportional hazards modeling was used to assess the relationship between outcomes and BB dose as a continuous variable, before and after adjustment for the variables found to be significantly associated with each endpoint. A p-value ≤0.05 was considered statistically significant for all analyses. The relationship between BB dose as a continuous variable and exercise parameters at 3, 12, and 24 months was analyzed using linear regression models that included the exercise parameters during follow-up as the response variables and the BB dose along with potential confounders as independent variables. Inverse probability weighting was used to adjust for missingness of the exercise parameters during follow-up. The exercise variables were transformed to achieve normality when needed. Statistical analysis was performed by the Duke Clinical Research Institute using SAS software version 9.2 (Cary, NC).
Results
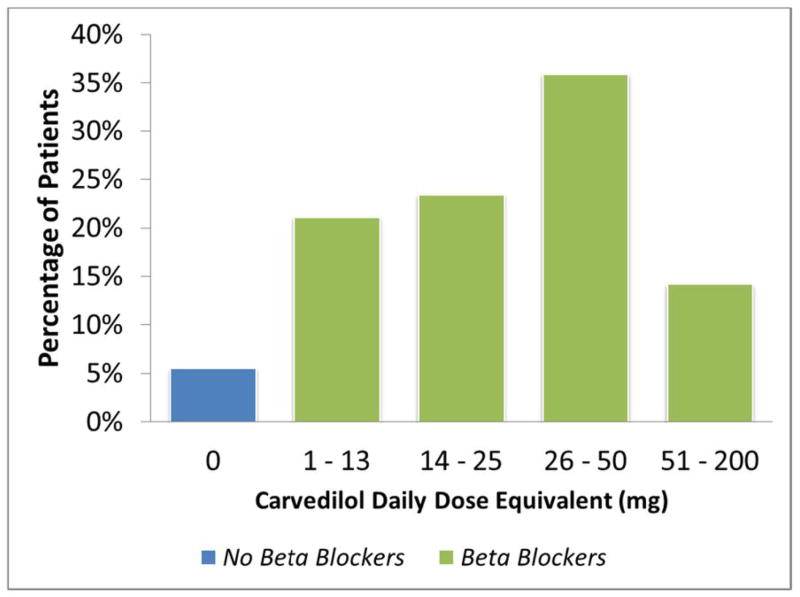
Of the 2331 patients enrolled in HF-ACTION, 2325 patients were included in this analysis; 6 patients with missing information at baseline were excluded. Only 128 (5.5%) patients were not on a BB. There was a broad distribution of doses in this cohort. Excluding those patients not receiving BB at randomization, the median BB dose was 38 mg carvedilol-equivalents daily (1st quartile = 25 mg, 3rd quartile = 50 mg). The most common dosing range was 26–50 mg daily (36%) followed by 14–25 mg daily (23%) (Figure 1). In total, 73% of patients did not change BB dose groups during the study, 6% died, and 15% had missing data; therefore, only 6% of patients changed dose groups by the last assessment.
Figure 1. Distribution of Doses.
Percentage of patients in each dose group (0 dose or no beta-blocker: 5.5%; 1–13 mg dose: 21%; 14–25 mg dose: 23%; 26–50 mg dose: 36%; 51–200 mg dose: 14%)
Baseline characteristics are shown in Table 1. Patients not on a BB were older and more often white, whereas blacks were more often in the highest dose group. There was no difference in dose distribution based on sex. Those not on a BB more often had a higher blood urea nitrogen level. Not surprisingly, patients in the highest BB dose group had a higher body mass index and were younger. There was no difference between the dose groups in baseline exercise parameters. There was a significant reduction in maximal heart rate at peak exercise with increasing doses, indicating the degree of beta-blockade was indeed greater in the higher dose groups.
Table 1.
Baseline Characteristics by Beta-Blocker Use at Randomization [n = 2325]
| Characteristics | Beta-Blocker Dose, mg
|
|||||
|---|---|---|---|---|---|---|
| No BBs (n = 128) | 1–13 (n = 490) | 14–25 (n = 544) | 26–50 (n = 834) | 51–200 (n = 329) | P Value | |
| Age, y | 64 | 62 | 60 | 58 | 57 | <0.0001 |
|
| ||||||
| Female sex, % | 27 | 32 | 27 | 29 | 24 | 0.11 |
|
| ||||||
| Black (n = 2290), % | 27 | 30 | 30 | 33 | 44 | — |
|
| ||||||
| BMI (n = 2318) | 28 | 29 | 29 | 31 | 33 | <0.0001 |
|
| ||||||
| NYHA HF class, % | ||||||
| II | 56 | 59 | 65 | 65 | 68 | — |
| III/IV | 44 | 41 | 35 | 35 | 32 | — |
|
| ||||||
| HF etiology, ischemic, % | 56 | 56 | 57 | 46 | 46 | <0.0001 |
|
| ||||||
| SBP (n = 2321), mmHg | 114 | 110 | 112 | 112 | 112 | 0.07 |
|
| ||||||
| LVEF (n = 2321), % | 23 | 24 | 25 | 25 | 24 | 0.01 |
|
| ||||||
| History of diabetes, % | 5 | 19 | 21 | 37 | 18 | 0.0006 |
|
| ||||||
| BUN (n = 2022), mg/dL | 23 | 21 | 20 | 20 | 19 | 0.002 |
|
| ||||||
| Sinus rhythm (n = 2287), % | 76 | 79 | 81 | 81 | 83 | 0.41 |
|
| ||||||
| HR at peak exercise (median)(n = 2323), bpm | 126 | 122 | 120 | 118 | 117 | 0.0001 |
|
| ||||||
| CPX duration (n = 2303), min | 9.0 | 9.3 | 9.7 | 10.0 | 9.2 | 0.05 |
|
| ||||||
| 6MWT distance (n = 2274), m | 351 | 366 | 372 | 375 | 374 | 0.37 |
|
| ||||||
| Peak VO2 (n = 2269), mL/kg/min | 14.6 | 14.0 | 14.7 | 14.5 | 14.3 | 0.46 |
BB = beta-blocker; BMI = body mass index; BUN = blood urea nitrogen; CPX = cardiopulmonary exercise; SBP = systolic blood pressure; HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; 6MWT = 6-minute walk test; peak VO2 = peak oxygen uptake.
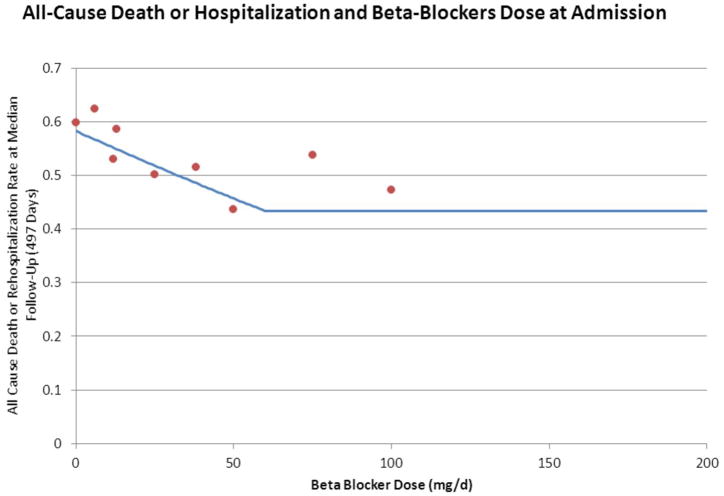
The relationship between all-cause death/hospitalization and dose is shown in Figure 2, and the clinical outcomes are shown in Table 2. The median follow-up was 2.5 years. A piecewise linear fit was used to model the relationship between the BB dose and the primary endpoint. When compared with a linear relationship, the piecewise linear model provided a better fit (likelihood ratio statistic = 25.8, p<0.0001). The same relationship was used for all-cause death. After adjusting for the variables found to be significantly associated with the primary endpoint of all-cause death or hospitalization, higher BB dose remained significantly associated with a lower rate of the primary endpoint (hazard ratio, 0.96; p=0.02). Although there was a significant inverse relationship between BB dose and all-cause death alone in a univariate model (p=0.004), it became non-significant after adjusting for confounding variables (p=0.65). This relationship remained significant when adjusted for sex, body mass index, loop diuretic dose, serum creatinine, and Canadian Cardiovascular Society angina class, and became non-significant after adjustment for CPX testing duration. Although each of the other cardiovascular endpoints showed a similar significant inverse relationship with BB dose in a univariate model, these also became non-significant when adjusted for other clinical variables (data not shown).
Figure 2. All-Cause Death or Hospitalization by Beta-Blocker Dose at Baseline.
Piecewise linear fit statistically significant. All-cause death or hospitalization by beta-blocker dose at baseline, showing a linear relationship up to 50 mg daily dose. Dots represent event rate at most common doses based on distribution of doses.
Table 2.
Outcomes and Beta-Blocker Dose at Randomization
| Chi-Square | P Value | HR (95% CI) [for 10 units increase in BB dose*] | |
|---|---|---|---|
| All-cause death or all-cause hospitalization (1430 events, 67%) | |||
| Unadjusted | 26.11 | <0.0001 | 0.93 (0.91–0.96) |
| Adjusted† | 5.43 | 0.02 | 0.96 (0.93–0.99) |
|
| |||
| All-cause death (307 events, 15%) | |||
| Unadjusted | 8.27 | 0.004 | 0.92 (0.88–0.98) |
| Adjusted‡ | 0.86 | 0.35 | 0.97 (0.92–1.03) |
Hazard ratios are applicable for doses up to 50 mg/d. For dose >50 mg/d, hazard ratio = 1.
Adjusting by Kansas City Cardiomyopathy Questionnaire symptom stability, left ventricular ejection fraction, region, sex, ventricular conduction, Weber class, blood urea nitrogen, and mitral regurgitation.
Adjusting by sex, body mass index, loop diuretics, Canadian Cardiovascular Society angina class, creatinine, exercise duration, ventricular conduction, and left ventricular ejection fraction.
BB = beta-blocker; CI = confidence interval; HR = hazard ratio.
The relationship between BB dose and exercise parameters (change in CPX duration, 6-minute walk test, and peak VO2 at 3, 12, and 24 months) was not significant when adjusted for baseline clinical variables, except for change in peak VO2 at 3 months. Those in the highest BB dose group had the greatest increase in peak VO2 at 3 months (3.9%; p=0.048), but the relationship between these variables was not significant at 12 or 24 months. No relationship was observed between changes in CPX duration or 6-minute walk distance and the dose of BB at 3, 12, or 24 months. The relationships between BB dose and cardiovascular adverse events are shown in Table 3. Patients who were not on a BB had the highest number of cardiovascular events, and those in the 26–50 mg daily dose group seemed to have the lowest cardiovascular adverse event rate. There was no increase in the incidence of bradycardia with increasing doses of BB.
Table 3.
Cardiovascular Adverse Events by Beta-Blocker Use at Randomization
| Event type, % | Beta-Blocker Dose, mg
|
|||||
|---|---|---|---|---|---|---|
| No BBs (n = 128) | 1–13 (n = 490) | 14–25 (n = 544) | 26–50 (n = 834) | 51–200 (n = 329) | P Value | |
| Cardiovascular | 45 | 43 | 41 | 33 | 43 | 0.0001 |
|
| ||||||
| Heart failure | 34 | 32 | 29 | 22 | 30 | 0.0001 |
|
| ||||||
| Myocardial infarction | 4 | 3 | 5 | 4 | 3 | 0.59 |
|
| ||||||
| Unstable angina | 7 | 7 | 10 | 6 | 7 | 0.15 |
|
| ||||||
| Arrhythmia | 18 | 16 | 13 | 13 | 14 | 0.56 |
|
| ||||||
| Bradycardia | 3 | 2 | 1 | 2 | 1 | 0.35 |
|
| ||||||
| Stroke | 2 | 2 | 3 | 2 | 5 | 0.09 |
|
| ||||||
| No. cardiovascular events | 0.004 | |||||
| 0 | 56 | 57 | 59 | 68 | 57 | |
| 1 | 13 | 16 | 18 | 13 | 19 | |
| 2 | 12 | 10 | 8 | 7 | 9 | |
| 3 or more | 20 | 17 | 16 | 13 | 15 | |
Discussion
There were several important findings from this study. First, there was a significant relationship between BB dose and the adjusted risk for the primary outcome of all-cause death or hospitalization; however, no significant relationship between BB dose and secondary outcomes was observed after adjustment for other clinical variables. Second, there was a significant relationship between BB dose and change in peak VO2 at 3 months, but not for other exercise or functional parameters. In addition, our study showed no increased incidence of bradycardia on higher doses of BB in this cohort; rather there were more cardiovascular and HF events in patients not on a BB compared with those on moderate-to-high doses even after adjustment for other key prognostic variables. Finally, approximately 50% of patients were not on target doses of BBs.
The relationship between higher doses of BB and an improvement in the adjusted risk for the primary composite endpoint of all-cause death or hospitalization appeared to be linear up to the highest dose group. Although guidelines recommend moderate-to-high doses of BB therapy, many patients are not titrated to doses demonstrated to be advantageous in large, multisite, randomized, controlled clinical trials and registries (11). Data from the OPTIMIZE-HF registry showed, in patients hospitalized for HF, that the mean daily dose of BBs before hospital admission was half the recommended target dose, and most patients were not titrated to target doses up to 90 days post-discharge. In fact, at 60 and 90 days post-discharge, only 17.5% and 7.9% of patients were receiving target doses of carvedilol and metoprolol, respectively (11). In part, this reluctance toward titration to evidence-based doses may be based on lack of definitive evidence that there is a dose-response relationship between BB therapy and clinical outcomes; health system barriers that prevent easy titration of the medications to target doses; and a concern about an increase in adverse events, particularly in those who are older and have significant comorbidities (12–14).
Recently, the CIBIS-ELD study evaluated the tolerability of bisoprolol and carvedilol in elderly patients; only 31% of patients were able to reach target doses (15). However, other studies have shown good BB tolerability in this patient population (16). The findings of CIBIS-ELD were more likely due to an aggressive titration schedule; this supports the European Society of Cardiology guidelines’ titration scheme, which allows for a slower titration to achieve dose targets (17).
The evidence for a dose-response relationship for cardiovascular drugs has been limited. Two studies have shown a benefit between higher doses of ACE inhibitors or angiotensin receptor blockers and clinical outcome (18,19); these showed an advantage on a composite endpoint of HF hospitalization and death but not on death alone. Very little evidence exists regarding BB dose and outcomes in HF patients. The REVERT trial examined the effects of BB therapy on LV remodeling in asymptomatic patients, and it showed that the benefits on LV end-systolic volume index and LVEF were dose-dependent (20). As previously mentioned, the MOCHA study demonstrated a positive dose-response relationship between BB dose and LVEF improvement as well as an improvement in survival (3). However, given that this was a study of only 300 patients with only 30 deaths, the findings regarding survival are difficult to interpret. A meta-analysis of BB dose and clinical outcome in HF patients found no significant relationship between all-cause mortality and BB dose (21); however, there were important limitations, as with any meta-analysis. In our study, all-cause mortality did not show a dose-related benefit with BB therapy when adjusted for other clinical variables, in particular when CPX duration was added to the model. This finding is not surprising, given that the strongest baseline predictor of both the primary and mortality endpoints in the HF-ACTION predictive model was exercise duration on CPX (10). Yet the primary endpoint of all-cause death/hospitalization remained significant even after adjustment for CPX duration, indicating this relationship was uniquely important in this cohort. There remains no definitive evidence of a dose-response relationship between BB therapy and outcomes in a randomized, controlled trial. In the absence of randomized testing, the fact that an association exists may indicate the effect could be more or less pronounced than our results demonstrate.
The clinical improvement found with higher doses was consistent with the improvement in exercise duration at 3 months with higher doses of beta-blockade. This finding is noteworthy as phase II studies of BB therapy have failed to demonstrate improvement in maximal exercise tolerance, peak VO2, or 6-minute walk with BB therapy vs. placebo but have shown favorable trends on submaximal exercise (22–24). Certainly one of the concerns about BB therapy is potential impairment in exercise performance at higher BB doses. Our finding that there is no attenuation of exercise performance with higher doses of BBs should allow clinicians to feel more comfortable with BB titration, particularly in those patients who have important reductions in baseline exercise capacity, as seen in the HF-ACTION study. Furthermore, our study showed no increased incidence of bradycardia with higher BB doses, supporting the concept that patients may receive more benefit than harm with moderate-to-higher doses. This is particularly reassuring if adverse events are a concern preventing dose titration. We found approximately half the patients were not at target doses. These findings suggest that there is considerable room for BB up-titration in clinical practice.
Interestingly, there appeared to be racial differences in the baseline dose of BB therapy. Black patients were more often receiving a higher baseline dose of BB than were whites or others. It is unclear whether this finding was related to the need to have relatively higher doses of BB therapy to achieve similar efficacy. There was no statistical interaction between race and BB dose. This is an interesting finding that requires further analysis to better understand the differences between BB dosing and outcomes in black patients.
Limitations
Our findings should be interpreted in the context of several potentially important limitations. First, this is a post-hoc analysis. Although this study population is broad, patients who were not ambulatory and patients with preserved systolic function were excluded. This may have limited the number of elderly patients enrolled and conferred a generally younger population with an average age of 58 years, compared with the BB clinical trials in which the average age was roughly 62 years. On the other hand, this study includes a relatively large cohort of women and blacks. Second, the use of dose conversions is an imperfect method for comparing doses. In this cohort, the median patient weight was 90 kg (1st quartile = 76 kg, 3rd quartile = 106 kg). Guideline dosing recommendations suggest that target carvedilol doses for patients >75 kg is 100 mg a day, relative to 200 mg a day for metoprolol. This would convert to a 2:1 dosing ratio with metoprolol. However, doses used in heart failure clinical trials may suggest a 4:1 conversion ratio. We therefore conducted a sensitivity analysis using both methods; there was no difference in heart rate reduction throughout the distribution of doses using a 4:1 conversion compared with the 2:1 conversion formula. There was also no difference in the clinical endpoint results. Finally, an important potential confounder is that sicker patients may be less able to tolerate higher BB doses. Although we adjusted for numerous known predictors of adverse outcome, the possibility of important unidentified prognostic indicators must be considered. A true dose-response relationship should be assessed in a randomized clinical trial.
Conclusions
Higher BB dose in ambulatory HF patients with reduced ejection fraction was associated with a significantly lower rate of all-cause hospitalization and all-cause death, even after adjustment for important prognostic covariates. Patients with a higher baseline BB dose also demonstrated longer exercise duration and higher peak VO2. Finally, higher doses were not associated with increased incidence of bradycardia, with the best cardiovascular event profile in patients at target doses. These data support the current clinical guideline recommendations that BB therapy should be titrated to moderate-to-high doses as used in randomized, controlled clinical trials.
Supplementary Material
Acknowledgments
Funding Source: The HF-ACTION trial was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute, Bethesda, MD.
The authors wish to thank Drs. Kerry Lee, Stephen Ellis, and Karen Chiswell for their thoughtful suggestions and statistical consultation and Morgan deBlecourt for her editorial assistance with the manuscript.
Abbreviations
- ACE
angiotensin-converting enzyme
- BB
beta-blocker
- CPX
cardiopulmonary exercise
- HF
heart failure
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NYHA
New York Heart Association Peak
- VO2
peak oxygen uptake
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11:200–205. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA investigators. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 4.Wikstrand J, Hjalmarson A, Waagstein F, et al. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the Experience in Metoprolol CR/XL Randomized Intervention Trial in Chronic Heart Failure (MERIT-HF) J Am Coll Cardiol. 2002;40:491–498. doi: 10.1016/s0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 5.Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–1433. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 6.Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005;26:2259–2268. doi: 10.1093/eurheartj/ehi386. [DOI] [PubMed] [Google Scholar]
- 7.Gullestad L, Wikstrand J, Deedwania P, et al. What resting heart rate should one aim for when treating patients with heart failure with a beta-blocker? Experiences from the Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT-HF) J Am Coll Cardiol. 2005;45:252–259. doi: 10.1016/j.jacc.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Whellan DJ, O’Connor CM, Lee KL, et al. Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION): Design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2011;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonarow GC, Abraham WT, Albert NM, et al. Dosing of beta-blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Am J Cardiol. 2008;102:1524–1529. doi: 10.1016/j.amjcard.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Fonarow GC, Albert NM, et al. Influence of patient age and sex on delivery of guideline-recommended heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Am Heart J. 2009;157:754–762. e752. doi: 10.1016/j.ahj.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030. e1023. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Yancy CW, Albert NM, et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail. 2008;1:98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 15.Dungen HD, Apostolovic S, Inkrot S, et al. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail. 2011;13:670–680. doi: 10.1093/eurjhf/hfr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krum H, Hill J, Fruhwald F, et al. Tolerability of beta-blockers in elderly patients with chronic heart failure: the COLA II study. Eur J Heart Fail. 2006;8:302–307. doi: 10.1016/j.ejheart.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 18.Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Atlas study group. Circulation. 1999;100:2312–2318. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 19.Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 20.Colucci WS, Kolias TJ, Adams KF, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: The REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation. 2007;116:49–56. doi: 10.1161/CIRCULATIONAHA.106.666016. [DOI] [PubMed] [Google Scholar]
- 21.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Nardi M, Giubbini R, Dei Cas L. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24:1678–1687. doi: 10.1016/0735-1097(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 23.Pollock SG, Lystash J, Tedesco C, Craddock G, Smucker ML. Usefulness of bucindolol in congestive heart failure. Am J Cardiol. 1990;66:603–607. doi: 10.1016/0002-9149(90)90488-m. [DOI] [PubMed] [Google Scholar]
- 24.Krum H, Sackner-Bernstein JD, Goldsmith RL, et al. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995;92:1499–1506. doi: 10.1161/01.cir.92.6.1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.