Abstract
This work demonstrates the first whole brain “high spatial resolution” 7Li MRSI in bipolar disorder subjects. The in vivo quantification is validated by a phantom containing 5 mM lithium salt using the identical RF sequence and imaging protocol. This study is the first demonstration of the 7Li distribution in the brain of bipolar disorder patients on lithium therapy using a 3D MRSI approach. The results show that brain lithium level is strongly correlated with serum lithium concentration. The brain-to-serum lithium ratio for the average brain and the local maximum were 0.39 ± 0.08 (r = 0.93) and 0.92 ± 0.16 (r= 0.90), respectively. The lithium distribution is found to be non-uniform throughout the brain for all patients, which is somewhat unexpected and highly intriguing. This uneven distribution is more evident in subjects at a higher therapeutic serum lithium level. This finding may suggest that lithium targets specific brain tissues and/or certain enzymatic and macromolecular sites that are associated with therapeutic effect. Further investigations of bipolar disorder patients on lithium therapy using 3D 7Li MRSI are warranted.
Keywords: Lithium, MRSI, Bipolar disorder, Fourier transform
Introduction
Lithium is a widely used first-line treatment for bipolar disorder; however, its mechanism of therapeutic action is largely unknown [1]. It is reasonable to believe that the lithium level and its spatial distribution in the brain play an important role in successful therapy. Furthermore, therapeutic response is quite variable, and it remains difficult for clinicians to accurately predict which patients will respond prior to a lengthy lithium trial. Therefore, the development of a technique to accurately determine the concentration and spatial distribution of lithium in the brain is desirable and may allow identification of imaging-based biomarkers to better identify potential treatment responders and maximize treatment efficacy.
Lithium-7 (7Li) MR spectroscopy (MRS) is a non-invasive method for measuring brain concentration of lithium [1–3]. Although still in a relatively early stage of development, this method has been previously used to measure both steady-state concentrations and pharmacokinetics of brain lithium in bipolar patients, but without precise localization to particular regions of the brain [1–3]. The 7Li MRS studies of humans that have appeared to date have been reviewed in several places [3–5], so will not be discussed in detail here. These studies, however, were either unlocalized or localized to a single, thick slice through the brain. They were largely aimed at demonstrating basic technical issues, gross correlation of lithium serum and brain concentrations, or measuring global pharmacokinetics of lithium uptake into the brain. Two early MRS Imaging (MRSI) studies were performed on a clinical scanner, both at the relatively low magnetic field strength of 1.5 T [6, 7]. Most recently, a one-dimensional (1-D) 7Li MRSI study using a surface coil approach at 3T was reported [8]. While these studies demonstrated the feasibility of the technique and localized signals from lithium were observed, the results were limited. Specifically, resolution was too poor and the signal strength too weak at 1.5 T to yield interpretable, clinically useful results regarding regional variability of lithium concentration. Nonetheless, these previous studies demonstrated the potential biomedical utility of in vivo 7Li MRSI, and serve to confirm that with sensitivity increases, 7Li MRSI might be usefully applied to regional analyses.
Here we developed 7Li 3D MRSI methodology to measure regional brain lithium concentrations and to demonstrate that sufficient whole brain spatial resolution (~12 ml) is achievable on a 4T MR scanner to distinguish brain regions of a size comparable to the typical resolution (~8 ml) employed for in vivo 1H MRS. We also report some preliminary results from patients with bipolar disorder who were on lithium medication.
Methods
Data Acquisition
Fifteen bipolar subjects (6M/9F, age 28 ± 9 yrs) in depressed or mixed mood states were recruited and consented for the study. Patients were on lithium treatment at the time of recruitment and were asked to skip a morning dose on the day of the scan if MRSI scans were performed in the morning. The medication duration and lithium dose ranged from 2 to 75 weeks and from 300 mg/day to 1200mg/day (mainly 900 mg/day). A few patients were also taking concomitant medications. All patients had blood drawn to measure their blood serum lithium to compare with MRSI results; eight of them had their blood drawn within 24 hours of the imaging scan. Three and seven patients received three and two scans, respectively, on different days and two subjects received a repeated scan on the same day for technical validation. All MR studies were performed using a 1H/7Li dual-tuned TEM volume head coil on a Varian INOVA 4T MRI system (Varian Medical Systems, Palo Alto, CA). For in vivo quantification, six 7Li phantom datasets were acquired over a span of about 6 months on an aqueous phantom containing 5 mM DL-lactic acid (lithium salt) using the same pulse sequences and coil as for the in vivo 7Li MRSI acquisition. All patients had a 1H 3D T1-weighted anatomical image acquired before 3D 7Li MRSI scan using a 3D MDEFT sequence (TMD=1.1 s, TR=13 ms, TE=6 ms, FOV=25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96 pixels, flip angle=20 degrees) [9]. MDEFT images were used for lithium map overlap as well as for brain tissue segmentation. The 7Li MRSI were obtained using a one-pulse 3D MRSI sequence acquired in a spherical sampling scheme (13×13×13 data matrix) over a field of view of 24×24×24 cm with a flip angle of 37.5° (pulse width 125 μs), 0.5 sec TR, 928 phase encoding steps (phase encoding duration 500 μs), and 6 averages. Total acquisition time for 7Li MRSI is about 46 minutes. Each FID has 512 complex points and a 2 KHz spectral width. The data analysis was performed in MATLAB (MathWorks, Natick, MA) using in-house software. The 3D MRSI matrix was zero-filled to 16 × 16 × 16 prior to processing with a 3D symmetric hamming filter using the MATLAB signal processing toolbox function. After a point spread function correction, the nominal voxel size was approximately 12 ml.
Data Analysis
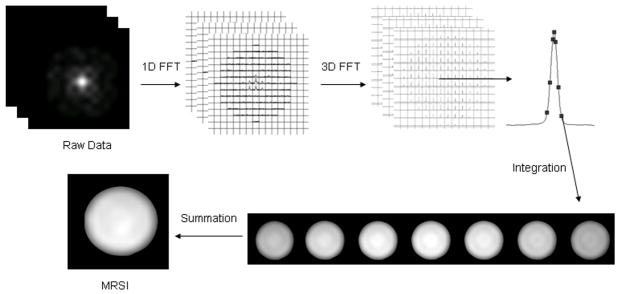
The raw data were analyzed by applying a 1D FFT (with a 15 Hz exponential broadening) on each FID and then integrating the MRSI by applying a 3D FFT on the data matrix (Figure 1), point by point, along a specified range that covered the entire lithium peak [10]. The matrices that had 3D FFT with a hamming filter were then summed to generate a spatial map of lithium concentration. The integration interval (i.e. frequency range) was determined using the 7Li spectra from the center of k-space. This allowed for a rough approximation of the frequency range of the lithium peak. Figure 1 shows a phantom lithium peak with seven sequential points in this case. At the bottom of Figure 1 are shown the seven maps generated from the individual points. These are then summed to generate a final lithium MRSI map. Increasing the number of points beyond those on and around the peak does not improve the map further since their intensities are at the noise level. The center individual map has the highest value and corresponds to the highest point of the lithium peak. Moving away from the center of the peak continues to generate maps but of decreasing value leading to a diminishing rate of return for increasing the integration interval. We used typically 12–16 points and 18–24 points to generate a final lithium MRSI map for phantom and in vivo samples, respectively. The result was confirmed by a more traditional approach, which applied a 3D FFT on the data matrix point by point along the entire time-domain data in k-space and then a 1D FFT was then applied on the FID data at each matrix location (10).
Figure 1.
Illustration of the 1D-3D FFT approach used in this study. Lithium MRSI was integrated from each and seven individual points in this case. See details in text.
Map Verification
To verify that the MRSI maps of lithium concentration were correct, a single voxel reconstruction technique was used to extract a single spectrum at a given location. Again, a 15 Hz exponential filter was applied prior to the 1D FFT on the spectra. Spectra were selected from multiple regions of different intensities to verify that the underlying spectra corresponded to the intensity map of the lithium concentration. This was done to verify that high intensity regions had a strong lithium signal and low intensity regions had a weak lithium signal (Figure 2c).
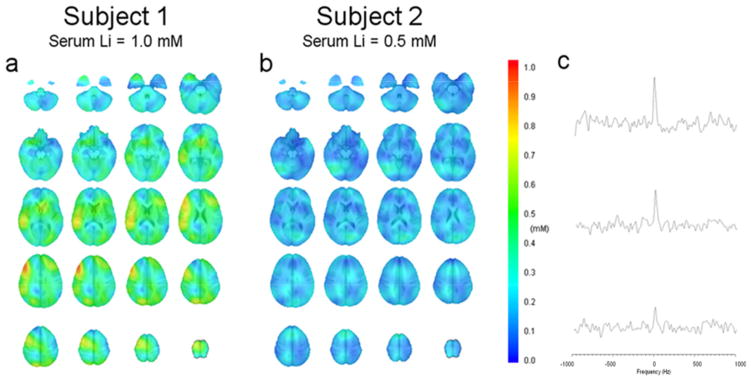
Figure 2.
Brain lithium distribution from two subjects with serum lithium of a) 1.0 mM and b) 0.5 mM. The color bar gives the concentration from 0 to 1 mM. c) Three representative spectra obtained by using a single voxel reconstruction technique to illustrate typical spectra for concentrations of 0.8, 0.5, to 0.2 mM from top to bottom, respectively.
Data calibration
To determine the lithium concentration of the in vivo data, we used the above mentioned phantom containing 5 mM lithium lactate as a reference. Phantom datasets were acquired on six different occasions immediately before or after patient scans using the same settings as the patient datasets. The phantom data used the same methods as described above to generate spatial concentration maps. These maps were then averaged to generate mean intensity values for each phantom. Due to small intensity variations in the phantom caused by B1 inhomogeneity that may be due to the spherical sampling scheme and some minor dielectric effects, the mean of these values was then used to calibrate the human data after correction for T1 differences. The T1s at 4T for human brain and phantom used to correct the human data were 4678 ms [11] and 1200 ms (measured from the phantom), respectively. Since we used a one pulse acquisition method for this study, the delay time between RF excitation and data acquisition is the sum of half of the duration of the hard pulse (125 μs) and the duration of phase encoding (500 μs). The contribution of T2* relaxation for absolute quantification, about 1–2% of calculated concentration, was also corrected by using the spectrum linewidth as an estimation of T2* for phantom and brain 7Li signals. The lithium concentration in cerebrospinal fluid (CSF) was found to be lower than for brain [12]. Furthermore, the 7Li T1 in CSF is much longer, on the order of 20 s [13]. At our typical TR of 0.5 s, the 7Li signal from CSF will be strongly attenuated relative to tissue signal because of the very long T1. There is essentially little contribution of CSF lithium signal to the lithium map. For the whole brain lithium quantification, we assume that there is no lithium from the CSF space. The high-resolution MDEFT anatomical images were analyzed by the software SPM5 to determine percent GM, WM, and CSF for regions of interest corresponding to the spatial location of each of the extracted voxels. Concentrations in units of mM (or milli-mole per liter of water) were then obtained by converting from milli-mole per liter brain tissue (i.e. non-CSF region only) assuming a brain water fraction of 0.75 [14, 15]. The scaled lithium maps of all subjects were then exported to and analyzed in AFNI [16].
Results
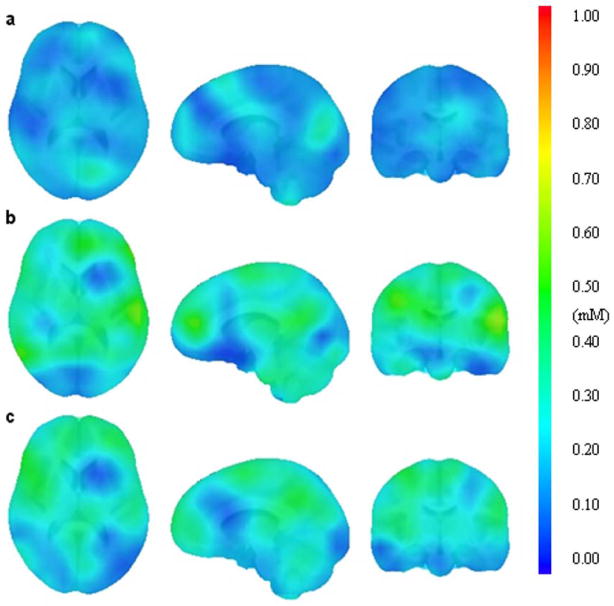
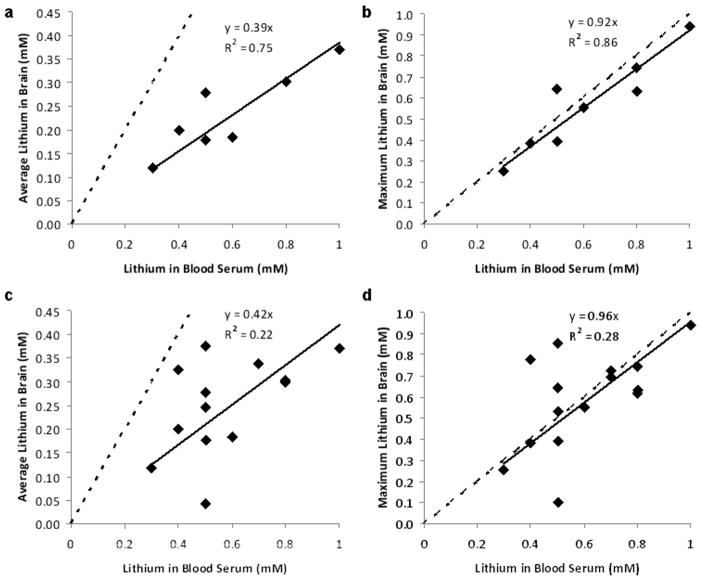
Figure 2 shows typical lithium axial images of the distribution of 7Li concentration from two subjects with high (1.0 mM) and low serum (0.5 mM) lithium level, respectively. The results show that human 7Li maps have uneven distributions throughout the brain, which are not observed in the phantom data shown in Figure 1. Inter-subject variations of brain lithium concentration and distribution were observed. Three representative spectra for high (0.8 mM), medium (0.5 mM), and low (0.2 mM) lithium concentration are illustrated in Figure 2c. The highest signal-to-noise ratio of the single voxel 7Li MRS was around 15. Figure 3 shows images from three scans for one subject separated by 15 weeks. Figure 3a is the initial scan, while Figures 3b and 3c are the repeated scans that were conducted on the same day and separated by 2 hours. In this case, the subject took a 30-minute break before reentering the magnet for the second scan. The subject’s serum levels were 0.5 mM and 0.8 mM for the first and second visits, respectively. The results show that brain lithium concentrations between scans on the same day are more comparable than those recorded on different days. The difference of average concentration for the entire brain was 0.02 mM between scans acquired on the same day (Fig. 3b vs Fig. 3c), compared to 0.09 mM between scans recorded on different days (Fig 3a vs Fig 3b). Intra-subject variations of brain lithium concentration and distribution between different days were also present and are probably associated with the brain lithium uptake level. Figures 4a and 4b illustrate the correlations between serum lithium level and the average brain and the local maximum concentration, respectively, for the eight subjects whose blood was drawn within 24 hours of the imaging scan. The brain-to-serum-lithium ratio for the average brain and the local maximum were 0.39 ± 0.08 and 0.92 ± 0.16 (solid lines in Figs 4a and 4b), respectively, which demonstrated a strong linear relationship with serum lithium level; however, latter (R2=0.86, r=0.93) was stronger than the former (R2=0.75, r=0.89). Additionally, Figures 4c and 4d present the correlations between serum lithium level and the average brain (R2=0.22, r=0.60) and the local maximum concentration (R2=0.28, r=0.63), respectively, for all subjects. The results show that the brain-to-serum-lithium ratios are similar between these two conditions. However, the correlation is much stronger when blood was drawn within 24 hours of the scan.
Figure 3.
Comparison brain lithium maps of one subject who has been scanned three times in two visits separated by 15 weeks. Lithium map a) was obtained on the first visit when serum level was 0.5 mM, while b) and c) were obtained on the second visit when serum level was 0.8 mM.
Figure 4.
The correlation (solid lines) between serum lithium level and a) the brain average lithium concentration and b) the brain local maximum lithium concentration for subjects (n=8) whose blood was drawn within 24 hours of the MRS scan. The correlation between serum lithium level and c) the brain average lithium concentration and d) the brain local maximum lithium concentration for all scans (n=22). Note: the number of data points may seem to be less than n since some data points overlap. Data with serum lithium concentration less than 0.3 mM were excluded from the analysis since the exact concentration was not given in the medical report (i.e. it reads < 0.3 mM). The solid lines represent the linear correlation between brain and serum lithium level and slopes give the brain-to-serum lithium ratios, while the dashed lines show the condition when the brain-to-serum lithium ratio is equal to one.
Discussion
This work demonstrates that high resolution whole brain 3D 7Li MRSI is possible and that using a lithium phantom as external reference is feasible for brain lithium concentration quantification. Several interesting results are observed. First, the inter-and intra-subject brain lithium level and distribution are quite variable and are strongly associated with serum lithium concentration. Therefore, it is important to draw blood as close as possible to the time of scan when comparing brain lithium level to serum concentration.
Second, the brain-to-serum lithium ratio for the entire brain is about 0.40 (Figs 4a and 4c). The finding for the brain-to-serum lithium ratio is near the lowest value reported from previous 7Li MRS work in humans [17–19]. However, if only considering the local maximum brain concentration of each individual (Figs. 4b and 4d), which may not be necessarily located at the same location among subjects, the brain-to-serum ratio for local maximum is about 0.95 (solid lines), which is close to 1 (dashed lines). The local maximum concentration is more than two times higher than the average brain concentration. This observation could explain the large variation in the ratio of brain to serum lithium levels reported previously, since earlier studies obtained either global values or partial measures with different locations in the brain [17–19]. Our results showed that the brain lithium level is strongly correlated with serum concentration which generally agrees with the previous studies [18–19]. It is possible to estimate the average brain and the local maximum lithium concentration by measuring serum lithium concentration. However, the position of the local maximum can only be determined by MRSI, which could be critical for the understanding the mechanism of lithium’s therapeutic effect. Since the exact concentration was not given in the medical report for serum lithium concentrations less than 0.3 mM, we have excluded these data from the correlation analysis.
Third, the brain lithium distribution in these subjects is not uniform, even though one might predict it would be since lithium is a simple cation and should diffuse freely within the water compartment. Nevertheless, an uneven spatial distribution of lithium in the rat brain after short-term lithium administration using 2D MRSI at high field strength has been reported [20]. The finding of a higher lithium concentration in certain regions of the brains of bipolar disorder patients, especially for those whose lithium serum levels were at a higher therapeutic range is noteworthy, since some of these regions are part of the anterior limbic network that maintains emotional homeostasis [21, 22]. This finding suggests that lithium may target specific brain tissues and/or certain enzymatic and macromolecular sites that are associated with therapeutic effect and hence is a subject worthy of further investigation. This hypothesis may be elucidated by investigating if the lithium distribution in the brain of healthy controls is also unevenly distributed.
Although we did not measure the brain 7Li T1 values for each individual subject but used the previous T1 value reported by Moore et al. [11], the error due to the difference in individual T1 values was insignificant because of the use of a small flip angle (37.5°), while TR was much shorter than the T1 values of both brain and phantom. Furthermore, the contribution of 7Li signal from CSF was ignored in this study due to its small contribution to the NMR signal. This may, however, underestimate the CSF lithium level in the lithium map of this study. In addition, the slight variation between the back-to-back scans (about 2 hours apart) is unclear. We have examined and eliminated the possible artifact caused by variation in chemical shift and/or linewidth from voxel to voxel. Although this has not been thoroughly investigated, it is possibly due to differential diffusion of Li+ among brain regions over time. Despite the promise of this technique for in vivo 7Li research in bipolar disorder, the relatively long scan time can be problematic for some patients. However, this limitation can potentially be overcome by using a parallel imaging approach and/or methods such as reported by Gao et al [23]. To the best of our knowledge, this is the first demonstration of 7Li whole brain mapping with a relatively high resolution in bipolar patients who are on lithium treatment.
Acknowledgments
This work was supported by the University of Cincinnati Bipolar Disorder Imaging and Treatment Research Center funded by the NIH (P50 MH077138).
References
- 1.Renshaw PF, Sachs GS, Gonzalez RG. In vivo MRS measurement of lithium levels in brain. In: Nasrallah HA, Pettegrew JW, editors. NMR Spectroscopy in Psychiatric Brain Disorders. Vol. 8. American Psychiatric Press; Washington: 1995. pp. 179–198. [Google Scholar]
- 2.Ikeda A, Kato T. Biological predictors of lithium response in bipolar disorder. Psychiatry Clin Neurosci. 2003;57:243–250. doi: 10.1046/j.1440-1819.2003.01112.x. [DOI] [PubMed] [Google Scholar]
- 3.Soares JC, Boada F, Keshavan MS. Brain lithium measurements with 7Li magnetic resonance spectroscopy (MRS): A literature review. European Neuropsychopharmacol. 2000;10:151–158. doi: 10.1016/s0924-977x(00)00057-2. [DOI] [PubMed] [Google Scholar]
- 4.Komoroski RA. Applications of 7Li NMR in biomedicine. Magn Reson Imaging. 2000;18:103–116. doi: 10.1016/s0730-725x(99)00116-2. [DOI] [PubMed] [Google Scholar]
- 5.Komoroski RA. Biomedical applications of 7Li NMR. NMR Biomed. 2005;18:67–73. doi: 10.1002/nbm.914. [DOI] [PubMed] [Google Scholar]
- 6.Komoroski RA, Newton JEO, Sprigg JR, Cardwell D, Mohanakrishnan P, Karson CN. In vivo 7Li nuclear magnetic resonance study of lithium pharmacokinetics and chemical shift imaging in psychiatric patients. Psychiatric Res: Neuroimaging. 1993;50:67–76. doi: 10.1016/0925-4927(93)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Girard F, Suhara T, Sassa T, Okubo Y, Obata T, Ikehira H, Sudo Y, Koga M, Yoshioka H, Yoshida K. 7Li 2D CSI of human brain on a clinical scanner. Magn Reson Mat Phys Biol Med. 2001;13:1–7. doi: 10.1007/BF02668644. [DOI] [PubMed] [Google Scholar]
- 8.Smith FE, Cousins DA, Thelwall PE, Ferrier IN, Blamire AM. Quantitative lithium magnetic resonance spectroscopy in the normal human brain on a 3 T clinical scanner. Magn Reson Med. 2011;66:945–949. doi: 10.1002/mrm.22923. [DOI] [PubMed] [Google Scholar]
- 9.Lee J-H, Garwood M, Menon R, Adriany G, Anderson P, Truwit CL, Ugurbil K. High Contrast and Fast Three-dimensional Magnetic Resonance Imaging at High Fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 10.Lee J-H, Norris MM, Adler CM, Macaluso EE, Chu W-J, Komoroski RA, Strakowski SM. 4T 7Li MRSI in the brains of bipolar disorder subjects. Proc Int Soc Magn Reson Med. 2010;18:593. doi: 10.1002/mrm.24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore CM, Frederick BD, Henry ME, Streeter CC, Cohen BM, Renshaw PF. Human in vivo lithium magnetic resonance spectroscopy at 4. 0 tesla: Initial findings. Biol Psychiatry. 2003;53:202S. [Google Scholar]
- 12.Wraae O. The pharmacokinetics of lithium in the brain, cerebrospinal fluid and serum of the rat. Br J Pharmacol. 1978;64:273–279. doi: 10.1111/j.1476-5381.1978.tb17300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullapalli RP, Hawk RM, Komoroski RA. A 7Li NMR study of visibility, spin relaxation, and transport in normal human erythrocytes. Magn Reson Med. 1991;20:240–252. doi: 10.1002/mrm.1910200207. [DOI] [PubMed] [Google Scholar]
- 14.Kreis R, Ernst T, Ross BD. Absolute quantification of water and metabolites in human brain. IIMetabolite concentrations. J Magn Reson B. 1993;102:9–19. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 15.Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative 31P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med. 2001;45:46–52. doi: 10.1002/1522-2594(200101)45:1<46::aid-mrm1008>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 17.Kushnir T, Itzchak Y, Valevski A, Lask M, Modai I, Navon G. Relaxation times and concentrations of 7Li in the brain of patients receiving lithium therapy. NMR Biomed. 1993;6:39–42. doi: 10.1002/nbm.1940060107. [DOI] [PubMed] [Google Scholar]
- 18.Riedl U, Barocka A, Kolem H, Demling J, Kaschka WP, Schelp R, Stemmler M, Ebert D. Duration of lithium treatment and brain lithium concentration in patients with unipolar and schizoaffective disorder – A study with magnetic resonance spectroscopy. Biol Psychiatry. 1997;42:844–850. doi: 10.1016/S0006-3223(96)00330-7. [DOI] [PubMed] [Google Scholar]
- 19.Soares JC, Boada F, Spencer S, Mallinger AG, Dippold CS, Wells F, Frank E, Keshavan MS, Gershon S, Kupfer DJ. Brain lithium measurements in bipolar disorder patients: Preliminary 7Li magnetic resonance spectroscopy studies at 3T. Biol Psychiatry. 2001;49:437–443. doi: 10.1016/s0006-3223(00)00985-9. [DOI] [PubMed] [Google Scholar]
- 20.Ramaprasad S, Ripp E, Pi J, Lyon M. Pharmacokinetics of lithium in rat brain regions by spectroscopic imaging. Magn Reson Imaging. 2005;23:859–863. doi: 10.1016/j.mri.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Mega MS, Cummings JL, Salloway S, Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatr Clin Neurosci. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Strakowski SM, Reeves SJ, Hetherington HP, Chu W-J, Lee J-H. Fast spectroscopic imaging using online optimal sparse k-space acquisition and projections onto convex sets reconstruction. Magn Reson Med. 2006;55:1265–1271. doi: 10.1002/mrm.20905. [DOI] [PubMed] [Google Scholar]