Abstract
Background
ALK+ Anaplastic Large Cell Lymphoma (ALCL) is usually a disease of young patients. We investigated phosphatidylinositol-3 kinase (PI3K)/Akt pathway-associated factors in pediatric cases and cell lines.
Procedure
Patient materials consisted of tissue slides of ALK+/CD30+ ALCL from 33 patients treated on Pediatric Oncology Group protocols (9219, n=8 and 9315, n=25). Slides were examined by immunohistochemistry for phospho(p)-Akt and PTEN, the primary feedback regulator of the pathway, as well as for p27kip1 and stathmin-1. ALCL cell lines SUDHL-1 and Karpas-299 were examined for ALK, pALK, pAkt, p27/Kip1, PTEN, pPTEN, CD30, pSTAT3 and pSTAT5; ALK inhibition was performed using compound PF-2341066 and PTEN genes were sequenced.
Results
A majority of patients expressed pAkt, PTEN and stathmin, with p27kip1 levels less than controls. Cell lines showed expression of ALK, pALK, pSTAT3, pSTAT5, CD30, pAkt, PTEN, and pPTEN, with p27 slightly less than positive controls, and germline PTEN DNA. There was evidence of phosphorylated PTEN (pPTEN) associated with inhibited function. Pharmacologic inhibition of activated ALK diminished pSTAT3, pSTAT5 and CD30 expression but not pAkt or pPTEN in cultured cell lines.
Conclusion
We conclude that the PI3K/Akt pathway is activated in many, though not all, pediatric ALK+ ALCL. Our data suggests that activation of this pathway involves post-translational regulation of PTEN. Pharmacologic inhibition of activated ALK does not reduce modest levels of activated Akt as it does with the more abundant levels of activated STAT3 or STAT5. Future therapy of ALCL might, in selected patients, best combine agents inhibiting PI3K/Akt with those targeting ALK.
Keywords: pediatric, anaplastic, lymphoma, signaling, PTEN
INTRODUCTION
Anaplastic large cell lymphoma (ALCL) was first described by Stein et al, in 1985 [1]. It is the most common peripheral T-cell lymphoma of children and adolescents, accounting for approximately 10% of pediatric non-Hodgkin lymphoma [2]. A majority exhibit chromosomal translocation t(2;5)(p23;q35), with fusion of the distal portion of the anaplastic lymphoma kinase (ALK) gene to the promoter region and proximal domain of the gene encoding nucleophosmin/B23 (NPM1) [2]. Variant translocations involving ALK also occur infrequently. Tumors are characterized by large anaplastic peripheral T cells with expression of the activation marker CD30 as well as ALK.
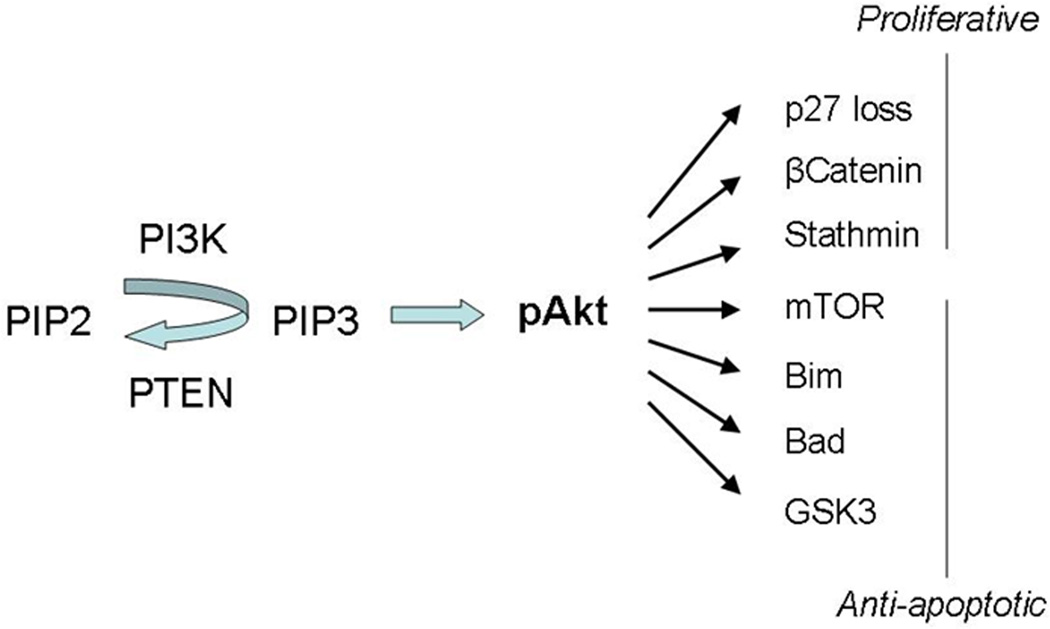
NPM-ALK is a receptor tyrosine kinase of the insulin-receptor superfamily. The pathogenesis of ALK+ ALCL is considered to be due to NPM-ALK (or variant) fusion protein with abnormal cell signaling involving multiple tumor promoter pathways [3,4]. The best characterized of these are the MEK/ERK, JAK3-STAT3, and PI3K/Akt pathways (Figure 1).
Figure 1.
Phosphatidylinositol-3,4,5-triphosphate (PIP3) is derived from phosphorylation of phosphatidylinositol-4,5-biphosphate (PIP2) by phosphatidylinositol 3-kinases (PI3Ks) in a reversible manner regulated by PTEN. PIP3 activates Akt/protein kinase B (pAkt) that in turn modulates a number of proliferative and anti-apoptotic intracellular processes.
The PI3K/Akt pathway is involved in regulation of both apoptosis and proliferation. Phosphorylation of phosphotidylinositol-4,5-biphosphate (PIP2) to phosphotidylinositol-3,4,5-triphosphate (PIP3) activates/phosphorylates Akt, which inhibits apoptosis through mTOR and Bcl-2 family members. It fosters proliferation through degradation of cell cycle inhibitor p27/kinase inhibitor 1 (kip1). The PI3K/Akt pathway is regulated by a PIP3 phosphatase known as phosphatase and tensin homolog deleted on chromosome ten (PTEN) that acts as a feedback inhibitor. Stathmin, a microtubule regulator, is increased by PI3K/Akt activity and/or functional PTEN loss (Figure 1). Previous studies have shown that NPM-ALK can activate the PI3K/Akt pathway, that PI3K inhibitors induce apoptosis in NPM-ALK-expressing lymphoma cells, and that dominant negative PI3K and Akt mutants suppress cell lines transfected with NPM-ALK [3].
It has been reported in a series of peripheral T-cell lymphoma that among ALCL (including ALK+ and ALK- cases), loss of p27 and PTEN expression may be related to adverse outcome [5]. We studied clinical cases of pediatric ALK+ ALCL to assess the frequency of involvement of the PI3K/Akt pathway and associated factors including activated Akt (pAkt), stathmin, p27 and PTEN. We further tested ALCL cell lines to test hypotheses generated from immunohistochemical (IHC) studies.
METHODS
Patient specimens
Histologic slides from 33 cases of ALCL treated on Pediatric Oncology Group (now part of Children’s Oncology Group) 9219 for localized lymphoma (n=8) or 9315 for advanced stage large cell non-Hodgkin lymphoma (n=25), were available subsequent to pathology review along with unstained slides adequate for several immunohistochemical stains [6]. All materials were obtained following appropriate institutional review. 20 cases were boys and 13 were girls. Median age was 14 years (range, 2–20). The majority of the diagnostic specimens (24) were lymph node biopsies, with 3 skin biopsies (two of which were associated with systemic disease), 2 lung, 1 abdominal mass, 1 left upper quadrant mass, 1 retroperitoneal (psoas) biopsy and 1 tibial bone biopsy. Pathologic diagnosis on protocol review was ALCL with various histologic patterns, expressed per current WHO guidelines [7]: 23 common, 1 common with Hodgkin lymphoma-like features, 8 lymphohistiocytic and 1 with a sarcomatoid pattern. All cases previously tested positively for CD30 and ALK and many were also previously studied for expression of activated STAT3 [8].
Cell lines
ALK+ ALCL cell lines SUDHL-1 and Karpas-299 (DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were tested by flow cytometry and Western blot analysis and SUDHL-1 was tested by immunohistochemistry [9-11].
Immunohistochemistry was performed on unstained slides using standard techniques. Heat-induced epitope retrieval was performed for all immunostains except the first 15 cases tested for pAkt. 3,3’-Diaminobenzidine/H2O2 (Biogenex, San Ramon, CA) was used as primary chromogen and hematoxylin as counterstain. Antibodies included pAkt (Ser 473), stathmin and pPTEN (Ser380/Thr382/383) (Cell Signaling Technology, Beverly, MA), PTEN (Thermo-Scientific, Fremont, CA) and p27kip1 (Cell Marque, Rocklin, CA). In cases with limited slides, antibody stains were performed by manually applying 2 different antibodies to different parts of the tissue, determined from H&E stains. Assay of pAkt was initially performed on a subset (15) of cases using manual technique and subsequently on an additional 13 using an automated platform (Ventana, Tucson AZ). Only cytoplasmic labeling of pAkt was scored due to between-run variability of nuclear staining (nuclear staining was not found for pAkt with the automated technique). Phospho-PTEN labeling was performed on a subset of 13 cases for which slides remained available after other studies. Antibodies were scored by light microscopy.
ALK inhibition was performed on cell lines using compound PF-2341066 (Selleck Chemicals LLC, Houston, TX). Karpas 299 and SUDHL-1 cells were cultured in RPMI 1640 +10% FCS with varied concentrations of compound PF-2341066 as well as DMSO (diluent of PF-2341066) for 24 hours. Assays for ALK, pALK, pAkt, PTEN, pPTEN, non-pPTEN, p27, CD30, pSTAT3 and pSTAT5 were performed by phospho flow cytometry and pALK and pPTEN also measured by Western blot (SUDHL-1 only). Dosing was based on concentrations determined to be at and below 50% inhibitory concentrations in proliferation assays at 48 hours, with signal measurements taken at 24 hours to minimize nonspecific effects due to cell death or apoptosis. Doses were limited to those not causing loss of cell integrity or viability: DMSO negative control (diluent for PF-2341066), 50 nM, 100 nM and 175 nM for SUDHL-1; control, 250 nM, 500 nM and 1000 nM, for Karpas-299. Doses of ALK inhibitor were higher for Karpas-299 due to its‘ greater tolerance of the compound. Flow cytometry experiments were performed in replicates of 5 and results expressed as median fluorescence +/- standard error.
Flow cytometry of cell lines was performed on an LSR-II flow cytometer (Becton Dickinsen, Franklin Lakes, NJ) using cytoplasmic labeling with fluorescent-labeled rabbit or mouse monoclonal antibodies (MoAb) against phospho-Akt (Ser 473), and phospho-STAT3 (Tyr 705) (Cell Signaling Technology, Beverly MA), p27 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), ALK, CD30, pSTAT5 (Y694) and PTEN (BD Biosciences, San Jose, CA), with appropriate isotype controls. Unlabeled rabbit MoAbs pALK (Tyr 1604), pPTEN (Ser380/Thr382/383) and non-pPTEN (Ser380/Thr382/383) were similarly detected by flow cytometry after detection by Alexa-fluor 488-labeled anti-rabbit IgG(H+L), F(ab’)2 antibody with appropriate rabbit isotype control (all Cell Signaling Technology). Fixation and preparation was performed using Phos-flow reagents and techniques (BD-Biosciences).
Western blot analysis utilized unlabeled antibodies of the same clones of rabbit monoclonal PTEN, pPTEN (Ser380/Thr382/383), ALK and pALK (Tyr 1604) (Cell Signaling Technology, Beverly MA ). Standard techniques were employed. The PTEN gene was sequenced from each cell line. DNA was extracted from cultured cells using Qiagen FlexiGene DNA kit (Qiagen, Valencia, CA). All PTEN coding exons were amplified with primers designed using the Vector NTI program (Invitrogen, Carlsbad, CA). Primers were purchased from Integrated DNA Technologies (IDT, Coralville, IA). PCR amplification of exon1 was performed using the Roche GC-rich PCR system (Roche Molecular Biochemicals, Indianapolis, IN). PCR amplification of exons 2 through 9 was performed using GoTaq (Promega, Madison, WI) and standard conditions. PCR products were cycle sequenced using the ABI BigDye terminator cycle sequencing kit (PE Applied Biosystems, Foster City, CA).
PIP3 phosphatase activity was measured chemically by the malachite green assay (Echelon Biological, Salt Lake City, UT) that assays production of free phosphate from a PIP3 substrate leading to generation of PIP2 [12]. Immunoprecipitation was performed utilizing anti-PTEN and anti-pPTEN mouse monoclonal antibodies (Cell Signaling Technology) and protein A conjugated 6% agarose beads (Pierce/Thermo, Rockford, IL). Free phosphate levels were measured in triplicate in an ELISA plate reader at 650nm. Absorbance was converted to pmol phosphate using a phosphate standard curve. Results were compared to a recombinant PTEN positive control (Echelon).
RESULTS
Results of immunohistochemical assays are shown in Table I. 18 of 27 (67%) of cases analyzed showed expression of cytoplasmic pAkt. (Figure 2) Twenty six of thirty three cases (79%) showed PTEN expression. 22/33 (67%) were positive for stathmin (cytoplasmic). Staining for p27 showed cytoplasmic staining in four of thirty cases tested (13%). An additional 7 (11/33; 33%) showed nuclear p27. No cases showed p27 expression equal to or greater than intrafollicular T-cells in tonsil positive control. Seven of 13 (subset of cases) were positive for pPTEN. Each of these cases was also positive for PTEN and 6 of 7 pPTEN+ cases were also positive for pAkt.
Table 1.
Immunohistochemical Results
| sex | study | age | Lab # | CD30 | ALK | PTEN | Stathmin | Nuc p27 | Cyto P27 | pAkt | p-PTEN | pSTAT3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 9315 | 6 | 00-09 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| Male | 9315 | 14 | 00-12 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| Male | 9315 | 14 | 00-37 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| Female | 9315 | 16 | 00-58 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Male | 9315 | 12 | 00-125 | 1 | 1 | 0 | 0 | 0 | 0 | 1, weak | 0 | |
| Male | 9315 | 16 | 00-126 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Female | 9315 | 16 | 01-019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Female | 9315 | 20 | 03-012 | 1 | 1 | 1 | 0 | 0 | 0 | 1, weak | 0 | |
| Male | 9315 | 15 | 97-029 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | |
| Female | 9315 | 11 | 97-196 | 1 | 1 | 1 | 1 | 0 | 0 | 1, weak | ||
| Female | 9219 | 16 | 98-028 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| Male | 9315 | 14 | 98-031 | 1 | 1 | 1 | 1 | 0 | 0 | 1, weak | 0 | |
| Male | 9315 | 12 | 98-112 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | ||
| Male | 9219 | 10 | 98-250 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| Male | 9219 | 16 | 98-252 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Male | 9315 | 10 | 98-298 | 1 | 1 | 0 | 0 | 0 | ||||
| Female | 9315 | 11 | 98-299 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Male | 9315 | 5 | 98-336 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Male | 9219 | 2 | 99-005 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Male | 9315 | 15 | 99-037 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| Male | 9219 | 13 | 99-045 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| Male | 9315 | 7 | 99-132 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| Male | 9315 | 5 | 99-159 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| Female | 9219 | 14 | 99-171 | 1 | 1 | 1 | 1 | 0 | 0 | |||
| Female | 9315 | 14 | 99-184 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | |
| Male | 9219 | 17 | 99-188 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| Female | 9315 | 15 | 99-190 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Female | 9315 | 13 | 99-193 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Female | 9315 | 8 | 99-218 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Male | 9315 | 16 | 99-229 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Female | 9315 | 13 | 99-265 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Male | 9315 | 11 | 99-275 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Female | 9315 | 3 | 99-281 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| SUDHL-1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Karpas-299* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| *(includes flow cytometry) | ||||||||||||
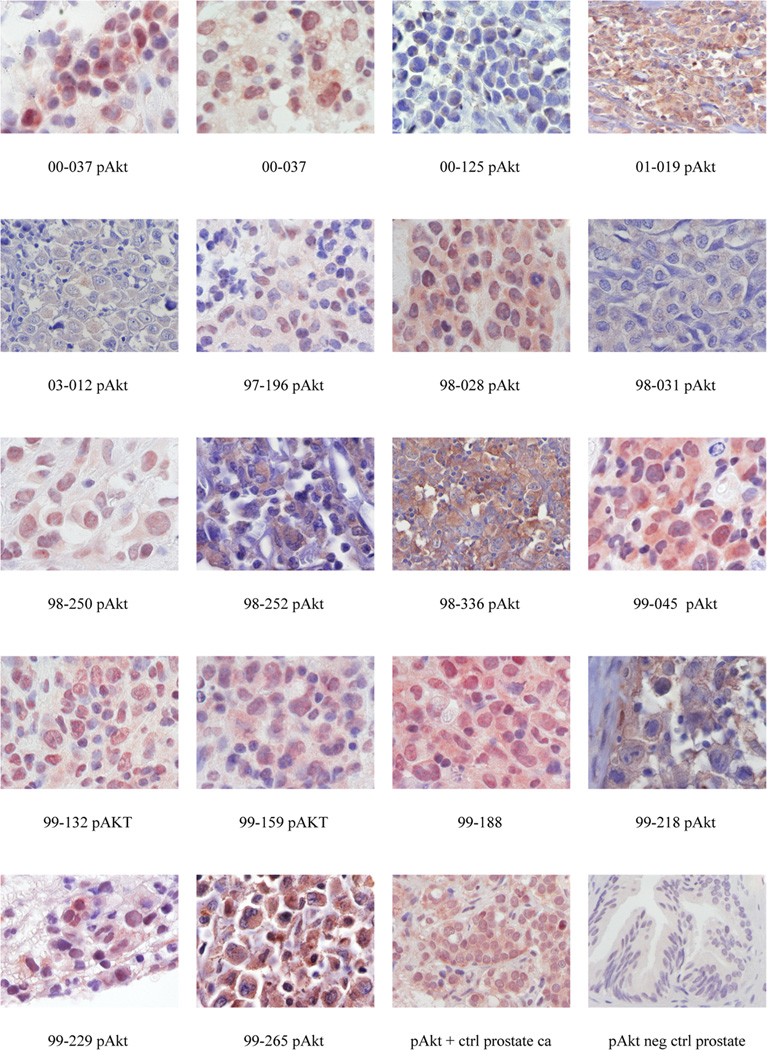
Figure 2.
Immunohistochemical results of pAkt of tumors with cytoplasmic positivity, as well as prostate carcinoma positive control and normal prostate negative control. Specimen numbers are unique laboratory-specific numbers that correspond to Table 1.
Immunohistochemistry of ALCL cell line SUDHL-1 showed cytoplasmic PTEN, pPTEN, stathmin and pAkt. SUDHL-1 also showed cytoplasmic (and nuclear) p27, but equal to or less than positive controls (tonsilar T-cells).
Flow cytometry of cell lines showed expression of ALK, pALK, PTEN, pPTEN, pSTAT3, pSTAT5, CD30, pAkt and p27. Karpas-299 showed variability of pAkt, often dim in healthy untreated cells (>90% viability), but moderately positive after 24 hours cultured in serum-free media or treated with PF-2341066.
Sequencing of the PTEN gene from SUDHL-1 and Karpas-299 was performed. Results showed germline configuration in each.
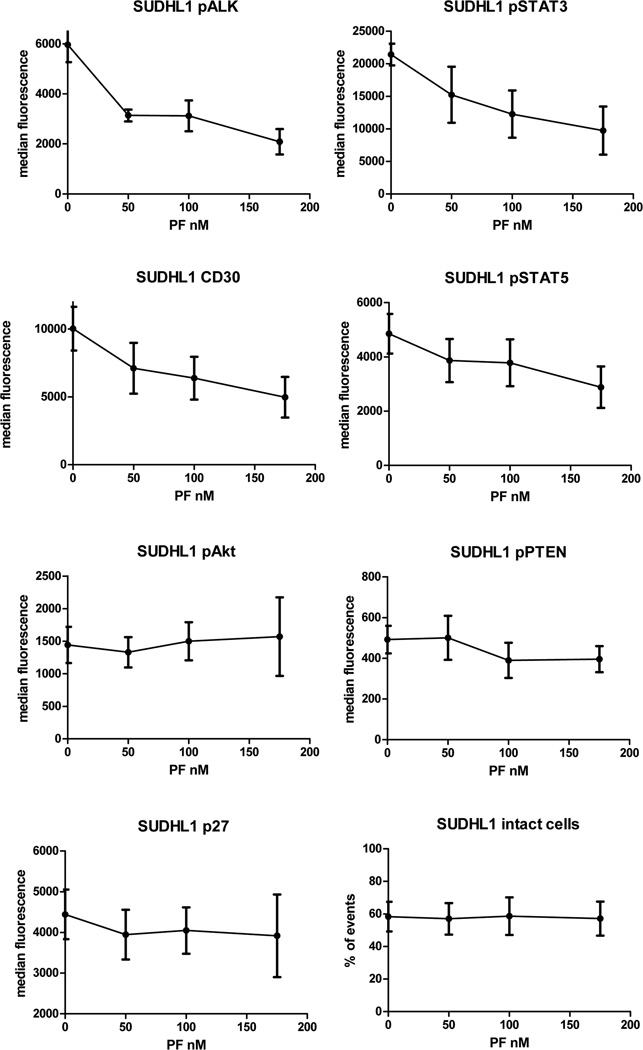
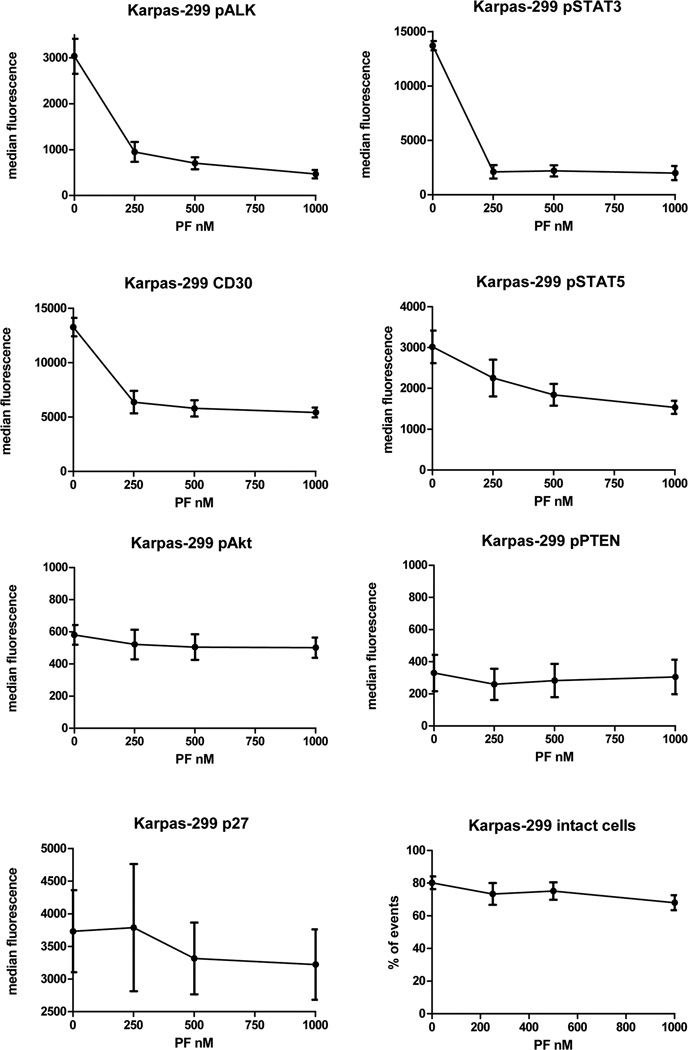
Results of flow cytometry following multi-dose treatment with PF-2341066 are summarized in Figures 3 and 4 and show decreases of pALK, pSTAT3, pSTAT5 and CD30 with increasing dosage but comparatively stable expressions of pAkt, pPTEN and p27. pAkt showed slight increase in SUDHL-1 and slight decrease in Karpas-299.
Figure 3.
ALK inhibition assays by phospho-flow cytometry show median fluorescence intensity of each measured protein at doses of PF-2341066: SUDHL-1; DMSO control, 50 nM, 100 nM, 175 nM
Figure 4.
ALK inhibition assays by phospho-flow cytometry show median fluorescence intensity of each measured protein at doses of PF-2341066: Karpas-299; DMSO control, 250 nM, 500 nM and 1000 nM
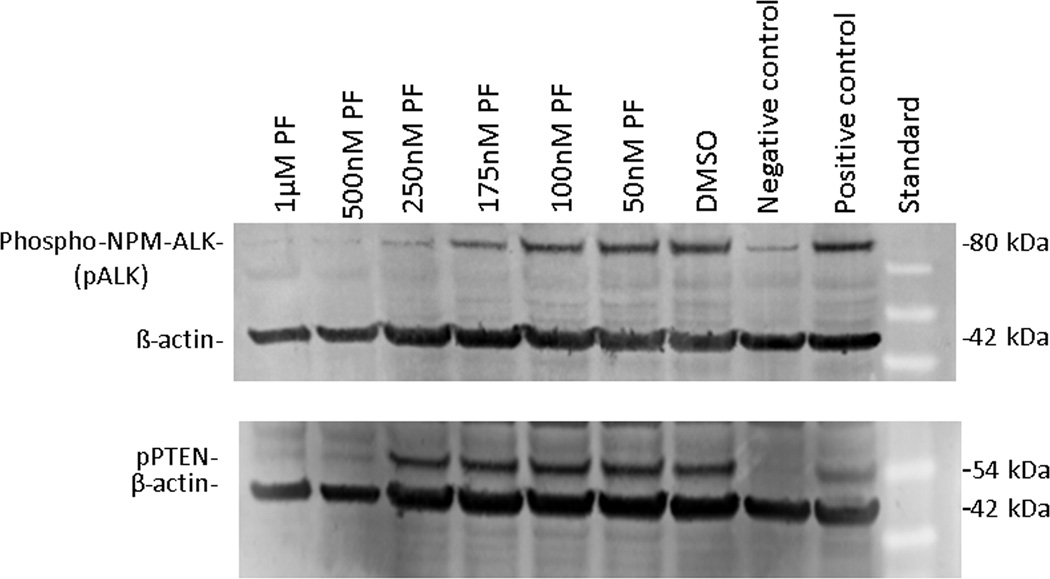
Western blots showed presence of total ALK, pALK, PTEN and pPTEN in both cell lines. Inhibition of ALK by PF-2341066 in SUDHL-1 resulted in reduction of pALK but not of pPTEN (Figure 5). Experiments at both 24 and 48 hours following treatment showed no apparent loss of PTEN or pPTEN (Supplemental Figure 1)
Figure 5.
Western blot analysis showing pALK and pPTEN expression in the SUDHL-1 cell line with and without ALK inhibition by PF-2341066. Expression of pALK was partially reduced in SUDHL-1 at 175 uM and substantially reduced at 250 nM. pPTEN was reduced by treatment with PF-2341066 only at 500 nM, which was, however, associated with loss of cell viability and integrity. Phospho-NPM-ALK positive control is cell line Karpas-299. pPTEN positive control is cell line MCF7. Negative control is MDA-MB-468.
PTEN PIP3 phosphatase assay of untreated SUDHL-1 cells showed mean phosphatase activity of 219 pmol/well for PTEN and 261 pmol/well for pPTEN immunoprecipitates. This compares to that 532 pmol/well for a recombinant PTEN positive control and 41 for a negative reagent control (Supplemental Figure 2).
DISCUSSION
The WHO classification, Tumours of Haematopoietic and Lymphoid Neoplasms, recognizes systemic ALCL as 2 diseases, ALK + ALCL and ALK - ALCL [7]. ALK+ ALCL occurs in younger patients and has a better prognosis than systemic ALK-disease. Age is likely one factor in survival differences [13], but the biology of ALK+ ALCL may be considered distinct [14]. Primary cutaneous ALCL (cutaneous CD30+ lymphoproliferative disorder) also occurs, is usually ALK- and shows entirely different clinical characteristics.
The PI3K/Akt pathway is active in ALK+ ALCL cell lines, affecting both proliferation and apoptosis (Figure 1) [15]. Class IA PI3Ks phosphorylate PIP2 to form PIP3 that in turn activates Akt. Resultant p27 degradation allows cyclin-CDK complex-directed cell cycle progression [16]. PTEN inhibits the pathway by de-phosphorylating PIP3 back to PIP2. Stathmin1 is not only a marker of PI3K pathway activity but is also involved in cell cycle progression and interacts with p27kip1 [17,18]. PTEN and p27 both act as tumor suppressors.
Results of our study show that clinical cases of pediatric ALK+ ALCL as well as established cell lines demonstrate frequent though not constant activation of the PI3K/Akt signaling pathway. We were surprised, however, to find that a majority of clinical cases also showed strong cytoplasmic expression of PTEN, the pathway’s primary inhibitor, and no increase in p27. These results suggested that the PTEN feedback loop is active but that PTEN is ineffective at blocking Akt pathway activation.
Several possibilities were considered regarding this anomaly: that PTEN may be mutated; that intracellular localization may be abnormal; that NPM-ALK signaling may overwhelm PTEN feedback regulation; or that PTEN may be otherwise rendered inactive.
PTEN directly opposes the activity of PI3Ks and is one of the most frequently mutated or inactivated tumor suppressor genes. Mutational loss of PTEN in some tumors drives PI3K pathway signaling [19]. Gene sequencing results in ALCL cell lines suggest that PTEN mutation is not likely a factor in ALCL. To our knowledge, there has not been any published report of PTEN mutation in ALCL tissues or cell lines (www.sanger.ac.uk/genetics/CGP/cosmic). PTEN mutation is not, however, entirely ruled out by DNA sequencing.
PTEN has both nuclear and cytoplasmic functions. Cytoplasmic PTEN behaves as a negative feedback regulator of PI3K and thus acts as a tumor suppressor [20,21]. Nuclear PTEN displays PI3K/AKT independent tumor suppressor activity and its’ loss is associated with aggressive tumor course. Monoubiquitination imports PTEN into the nucleus, whereas polyubiquitinated PTEN remains in the cytoplasm and is degraded by the proteasome [22]. PTEN shuttling between the nucleus and cytoplasm may be important in cell cycle regulation. All of our positive cases showed cytoplasmic PTEN localization consistent with PI3K feedback. The effects of concurrent nuclear PTEN expression in a minority are not known.
We hypothesized that ALK signaling may strongly drive the pathway and overwhelm PTEN inhibition. To test this, we suppressed ALK activation using compound PF-2341066, a small molecule inhibitor of ALK and c-MET. We anticipated that ALK inhibition would also lead to suppression of pAkt, increased p27 and possibly decreased PTEN expression. What we found is that inhibition of activated ALK diminishes expression of pSTAT3, pSTAT5 and CD30 but not of the more modest levels of pAkt, PTEN or pPTEN, and p27 does not increase. This agrees with previous findings of Christensen, et. al., who found that in xenografts of Karpas 299, Akt was less affected by ALK inhibition than were STAT3 and others, and suggesting downstream influences on Akt activation other than ALK [23].
There is abundant evidence that NPM-ALK is associated with PI3K/Akt pathway activation and regulation [24,25]. NPM-ALK associates with phospholipase C (PLC-γ) at position Y664 of NPM-ALK. It’s activation induces hydrolysis of PIP2 to inositol triphosphate (IP3) and diacylglycerol (DAG), which are involved in signal transduction [26]. Additionally, NPM-ALK recruits the C-terminal SH2 domain of the PI3K p85 subunit with activation of Akt. This results in antiapoptotic signals regulating caspase 9, BAD, NF-kB, Fas ligand and others [25,26].
It is also well-known that NPM-ALK activates signal transducers and activators of transcription (STATs), particularly STAT3 but also STAT5. STAT3 is a key effector molecule of NPM-ALK signaling, being phosphorylated by NPM-ALK directly or through activation of JAK3 [26]. Downstream effects of STAT3 phosphorylation include increased levels of BCL2, BCL-XL, survivin and MCL-1.
Recently developed small molecule inhibitors of NPM-ALK principally act by impeding access of ATP to the ATP-binding pocket of the tyrosine kinase molecule, thus blocking activating phosphorylation [27]. Our cell line experiments utilizing PF-2341066 suggest that the previously documented association of NPM-ALK with PI3K/Akt activation is not due to activated ALK, but to other mechanisms likely including interactions of NPM-ALK with PLC-γ and PI3K, as described above.
It is also possible that abnormal NPM1/B23 may play a role in PI3K/Akt pathway regulation. NPM is a molecular chaperone involved in the shuttling of preribosome subunits from the nucleus to the cytoplasm during ribosome biogenesis and is involved in DNA repair, transcription and genomic stability [28]. NPM is a physiologic receptor for both PIP3 and for Akt, helps maintain a fine balance in Akt activation and may function as either an oncogene or a tumour suppressor [29] [30]. In the usual translocation affecting ALCL, the functional carboxy terminal portion of NPM1 is almost entirely replaced by oncogenic ALK [29]. It is not known if NPM1 is involved directly in Akt activation or in PTEN regulation.
PTEN phosphorylation has been proposed as a cause of decreased PTEN functional activity and associated Akt activation in Hodgkin lymphoma, and contributes to CD30 expression/activation [21]. Similarly, a study of T-lymphoblastic leukemia (T-ALL) noted that PI3K/Akt activation is common in that disease and usually accompanied by high expression of unmutated PTEN. In T-ALL, PI3K/Akt activation occurs through varied mechanisms including PTEN phosphorylation, PTEN mutation (uncommonly), Notch1 mutation and PTEN oxidation by reactive oxygen species (ROS)[31].
The PTEN molecule carries a carboxy-terminal regulatory tail that, when phosphorylated, decreases PTEN activity and increases stability, leading to accumulation [32]. Dephosphorylation triggers electrostatic binding to the plasma membrane, PIP3 dephosphorylation and PTEN degradation [33]. Phosphorylation of regulatory sites is not known to abrogate phosphatase activity and our finding that PTEN in SUDHL-1 retains PIP3 phosphatase activity shows that the molecule is functionally intact.
In one previous study, SUDHL-1 and Karpas-299 showed differences in levels of PTEN expression [34]. SUDHL-1 cells expressed high levels of PTEN and Karpas-299 did not express PTEN, while the Akt pathway was considered to be constitutively activated in both. In our hands, both of these cell lines are seen to express PTEN.
The significance of finding p27 expression by immunochemistry in both cell lines but only in a minority of patient samples is unclear, possibly related to better sensitivity when testing fresh cells and freshly fixed material versus archived paraffin-embedded samples. Lack of strong or frequent p27 labeling in patient materials likely is a reflection of Akt activation and of the proliferative nature of the disease.
There has been tremendous progress in our understanding of the biology and genetics of ALCL since the original morphologic description in 1985. ALCL has been described as a tumor of good prognosis and, indeed, in an international study of NHL outcomes in 1997 showed the best overall survival, not stratified for age [35]. Treatment outcomes for this disease, however, have not kept pace with improvements in other pediatric lymphomas. Even with 60-70% long term event free survival, the prognosis is now less favorable compared to other pediatric lymphomas, as recent treatment studies for Diffuse Large B-cell, Burkitt, Lymphoblastic and Hodgkin lymphomas have all approached, and in some exceeded 90% event free survival [36].
The results of the present study show that two thirds of cases of pediatric ALCL exhibit PI3K/Akt pathway activation, show retained PTEN expression that is predominantly localized to the cytoplasm, and at least some (7 of 13 patients tested) contain a component of a phospho form associated with decreased activity.
Our results also suggest that targeting ALK will not predictably inhibit the PI3K/Akt pathway, although it does inhibit STAT signaling, and that there may be benefit in targeting PI3K/Akt in other ways. It also suggests that targeted therapies may best be combined in a patient-individualized manner based on biologic studies of patient material.
Supplementary Material
Supplemental Figure 1. PTEN and pPTEN did not show decreased expression in SUDHL-1 48 hours after treatment with PF-2341066 compared to that 24 hours after treatment with 50 and 175 nM doses. MCF-7 is positive control for both PTEN and pPTEN blots and MDA-MB-468 is negative control for both.
Supplemental Figure 2. PTEN and pPTEN immunoprecipitates from cultured SUDHL-1 cells showed PIP3 phosphatase activity as illustrated, compared to a recombinant PTEN positive control and negative reagent control.
ACKNOWLEDGEMENTS
Kim Cripp, BS, and Nazanin Khajouee-Nejad for technical assistance, and Peter Hahn, PhD, for editorial assistance and advice.
Funded in part by the Paige Yeomans-Arnold Cancer Research Fund and by the Children’s Oncology Group/NIH Chair’s Grant U10 CA98543.
Footnotes
Conflict of Interest Statement
All authors disclose that they have no conflicts of interest.
REFERENCES
- 1.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–858. [PubMed] [Google Scholar]
- 2.Stein H, Foss HD, Durkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681–3695. [PubMed] [Google Scholar]
- 3.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110(7):2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiarle R, Voena C, Ambrogio C, et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 5.Uner AH, Saglam A, Han U, et al. PTEN and p27 expression in mature T-cell and NK-cell neoplasms. Leuk Lymphoma. 2005;46(10):1463–1470. doi: 10.1080/10428190500144813. [DOI] [PubMed] [Google Scholar]
- 6.Laver JH, Kraveka JM, Hutchison RE, et al. Advanced-stage large-cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate-dose methotrexate and high-dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. J Clin Oncol. 2005;23(3):541–547. doi: 10.1200/JCO.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow SH, Campo E, Harris NL, et al. Lyon: International Agency for Research on Cancer; 2008. WHO classification of tumours of hematopoietic and lymphoid tissues; p. 441. [Google Scholar]
- 8.Nasr MR, Laver JH, Chang M, et al. Expression of anaplastic lymphoma kinase, tyrosine-phosphorylated STAT3, and associated factors in pediatric anaplastic large cell lymphoma: A report from the children's oncology group. Am J Clin Pathol. 2007;127(5):770–778. doi: 10.1309/FNY8Y4H6PK1V2MGE. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AL, Levy R, Kim H, et al. Biology of the human malignant lymphomas. IV. Functional characterization of ten diffuse histiocytic lymphoma cell lines. Cancer. 1978;42(5):2379–2391. doi: 10.1002/1097-0142(197811)42:5<2379::aid-cncr2820420539>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Hecht BK, Epstein AL, Berger CS, et al. Histiocytic lymphoma cell lines: immunologic and cytogenetic studies. Cancer Genet Cytogenet. 1985;14(3–4):205–218. doi: 10.1016/0165-4608(85)90186-4. [DOI] [PubMed] [Google Scholar]
- 11.Fischer P, Nacheva E, Mason DY, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72(1):234–240. [PubMed] [Google Scholar]
- 12.Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278(36):33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- 13.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 14.Benharroch D, Meguerian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91(6):2076–2084. [PubMed] [Google Scholar]
- 15.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 16.Rassidakis GZ, Feretzaki M, Atwell C, et al. Inhibition of Akt increases p27Kip1 levels and induces cell cycle arrest in anaplastic large cell lymphoma. Blood. 2005;105(2):827–829. doi: 10.1182/blood-2004-06-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104(18):7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–250. doi: 10.1002/jcb.20187. [DOI] [PubMed] [Google Scholar]
- 19.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65(18):8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 21.Georgakis GV, Li Y, Rassidakis GZ, et al. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. Br J Haematol. 2006;132(4):503–511. doi: 10.1111/j.1365-2141.2005.05881.x. [DOI] [PubMed] [Google Scholar]
- 22.Trotman LC, Wang X, Alimonti A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6(12 Pt 1):3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 24.Bai RY, Ouyang T, Miething C, et al. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96(13):4319–4327. [PubMed] [Google Scholar]
- 25.Slupianek A, Nieborowska-Skorska M, Hoser G, et al. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 2001;61(5):2194–2199. [PubMed] [Google Scholar]
- 26.Barreca A, Lasorsa E, Riera L, et al. Anaplastic lymphoma kinase in human cancer. J Mol Endocrinol. 2011;47(1):R11–R23. doi: 10.1530/JME-11-0004. [DOI] [PubMed] [Google Scholar]
- 27.Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9(3):331–356. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grisendi S, Mecucci C, Falini B, et al. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6(7):493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 29.Falini B, Nicoletti I, Bolli N, et al. Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica. 2007;92(4):519–532. doi: 10.3324/haematol.11007. [DOI] [PubMed] [Google Scholar]
- 30.Ahn JY, Liu X, Cheng D, et al. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol Cell. 2005;18(4):435–445. doi: 10.1016/j.molcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vazquez F, Ramaswamy S, Nakamura N, et al. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20(14):5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci U S A. 2003;100(13):7491–7496. doi: 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turturro F, Frist AY, Arnold MD, et al. Biochemical differences between SUDHL-1 and KARPAS 299 cells derived from t(2;5)-positive anaplastic large cell lymphoma are responsible for the different sensitivity to the antiproliferative effect of p27(Kip1) Oncogene. 2001;20(33):4466–4475. doi: 10.1038/sj.onc.1204582. [DOI] [PubMed] [Google Scholar]
- 35.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 36.Jaglowski SM, Linden E, Termuhlen AM, et al. Lymphoma in adolescents and young adults. Semin Oncol. 2009;36(5):381–418. doi: 10.1053/j.seminoncol.2009.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PTEN and pPTEN did not show decreased expression in SUDHL-1 48 hours after treatment with PF-2341066 compared to that 24 hours after treatment with 50 and 175 nM doses. MCF-7 is positive control for both PTEN and pPTEN blots and MDA-MB-468 is negative control for both.
Supplemental Figure 2. PTEN and pPTEN immunoprecipitates from cultured SUDHL-1 cells showed PIP3 phosphatase activity as illustrated, compared to a recombinant PTEN positive control and negative reagent control.