Summary
The global logic used by the brain for differentially encoding positive and negative experiences remains unknown along with how such experiences are represented by collections of memory traces [1] at the cellular level. Here we contrast the cellular memory traces that form in the dorsal paired medial (DPM) neurons of Drosophila [2] after conditioning flies with odors associated with aversive or appetitive unconditioned stimuli (US) [3,4]. Our results show that the appetitive DPM neuron trace is distinguished from the aversive trace in three fundamental ways: (1) The DPM neurons do not respond to an appetitive US of sucrose by itself, in contrast to their robust response to an aversive US of electric shock. (2) The appetitive trace persists for twice as long as the aversive trace. (3) The appetitive trace is expressed in both neurite branches of the neuron, rather than being confined to a single neurite branch like the aversive trace. In addition, we demonstrate that training flies with non-nutritive sugars that elicit a behavioral memory that decays within 24 hr [5,6] generates, like aversive conditioning, a short-lived and branch-restricted memory trace, whereas training with nutrient sugars generates non-decaying behavioral memory and the more persistent and cell-wide memory trace. These results indicate that the persistence and breadth of the DPM neuron memory trace influences the duration of behavioral memory.
Results and Discussion
The initial question we asked was do the DPM neurons form an olfactory, cellular memory trace after appetitive conditioning? We have previously characterized several different cellular memory traces, defined as a change in response properties to the learned odor after conditioning, that form from aversive conditioning [1]. We imaged neurites of DPM neurons in flies receiving appetitive olfactory conditioning using the G-CaMP1.6 reporter (Figure 1A). Since the initial performance indices (PI) after appetitive conditioning are low [7, 8, 9], we performed the essential test-retest experiment [10, 11]. The results of the test-retest experiment indicate that essentially all flies acquire memory and that the PI indicates the probability that learned flies will make the proper decision at testing (Figure S1A). All trained flies were therefore used for imaging experiments.
Figure 1.
Forward Appetitive Conditioning Produces a Cell-Wide, Calcium-Based Memory Trace in the DPM Neuron Processes.
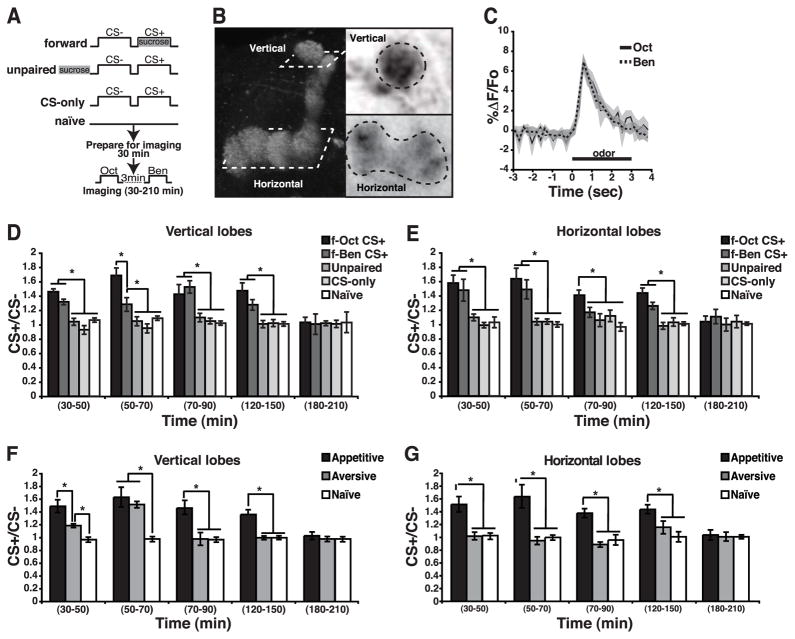
(A) Diagram illustrating the appetitive olfactory conditioning protocols used in this study. Starved flies were trained using forward, unpaired or CS-only conditioning. Naïve animals were used as control. The DPM neuron responses were subsequently recorded by presenting each odor for 3 sec separated by 3 min.
(B) Image of the DPM neuron innervation of the MB neuropil indicating the regions of interest subjected to imaging. The left panel illustrates the lobes of the MBs with the planes of imaging outlined. The right panels illustrate the regions of interest as viewed from the dorsal perspective.
(C) Time course of the fluorescence response of G-CaMP to Oct and Ben in the vertical branch of the DPM neuron calculated as the %ΔF/Fo. The shaded area around each line indicates the standard error of the mean across time.
(D) Bar graph of the response ratio of the CS+/CS− as a function of time after appetitive conditioning measured in the DPM vertical branch neurons after forward (f-Oct+, f-Ben+), unpaired, CS-only, and naïve conditioning. A robust increase in calcium influx was detected in the vertical branch at 30–50 min after forward conditioning with either CS+ odor. The enhanced response to the trained odor remained significant at 50–70 min, 70–90 min and 120–150 min after conditioning. The increased was not observed after unpaired or CS-only conditioning, or in naïve flies. Asterisks indicate a statistically significant difference as determined by Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.02). n=8–12 for all groups.
(E) Bar graph of the response ratio of the CS+/CS− after appetitive conditioning measured horizontal branch of the DPM neurons. An increase in calcium was detected in the horizontal branch at 30–50, 50–70, 70–90 and 120–150 min after forward conditioning. Asterisks indicate a statistically significant difference as assessed by Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.0031). n=6–10 for all groups.
(F) The DPM neuron vertical branch exhibited an increased response to the trained odor after appetitive and aversive conditioning. This increased response was observed with both training protocols at 30–50 and 50–70 min after training. In contrast with the aversive memory trace, the appetitive memory trace persisted in the vertical lobes during the 70–90 and 120–150 min time windows and became undetectable by 180–210 min. Asterisks indicate statistically significant differences as determined with Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.04). n=8–9 for all groups.
(G) The DPM neuron horizontal branch exhibited an increased response to the trained odor after appetitive but not aversive conditioning. Appetitive conditioning induced an increased response to the trained odor in the horizontal branch at 30–50, 50–70, 70–90, and 120–150 min after conditioning. In contrast, aversive olfactory conditioning failed to induce a memory trace in the DPM horizontal branch. Asterisks indicate statistically significant differences as determined with Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.009). n=6–15 for all groups. For all panels, error bars indicate the standard error of the mean. See also Figure S1.
We tested the responses of the DPM neurons at various times beginning 30 min post-conditioning by the presenting the CS+ and CS− odors. A DPM neuron schema and the imaged region of interest are shown in Figure 1B. Figure 1C illustrates the typical response of DPM neurons of naïve flies to the two odors used in the experiments. The response magnitude to odor varied significantly between individuals (Figure S1B). Nevertheless, the ratio of the response between the two odors used was similar within individual flies, such that the average ratio in the response between the odors was ~1.0 (Figure S1B). The similarity in response magnitude between odors within a fly and the stable response to the CS− odor offered the opportunity to use the CS− response as an internal control for each individual fly by calculating the CS+/CS− response ratio.
Flies trained in a forward manner formed a memory trace (as the CS+/CS− response ratio) in the vertical processes of the DPM neurons (Figure 1D). Identical conclusions were reached by quantifying the average response magnitude for each group of flies to the CS+ and CS− odors (Figure S1C). The memory trace was observed at 30 min and persisted until 150 min after conditioning (Figure 1D). As c316-gal4 has expression outside DPM neurons including MB cells [12], we obtained similar results when a more specific driver, VT64246-gal4, was used [13] (Figure S1D). In addition, comparable results were obtained with the G-CaMP3.0 reporter (Data not shown). Flies receiving unpaired or CS-only conditioning failed to exhibit the same increase in the CS+/CS− ratio (Figure 1D). Surprisingly, we also found evidence for a memory trace after appetitive conditioning in the DPM neuron horizontal branch (Figure 1E, S1D). An increase in calcium response to the CS+ odor was observed in the horizontal lobe from 30–150 min after conditioning.
The above data suggest that there exist qualitative and quantitative differences in the aversive and appetitive memory traces that form in the DPM neurons. However, our previous characterization of the aversive memory trace used conditioning while under the microscope and a within-animal protocol, in which the response to odors is evaluated before and after conditioning for each fly [2]. The appetitive memory trace described above utilized en masse training and a between-group experimental protocol. To confirm that the differences observed are authentic, we revisited the traces formed after aversive and appetitive conditioning by measuring them in parallel training the flies en masse.
An increase in the CS+/CS− response ratio in the processes of the vertical lobe was observed 30 to 70 min after aversive conditioning (Figure 1F, S1D). This increase was absent at subsequent time windows. In contrast, an increase of the CS+/CS− response ratio was observed from 30 to 150 min after appetitive conditioning (Figure 1F, S1D). Confirming our prior within-animal experiments [2], we found no evidence for a memory trace in the DPM horizontal lobes after aversive conditioning (Figure 1G, S1D). The differences observed between the aversive and the appetitive traces cannot be attributed to differences in the duration of the CS/US presentation, since no differences were observed in behavioral memory or in the DPM memory trace in flies trained using either a 1 or 2 min aversive training protocol (2 min CS+ along with 24 electric-shock pulses, 30 s of air and 2 min of CS−) (Figure S1E).
We draw three important conclusions from this set of data. First, a memory trace does form in the DPM neurons when flies are trained using a US of positive value. Second, the appetitive trace forms by 30 min after conditioning and persists to at least 150 min, longer than the trace generated by aversive conditioning [2]. Third, the memory trace is observed in the DPM neuron processes that innervate both the vertical and horizontal lobes of the MB’s, unlike the aversive trace that is delimited to the vertical processes.
The memory trace differences are intriguing as related to the known differences in behavioral memory produced from aversive or appetitive conditioning. The most interesting distinction is persistence: memory of a single training trial of aversive conditioning decays quickly [3, 10]. In contrast, memory after a single trial of appetitive conditioning forms stable, long-term memory (LTM) [4, 8, 9]. In addition, dopamine is required for aversive and octopamine for appetitive conditioning [14, 15, 16]. The difference in neuromodulator requirement may be related to memory trace differences.
We previously reported that the DPM memory trace formed after aversive conditioning is dependent on the amn gene product [2]. We wondered whether this would also hold true for the appetitive memory trace. The typical increased in response to the CS+ odor observed across a 30 to 90 min time window after appetitive conditioning in control flies (c316-gal4 flies) was absent in amnX8 mutant flies in both the vertical and horizontal branches (Figure S1F). However, providing the wild-type amn gene (uas-amn1 transgene) along with, c316-gal4 (DPM neuron driver) throughout development rescued the normal increase in the CS+/CS− ratio. These data show that the olfactory memory traces formed in the DPM neurons after appetitive and aversive conditioning are dependent on the function of the amn gene in these neurons. It has been reported that amnX8 mutant has some Gal4 activity in MBs [17], giving a potential risk of contamination in imaging experiments. However, the calcium-based memory traces representing short- and intermediate-term memory [1] known to form in the MB neurons are distinct in time course and localization within the lobes compared to the DPM memory trace [18], making it highly unlikely that the results obtained stem from this contaminating Gal4 activity.
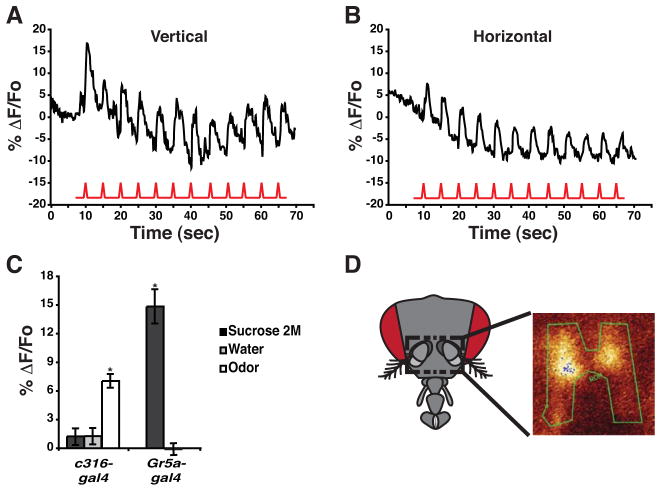
Our previous results [2] showed that the DPM neurons respond with calcium influx to electric shock pulses. We confirmed here their responsiveness to the US of electric shock in both the vertical and horizontal lobes (Figure 2A, B). These data offer the possibility that DPM neurons might integrate the CS and US stimuli independently of other neurons within the olfactory system [2]. This led us to address the issue of whether these neurons also respond to the US used for appetitive conditioning. For this purpose, flies were stimulated with water or a 2 M sucrose solution, delivered to the gustatory sensilla of the proboscis. Surprisingly, the change in fluorescence observed in the DPM neurons upon stimulation with water or sucrose was not significantly different from zero, although the same flies subsequently responded to odor (Figure 2C). To ensure that the peripheral sensory system remained responsive during the recordings, we monitored the response of flies expressing G-CaMP in the gustatory receptor neurons identified by the Gr5a-gal4 transgene [19]. Stimulation with sucrose but not water elicited a response in Gr5a-gal4 neurons (Figure 2C, D). These data indicate that the DPM neurons fail to respond to the US of sucrose during appetitive conditioning. Furthermore, they are inconsistent with the possibility that the DPM neurons integrate CS+ and US during appetitive conditioning [2], at least using G-CaMP responsiveness to assay this possibility.
Figure 2.
DPM Neurons Do Not Respond to the Appetitive Unconditioned Stimulus.
(A) Calcium influx into the DPM neuron processes that innervate the vertical lobes of the MB’s. Electric shock pulses of 90 V and 1.25 sec duration (red trace) were delivered every 5 sec. The upper trace represents the average %ΔF/Fo across the region of interest, which included the distal end of the vertical lobes. An obvious calcium response was observed with each shock pulse riding on the top of a decaying background due to bleaching over a 70 sec scanning period.
(B) Calcium influx into the DPM neuron processes that innervate the horizontal lobes of the MB’s. Electric shock pulses of 90 V and 1.25 sec duration (red trace) were delivered every 5 sec. The upper trace represent the average %ΔF/Fo across the region of interest, which included the area occupied by the horizontal lobes. An obvious calcium response was observed, with each shock pulse riding on the top of a decaying background due to bleaching over a 70 sec scanning period.
(C) Summary of DPM and taste neuron responses to sucrose, water, and odor stimuli. Flies were stimulated with water or a 2 M sucrose solution to the gustatory sensilla of the proboscis and the response of the neurons calculated as a ΔF/Fo. The responses of the DPM neurons (c316-gal4) to sucrose and water were not significantly different from zero (Wilcoxon signed rank test p=0≥0.0547 respectively). However, the DPM neurons tested in the same flies previously stimulated with sucrose exhibited a robust response to odor (Oct), providing assurance that the flies had the capability of responding. The response to odor was significantly different from the responses to water or sucrose (Kruskal-Wallis statistic of 13.37, p=0.0013; Mann-Whitney pairwise comparison, p=0.0006). As a positive control, the response of taste neurons (Gr5a-gal4) to sucrose stimulation was recorded. Flies were prepared as shown in panel D and stimulated with water or a saturated solution of sucrose. The Gr5a-gal4 neurons exhibited a significant increase in calcium influx in response to sucrose compared to the lack of response to water (Mann-Whitney pairwise comparison, p=0.0007). Error bars indicate the standard error of the mean. Asterisks indicate statistically significant differences. n=7–10 for all groups.
(D) Schematic illustration of a fly head showing the area of cuticle that was removed to image the Gr5a-gal4 neurons in the subesophageal ganglion. Removal of antennae and surrounding cuticle allowed visual access to this area of the brain. Gr5a-gal4 taste neurons were visualized in vivo using the G-CaMP reporter to monitor the change in calcium influx in response to sucrose. The green trace represents the region of interest selected for scanning.
Previous studies have shown that DPM synaptic activity is required between acquisition and retrieval for normal 3 hr aversive or appetitive memory. Additionally, blocking DPM neuron during retrieval of 1 h or 3 h memory has no effect (Figure S2), [20]. More specifically, blocks in synaptic transmission during the first hour after training are sufficient to impair 3 hr aversive and appetitive memory [2, 17, 20]. Unfortunately, none of these time window blocks discriminate between the unique time differences in the existence of the DPM memory trace for aversive and appetitive memory described above. If the differences in the time of existence of the DPM memory trace for aversive and appetitive memory are truly relevant for behavioral memory, then blocking synaptic transmission during time windows unique to the appetitive trace should selectively alter appetitive but not aversive olfactory memory.
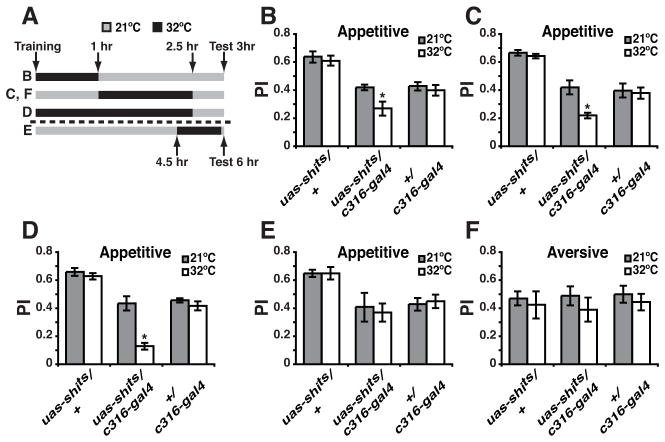
We therefore imposed synaptic transmission blocks, using uas-shits, in DPM neurons across various time windows after conditioning (Figure 3A). No significant within-genotype effect of temperature was observed in any experiment in the control flies carrying only the c316-gal4 or the uas-shits element (Figure 3B–F). For the bigenic flies, blocking DPM synaptic transmission immediately after conditioning across a 1 hr window or during the time period that the appetitive memory trace extends beyond the aversive trace (1 to 2.5 hr after training) impaired appetitive memory tested at 3 hr (Figure 3B, C). Blocking synaptic transmission across the complete time window of appetitive memory trace existence (to 2.5 hr) nearly abolished 3 hr memory (Figure 3D). As predicted, a block of DPM signaling from 4.5 to 6 hr after conditioning, a time window outside the memory trace existence, failed to impair 6 hr appetitive memory (Figure 3E). Finally, blocking synaptic transmission from DPM neurons across the period of the extended appetitive trace (1 to 2.5 hr after conditioning) but after aversive conditioning was without effect on aversive memory tested at 3 hr (Figure 3F). Prior studies have reported that a synaptic blockade across a 60–90 min time window disrupts performance tested later [13]. The basis for the discrepant results between the two studies is, at present, unclear. Our results document that there exists a differential time requirement for DPM neuron synaptic transmission for the formation of olfactory memories of opposed value.
Figure 3.
The Appetitive Memory Trace of the DPM Neuron Defines the Time Window Over Which Synaptic Transmission is Required for Normal Appetitive Memory.
(A) Schematic illustration of the conditioning protocols with temperature shifts that were used for these experiments. All flies were trained at the permissive temperature (21°C) and then shifted to restrictive temperature (32°C) for the times indicated. Retrieval tests were all performed at 21°C at 3 or 6 hr after training and a Performance Index (PI) calculated. The letters (B–E) at the left side of the illustration are a cross-reference to the data panels B–E. For reasons that are unclear, the uas-shits/+ genotype often performed at higher levels than the c316-gal4/+ control or the experimental genotype under permissive conditions. However, the relevant comparisons are within-genotype and between temperatures.
(B) Flies were trained using the appetitive protocol at 21°C, transferred to 32°C immediately after training and returned to 21°C after 1 hr. Blocking DPM synaptic transmission across this time window significantly reduced 3 hr appetitive memory (Mann-Whitney pairwise comparison, p=0.041) for the experimental group. No significant difference was observed between permissive and restrictive temperatures for control groups (Mann-Whitney pairwise comparisons, p≥0.4557).
(C) Flies were trained using the appetitive protocol at 21°C, transferred to 32°C at 1 hr after training and returned to 21°C after 2.5 hr. Blocking DPM synaptic transmission across this time window significantly reduced 3 hr appetitive memory (Mann-Whitney pairwise comparison, p=0.0041) for the experimental group. No significant difference was observed between permissive and restrictive temperatures for control flies (Mann-Whitney pairwise comparison, p≥0.3939).
(D) Flies were trained using the appetitive protocol at 21°C, transferred to 32°C immediately after training and returned to 21°C after 2.5 hr. Blocking DPM synaptic transmission across this time window nearly abolished 3 hr appetitive memory (Mann-Whitney pairwise comparison, p=0.0022) for the experimental group. The performance of c316-gal4/uas-shits flies at the restrictive temperature was significant different from zero (Wilcoxon signed rank test, p=0.0156). No significant difference was observed between permissive and restrictive temperatures for control flies (Mann-Whitney pairwise comparison, p=≥0.5887).
(E) Flies were trained using the appetitive protocol at 21°C, transferred to 32°C at 4.5 hr after training and returned to 21°C before testing. Blocking DPM synaptic transmission from 4.5 to 6 hr after training did not alter 6 hr appetitive memory (Mann-Whitney pairwise comparison, p=0.6991). No significant difference was observed between permissive and restrictive temperature for control flies (Mann-Whitney pairwise comparison, p=≥0.6991).
(F) Flies were trained using the aversive protocol at 21°C, transferred to 32°C 1 hr after training and returned to 21°C after 2.5 hr. Blocking DPM synaptic transmission from 1 to 2.5 hr after training did not impair 3 hr aversive memory (Mann-Whitney pairwise comparison, p=0.2786) for the experimental group. No significant difference was observed between permissive and restrictive temperature for control flies (Mann-Whitney pairwise comparison, p≥0.5737). Error bars indicate the standard error of the mean. n=6–8 for all groups. See also Figure S2.
The results described above are consistent with the hypothesis that the time-extended and broad appetitive memory trace is responsible for the remarkable stability of appetitive olfactory memory that is instilled independent of the time-extent of odor-sucrose pairing or sucrose concentration (Figure S3). We further tested this hypothesis by varying the nutritional quality of the sugar used as the US. Two recent studies claimed that the stability of appetitive memory is related to the nutritional value of the sugar used as the US [5, 6].
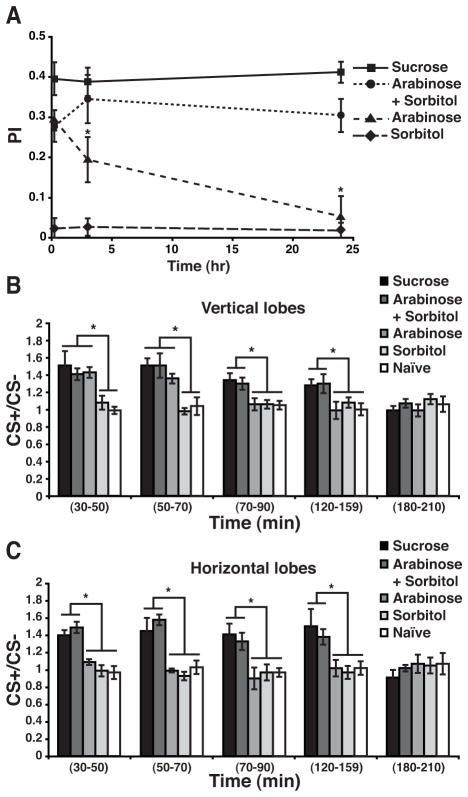
In contrast to the very stable memory observed after conditioning with sucrose, flies conditioned with D-arabinose, a non-nutritive sugar, exhibited a decaying memory (Figure 4A). After conditioning with arabinose supplemented with sorbitol as a tasteless but nutritionally valuable alcohol, memory stability was restored (Figure 4A). Finally, no behavioral memory was observed when flies were trained with sorbitol alone (Figure 4A); although it has been reported that sorbitol-reinforced memory can be formed after multiple-spaced training sessions [5]. If the extended and broader DPM trace observed after appetitive conditioning contributes to more stable behavioral memory, we predicted that flies conditioned with a non-nutritive sugar would form the ‘short’ trace and that the trace would be restricted to the vertical lobes.
Figure 4.
The Duration of DPM Neuron Memory Trace Dictates Memory Stability.
(A) Memory decay of flies conditioned with solutions of sucrose, arabinose+sorbitol, arabinose or sorbitol. No memory decay was observed in the first 24 hr after training when flies were conditioned using sucrose or arabinose+sorbitol as the US. In contrast, memory decayed to baseline at 24 hr when flies were conditioned with the non-nutritional sugar arabinose. Three minutes and 24 hr memory of flies trained with arabinose was significantly lower than flies trained with sucrose or arabinose+sorbitol (Two-way ANOVA p<0.0001; Bonferroni post-test, p<0.05). No memory was observed when flies were trained using sorbitol as the US. The PI at 3 min, 3 hr or 24 hr was not significantly different from zero (Wilcoxon signed rank, p≥0.1562).
(B) The CS+/CS− response ratios of the DPM neurons at different times after conditioning using different sugars as the US with naïve flies as a control group. A robust increase in calcium influx in response to the CS+ was detected in the DPM neuron vertical branch at 30–50 min after conditioning using sucrose, arabinose and arabinose+sorbitol. This enhancement in the response to the trained odor remained significant at 50–70 min for the three groups. In contrast, only the flies trained with sucrose or arabinose+sorbitol showed an increased calcium response to the trained odor at 70–90 and 120–150 min. No significant increase was detected using flies trained with arabinose for these time windows. No group exhibited a significant difference from the naïve group at 180–210 min. Error bars indicate the standard error of the mean as determined with Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.0207). Asterisks indicate a statistically significant difference. n=6–10 for all groups
(C) A robust increase in calcium influx to the CS+ was detected in the horizontal branch at 30–50 min after conditioning using sucrose or arabinose+sorbitol as US. This enhancement remained significant at 50–70, 70–90, 120–150 min after conditioning. No significant differences were detected after 180 min. The increased calcium response was not observed after conditioning using arabinose alone or sorbitol as US, or in naïve flies. Error bars indicate the standard error of the mean as determined with Kruskal-Wallis analysis followed by Mann-Whitney pairwise comparisons (p≤0.0411). Asterisks indicate a statistically significant difference. n=6–10 for all groups. See also Figure S3.
We thus measured the duration of the DPM trace after flies were trained with nutritive vs non-nutritive sugars. The trace formed after conditioning with arabinose did not persist past the 30–70 min time window and was present only in the vertical lobes (Figure 4B, C). In contrast, flies conditioned with arabinose supplemented with sorbitol exhibited a trace in both lobes that persisted at 150 min after training (Figure 4B, C). No trace was detected when flies were trained with sorbitol alone (Figure 4B, C). These results provide powerful evidence for the conclusion that the duration and the breadth of the DPM neuron memory trace underlies the stability of memory: a dietary manipulation that eliminates appetitive memory measured at 24 hr also shortens the duration and breadth of the DPM neuron memory trace. Restoring the nutritive value to the US restores the stability of appetitive memory and expands the cellular space and the duration of the DPM neuron memory trace to a time window of 2.5 hr after conditioning.
These results address the perplexing question of how the brain encodes memories of positive or negative value. They show that there is an overlap in the neurons that encode memories of opposite value. An alternative possibility was that appetitive and aversive memory traces might form in non-overlapping sets of neurons. A second important conclusion is that the cellular changes that occur with learning in these neurons are qualitatively and quantitatively different depending on the valence of the conditioning. The appetitive memory trace forming in the DPM neurons is more persistent than the aversive trace, existing across a time window of ~150 min after conditioning, and is cell wide, forming in the two major branches of the DPM neuron that innervate the vertical and horizontal lobes of the MB’s. The appetitive memory trace is also dependent on the amn gene product and influenced by the nutritive value of the sugar used for reinforcement. The observations together lead to the major conceptual conclusion that appetitive cellular memory traces form in at least some of the same neurons that encode aversive memories, but they differ in essential features like persistence and cellular expanse.
Although the molecular mechanisms underlying these differences remain unknown, we provide compelling evidence that the persistent and cell-wide DPM appetitive memory trace underlies time-stable, appetitive behavioral memory. The simplest model to explain this is that conditioning instills an increase in DPM neuron excitability, for an hour after aversive conditioning and for 2.5 hr after appetitive conditioning. This increase in excitability must translate to increased spontaneous synaptic activity onto follower neurons, presumably the MB neurons, to help stabilize memory. We provided evidence for this model by synaptic blocking experiments across different time intervals after conditioning and by relating the persistence and spatial extension of the memory trace to the stability of behavioral memory. We demonstrated that training flies with non-nutritive sugars that produce a decaying memory generate a short-lived and branch-restricted trace. When supplemented with a nutritive carbohydrate source, a non-nutritive sugar is capable of engendering a longer-lasting trace along with robust stable behavioral memory. Thus, there exists an intimate relationship between the long-lasting and cell-wide DPM neuron memory trace and stable behavioral memory.
Attached to the relationship between the persistent and cell-wide DPM neuron memory trace and the marked stability of appetitive behavioral memory, is the fly’s perception of nutrient value. Sugars like sucrose may provide two types of information that are integrated with the odor CS: sweetness and nutrient value. Sweetness, per se, appears sufficient to induce the short-lived trace and decaying behavioral memory while nutrient value is required for the formation of the extended memory trace and stable behavioral memory. This suggests that the fly assesses the nutrient value of the appetitive US using unknown mechanisms and then passes this assessment onto the DPM neurons to influence the duration of the memory trace.
Experimental Procedures
Detailed information can be found in Supplemental Experimental Procedures. Standard, two-odor discriminative, and negatively or positively reinforced classical conditioning was employed for behavioral conditioning. Functional imaging procedures were performed as previously described [2, 21].
Supplementary Material
Highlights.
A memory trace forms in both branches of DPM neurons after appetitive conditioning.
A memory trace forms in only one branch of DPM neurons after aversive conditioning.
The appetitive memory trace persists twice as long as the aversive trace.
More persistent appetitive trace is critical for non-decaying behavioral memory.
Acknowledgments
This research was supported by grant NS052351 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 4.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 6.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Buchanan ME, Han KA, Davis RL. The GABAA receptor RDL suppresses the conditioned stimulus pathway for olfactory learning. J Neurosci. 2009;29:1573–1579. doi: 10.1523/JNEUROSCI.4763-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colomb J, Kaiser L, Chabaud MA, Preat T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 2009;8:407–415. doi: 10.1111/j.1601-183X.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 9.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 12.Wu CL, Shih MF, Lai JS, Yang HT, Turner GC, Chen L, Chiang AS. Heterotypic gap junctions between two neurons in the drosophila brain are critical for memory. Curr Biol. 2011;21:848–854. doi: 10.1016/j.cub.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Lee PT, Lin HW, Chang YH, Fu TF, Dubnau J, Hirsh J, Lee T, Chiang AS. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci U S A. 2011;108:13794–13799. doi: 10.1073/pnas.1019483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.