Abstract
We hypothesized that aliskiren provides renoprotection in diabetic animals that did not receive sufficient renoprotection by AT1-receptor antagonist treatment. Type 2 diabetic KKAy mice were treated with group 1: vehicle or group 2: valsartan (15 mg/kg per day) from 12 to 16 weeks of age. The mice were subsequently divided into 4 groups and treated with the following combinations of drugs for another 6 weeks: 1: group 1 kept receiving vehicle, 2: group 2 continuously received 15 mg/kg per day of valsartan (Val-Val15), 3: group 2 received 50 mg/kg per day of valsartan (Val-Val50), 4: group 2 continuously received 15 mg/kg per day of valsartan with 25 mg/kg per day of aliskiren (Val-Val+Ali). Aliskiren exerted significant anti-albuminuric effects, whereas valsartan failed to ameliorate the albuminuria in the first four weeks. Surprisingly, the increasing dosage of valsartan in the Val-Val50 group showed non-significant tendencies to attenuate the albuminuria compared with vehicle infusion. Val-Val+Ali significantly suppressed the development of albuminuria and podocyte injury. Val-Val50 and Val-Val+Ali showed similar suppression of angiotensin II contents in the kidney of KKAy mice. In conclusion, the anti-albuminuric effect that was observed in the type 2 diabetic mice showing no anti-albuminuric effect by valsartan can be attributed to the add-on aliskiren.
Keywords: aliskiren, valsartan, diabetic nephropathy
Introduction
Diabetic nephropathy, one of the main complications of diabetes, develops in a time-dependent manner, and the number of patients requiring dialysis or transplantation is steadily increasing. Increases in urinary excretion of albumin and total protein occur during the development of diabetic nephropathy (1 – 4). Both basic and clinical studies have demonstrated that treatment with renin–angiotensin system (RAS) inhibitors delays the development and progression of albuminuria (5 – 9). However, significant numbers of patients are resistant to treatments for reduction of albuminuria with AT1-receptor antagonists or angiotensin-converting enzyme (ACE) inhibitors. A recent clinical trial, named the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study, reported that treatment with aliskiren, a renin inhibitor, in addition to losartan (100 mg daily, a maximal recommended renoprotective dosage in clinical medicine) prevented the decrease in the estimated glomerular filtration rate and increased the number of type 2 diabetic hypertensive patients with overt albuminuria who showed 50% albuminuria reduction during the 6-month observation period (10), thereby demonstrating the renoprotective benefit of aliskiren treatment in patients already receiving RAS inhibitors. On the other hand, the blood pressure of patients receiving placebo instead of aliskiren in the AVOID study was kept under control by the original dose of losartan or by adding a non-RAS-blocking anti-hypertensive drug. Thus, it is still premature to conclude that losartan monotherapy perfectly blocks RAS in the kidney of patients. Therefore, it remains unknown whether aliskiren exerted its RAS-inhibiting effect to compensate for insufficient RAS inhibition by AT1-receptor antagonist monotherapy or whether aliskiren had a totally different mechanism of renoprotection from its RAS-blocking effect. Regarding this issue, there will be clinical cases that require a decision for whether to 1) increase the dosage of the currently administered AT1 antagonist or 2) additionally treat with aliskiren together with the currently administered AT1 antagonist to protect the kidney of type 2 diabetic patients. However, no studies have compared the efficacy of these two treatments to clarify this issue.
The present study was conducted to compare the efficacy of increasing the dosage of valsartan, an AT1-receptor antagonist, with that of add-on treatment with aliskiren in type 2 diabetic KKAy mice with overt albuminuria that had been treated with an “insufficient” dosage of valsartan. We report that additional treatment with aliskiren elicited a stronger renoprotective effect than increasing the dosage of valsartan even with dosages that similarly suppressed the renal angiotensin II (AngII) level.
Materials and Methods
Animals and experimental design
All experimental procedures were performed according to the guidelines for the care and use of animals established by Kagawa University. Twelve-week-old male KKAy and C57BL/6J mice (C57BL) were purchased from CLEA Japan (Tokyo). Our preliminary experiments as well as reports from the other groups (11, 12) have shown that KKAy mice at this age do not show any increase in serum creatinine levels (C57BL vs. KKAy: 0.12 ± 0.01 vs. 0.10 ± 0.01 mg/dL, n = 6). In the first set of experiments, KKAy mice were randomly divided into two experimental groups as follows: 1) group 1 was the vehicle group (n = 10), and 2) group 2 was the valsartan-treated group (15 mg/kg per day, p.o., n = 30). At 4 weeks after starting the first experiment, the mice were divided into four groups and treated for a further 6 weeks as follows: 1) group 1 mice in the first phase receiving vehicle (vehicle, n = 10), 2) group 2 mice in the first phase receiving 15 mg/kg per day of valsartan (Val-Val15, n = 10), 3) group 2 mice in the first phase receiving an increased dosage of valsartan (50 mg/kg per day; Val-Val50, n = 10), and 4) group 2 mice in the first phase receiving add-on treatment with aliskiren (25 mg/kg per day; Val-Val+Ali, n = 10). C57BL mice were used as non-diabetic controls (n = 10). Another set of KKAy mice were treated as follows: 1) aliskiren treatment (25 mg/kg per day) for 10 weeks (Ali-mono, n = 8), and 2) aliskiren treatment (25 mg/kg per day) for 10 weeks and valsartan (15 mg/kg per day) added from week 4 (Ali-Ali+Val, n = 8). The mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and osmotic minipumps (Models 1002 and 1004; ALZET, Cupertino, CA, USA) were implanted subcutaneously at the dorsum of the neck to infuse vehicle or aliskiren. The osmotic minipumps were replaced at weeks 4 and 8.
Systolic blood pressure (SBP) was measured in conscious mice by tail-cuff plethysmography (BP-98A; Softron, Tokyo) and 24-h urine samples were collected at weeks 0, 2, 4, 6, 8, and 10. Postprandial blood glucose was measured with a glucometer (Sanwa-Kagaku, Nagoya) at weeks 0, 2, 4, 6, 8, and 10. All animals underwent a 12-h acclimatization period in metabolic cages prior to urine collection. Blood and kidney samples were harvested at week 10. Half of the kidney was dissected immediately after blood collection and snap-frozen in liquid nitrogen for measurement of the renal AngII content. Kidney sections were fixed in 10% formalin (pH 7.4) for histological examination or frozen in Tissue-Tek OCT compound (Sakura Finetech, Tokyo) for senescence-associated β-galactosidase (SABG) staining.
Immunohistochemistry for desmin
Immunohistochemistry for desmin was performed using a Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences, Tokyo) as previously described (13, 14). Deparaffinized sections were incubated with 0.1% hydrogen peroxide in methanol for 10 min to block endogenous enzymes. After blocking, the sections were incubated with the primary antibody overnight at 4°C. Bound antibodies were visualized using the substrate DAB (DakoCytomation, Glostrup, Denmark). Counterstaining was performed with hematoxylin (DakoCytomation). Sections incubated without the primary antibody were used as controls. The antibody-positive areas were calculated from 20 randomly selected microscopic fields (× 200) in each section. The histological analysis was evaluated using a color image analysis system (Image J) in a blind manner.
SABG staining
Kidney cryosections (16 µm) were washed with PBS and fixed with 0.5% glutaraldehyde for 15 min. The sections were then immersed in β-galactose staining solution (pH 6.0) containing 1 mg/mL 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside, 5 mM potassium ferrocyanide, 150 mM NaCl, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet-40 for 12 h at 37°C (15, 16).
Real-time RT-PCR
The p21, renin, and β-actin mRNA expression levels were analyzed by real-time PCR using a LightCycler FastStart DNA Master SYBR Green I Kit (Applied Biosystems, Foster City, CA, USA) as previously described (17). The primer sequences for p21, rennin, and β-actin were as follows: p21 forward, 5′-TCCTATACCGGAGGAACAGTCCTA-3′ and reverse, 5′-AAGGAACCAGGCAGGGAATG-3′; renin forward, 5′-AGGTAGCGACCCGCAGCATTATC-3′ and reverse, 5′-ACCCCCTTCATCGTGATCTGCCA-3′; and β-actin forward, 5′-CCCGCGAGCACAGCTTCTTTG-3′ and reverse, 5′-ACATGCCGGAGCCGTTGTCGAC-3′. All data were expressed as relative differences to vehicle-infused rats after normalization for β-actin expression.
Other analytical procedures
Urinary albumin excretion was determined using a commercially available ELISA kit (Shibayagi, Shibukawa). The urinary creatinine concentration was measured using an assay kit (Creatinine-test; Wako Co., Tokyo). The AngII level in the kidney was measured using a radioimmunoassay as described (18).
Statistical analysis
Data are expressed as means ± S.E.M. The statistical significance of differences was assessed by analysis of variance, followed by the Tukey-Kramer test. Values of P < 0.05 were considered to indicate statistical significance.
Results
Vehicle-treated KKAy mice showed higher SBP at weeks 4 and 10 than C57BL mice (Table 1). Valsartan at 15 mg/kg per day for the first 4 weeks did not affect SBP in KKAy mice. Val-Val15 also did not affect the increased SBP. Val-Val50 significantly reduced SBP at week 10. Val-Val+Ali caused a remarkable reduction in SBP in KKAy mice. Significant anti-hypertensive effects were also observed in Ali-mono, the group that received only aliskiren (25 mg/kg per day) during the whole experimental period (92 ± 5 mmHg), and Ali-Ali+Val, the group that received aliskiren during the whole experimental period and add-on valsartan (15 mg/kg per day) from week 4 (94 ± 3 mmHg).
Table 1.
Changes in blood pressure, body weight, blood glucose, and renal renin mRNA expression
| C57BL | Vehicle | Val-Val15 | Val-Val50 | Val-Val+Ali | ||
|---|---|---|---|---|---|---|
| baseline | 93 ± 3 | 111 ± 3* | 110 ± 4* | 113 ± 2* | 108 ± 5* | |
| SBP (mmHg) | week 4 | 94 ± 3 | 120 ± 2* | 121 ± 6* | 119 ± 4* | 119 ± 4* |
| week 10 | 97 ± 3 | 129 ± 2* | 128 ± 3* | 109 ± 4# | 86 ± 3# | |
| baseline | 27.6 ± 0.4 | 42.9 ± 1.0* | 40.8 ± 0.7* | 42.6 ± 0.9* | 41.4 ± 0.8* | |
| BW (g) | week 4 | 28.9 ± 0.5 | 48.1 ± 1.1* | 45.3 ± 0.7* | 47.4 ± 1.1* | 47.4 ± 0.7* |
| week 10 | 30.8 ± 0.6 | 49.6 ± 0.8* | 50.2 ± 1.0* | 52.5 ± 1.3* | 47.3 ± 1.8* | |
| baseline | 125 ± 4 | 386 ± 25* | 431 ± 23* | 444 ± 25* | 423 ± 44* | |
| BG (mg/dL) | week 4 | 117 ± 4 | 448 ± 19* | 411 ± 30* | 460 ± 29* | 496 ± 14* |
| week 10 | 123 ± 5 | 487 ± 16* | 449 ± 16* | 454 ± 39* | 216 ± 32*# | |
| Renin (fold) | week 10 | 1.00 ± 0.10 | 1.02 ± 0.11 | 1.54 ± 0.14 | 1.67 ± 0.08* | 3.06 ± 0.28*# |
All data are expressed as relative differences to vehicle-infused rats after normalization for β-actin expression and are shown as means ± S.E.M.
P < 0.05 vs. C57BL mice.
P < 0.05 vs. vehicle group.
SBP: systolic blood pressure, BW: body weight, BG: blood glucose.
KKAy mice at baseline showed significantly greater body weight (BW) than C57BL. Neither Val-Val15, Val-Val50, nor Val-Val+Ali showed changes in BW throughout the experimental period. Ali-mono and Ali-Ali+Val showed significantly smaller BW than the vehicle group both at week 4 (42.7 ± 2.1 and 42.5 ± 1.1 g, respectively) and 10 (44.2 ± 2.1 and 45.7 ± 1.1 g, respectively).
Vehicle-treated KKAy mice showed significantly higher postprandial blood glucose levels at weeks 4 and 10 than C57/BL mice (Table 1). Neither Val-Val15 nor Val-Val50 affected the postprandial blood glucose. In contrast, Val-Val+Ali significantly decreased the postprandial blood glucose at week 10 in KKAy mice. Anti-hyperglycemic effects were also observed in Ali-mono (189 ± 48 mg/dL) and Ali-Ali+Val (225 ± 50 mg/dL).
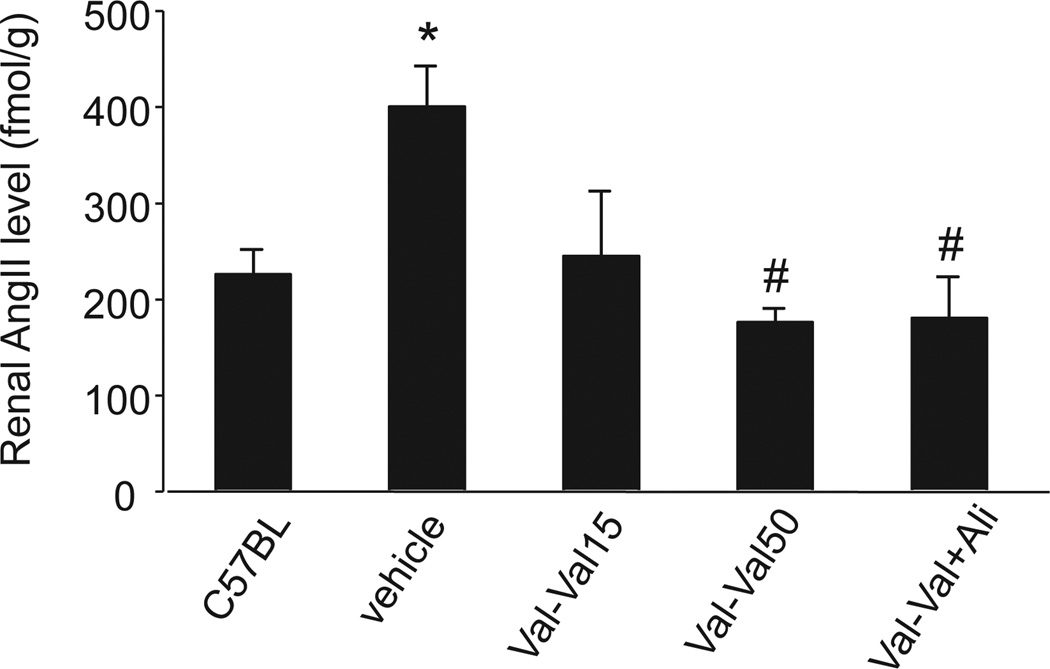
Vehicle-treated KKAy mice showed a significant increase in renal AngII compared with C57BL mice (Fig. 1). The elevated renal AngII was significantly inhibited in all treatment groups, including Ali-mono (198 ± 29 fmol/g) and Ali-Ali+Val (200 ± 24 fmol/g), with no significant difference among the treatment groups. On the other hand, Val-Val+Ali, Ali-mono (2.56 ± 0.26-fold), and Ali-Ali+Val (3.22 ± 0.33-fold) showed significant increases in renal renin mRNA expression compared with the vehicle-treated group (Table 1). Val-Val15 and Val-Val50 tended to increase renal renin mRNA expression, but the differences were not significant.
Fig. 1.
Renal angiotensin II (AngII) contents. The vehicle-treated mice show a greater AngII content in the kidney than C57BL mice. Val-Val15 tends to suppress the renal AngII level in type 2 diabetic KKAy mice, but the effect is not significant. Both Val-Val50 and Val-Val+Ali normalize the accumulation of AngII in the kidney. *P < 0.05 vs. C57BL mice, #P < 0.05 vs. vehicle group. Val-Val15: 15 mg/kg per day of valsartan for 10 weeks, Val-Val50: treatment with 15 mg/kg per day of valsartan for the first 4 weeks and treatment with 50 mg/kg per day of valsartan from weeks 4 to 10, Val-Val+Ali: treatment with 15 mg/kg per day of valsartan for 10 weeks and additional treatment with 25 mg/kg per day of aliskiren from weeks 4 to 10.
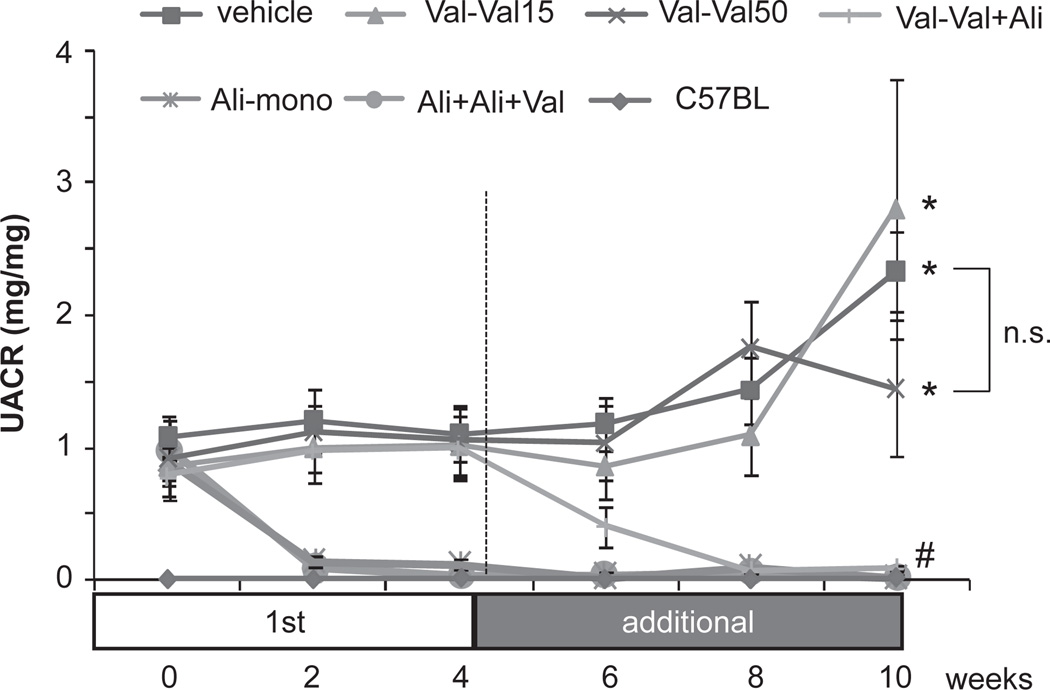
Vehicle-treated KKAy mice showed age-dependent increases in the urinary albumin/creatinine ratio (UACR) and their values were significantly higher than those of C57BL mice throughout the experimental period (Fig. 2). Val-Val15 and Val-Val50 both failed to attenuate albuminuria in KKAy mice. In contrast, Val-Val+Ali elicited a significant decrease in the UACR in KKAy mice. Similar significant anti-albuminuric effects were observed in Ali-mono and Ali-Ali+Val.
Fig. 2.
Urinary albumin excretion/creatinine ratios (UACRs). The vehicle-treated mice show more urinary albumin excretion than C57BL mice. Both low (Val-Val15) and high (Val-Val50) dosages of valsartan do not attenuate the albuminuria. In contrast, combined treatment with aliskiren and a low dosage of valsartan (Val-Val+Ali) shows dramatic inhibition of albuminuria. Aliskiren treatment started from week 0 (Ali-mono and Ali-Ali+Val) showed strong anti-albuminuric effects and maintained albuminuria at normal value throughout the experimental period. *P < 0.05 vs. C57BL mice, #P < 0.05 vs. vehicle group, n.s.: not significant. Val-Val15: 15 mg/kg per day of valsartan for 10 weeks, Val-Val50: treatment with 15 mg/kg per day of valsartan for the first 4 weeks and treatment with 50 mg/kg per day of valsartan from weeks 4 to 10, Val-Val+Ali: treatment with 15 mg/kg per day of valsartan for 10 weeks and additional treatment with 25 mg/kg per day of aliskiren from weeks 4 to 10, Ali-mono: 25 mg/kg per day of aliskiren for 10 weeks, Ali-Ali+Val: treatment with 25 mg/kg per day of aliskiren for 10 weeks and additional treatment with 15 mg/kg per day of valsartan from weeks 4 to 10.
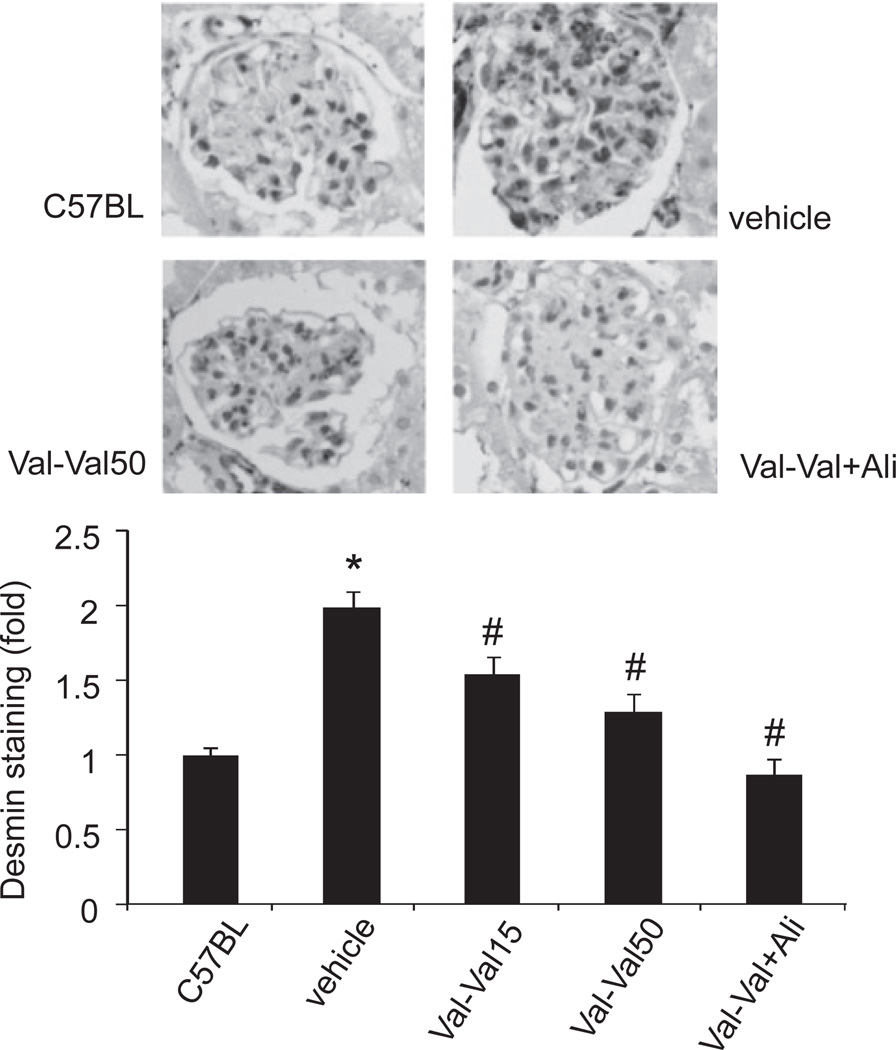
Since aliskiren strongly suppressed albuminuria in KKAy mice, we evaluated the glomerular podocyte damage by analyzing desmin immunostaining (19). At week 10, the desmin-positive areas were markedly greater in vehicle-treated KKAy mice than in C57BL mice (Fig. 3). Val-Val15 did not show any changes in the desmin-positive area. The increased desmin-positive area was significantly attenuated in Val-Val50 and normalized in Val-Val+Ali. Significant attenuations were observed in Ali-mono (1.01 ± 0.07-fold) and Ali-Ali+Val (0.72 ± 0.10-fold).
Fig. 3.
Desmin immunostaining. Podocyte injury revealed by desmin (dark staining) is increased in the vehicle-treated mice compared with C57BL mice. All the drug treatment groups, including Val-Val15, Val-Val50, and Val-Val+Ali, show significant decreases in the desmin-positive areas in the glomeruli. Val-Val+Ali tends to show stronger inhibition of the positive areas than Val-Val15 and Val-Val50. *P < 0.05 vs. C57BL mice, #P < 0.05 vs. vehicle group. Val-Val15: 15 mg/kg per day of valsartan for 10 weeks, Val-Val50: treatment with 15 mg/kg per day of valsartan for the first 4 weeks and treatment with 50 mg/kg per day of valsartan from weeks 4 to 10, Val-Val+Ali: treatment with 15 mg/kg per day of valsartan for 10 weeks and additional treatment with 25 mg/kg per day of aliskiren from weeks 4 to 10.
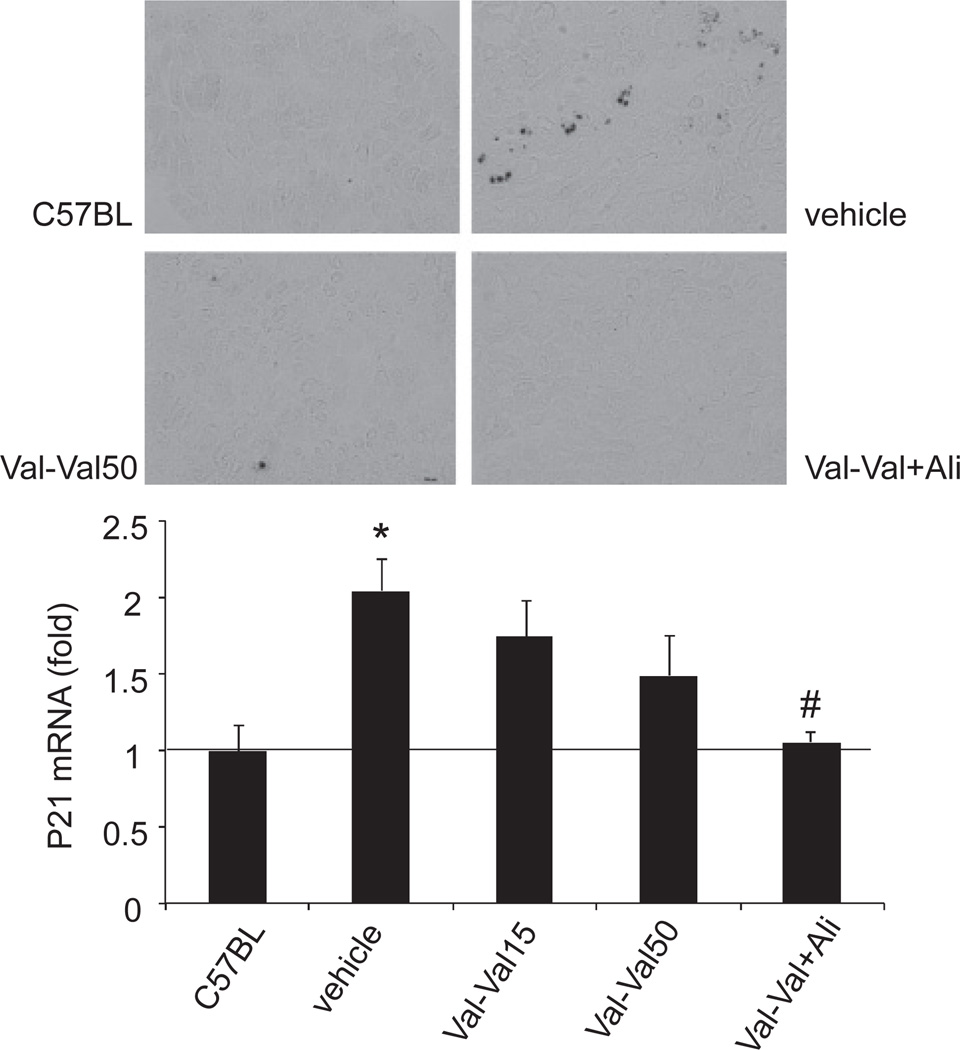
The kidney in type 2 diabetic patients has been reported to show accumulation of senescent cells (20). Therefore, we assessed the renal senescence status by SABG staining and expression of p21, a cyclin-dependent kinase inhibitor that regulates the cell cycle transition from G1 to S phase. The SABG-positive areas were significantly greater in kidney sections from vehicle-treated KKAy mice than in those from C57BL mice (Fig. 4, top). Val-Val15 did not affect the staining in KKAy mice (data not shown). Val-Val50 showed a comparable reduction in the SABG-positive area to the vehicle group. Val-Val+Ali abolished the increase in the SABG-positive area. The p21 mRNA expression level was significantly increased in the kidney of vehicle-treated KKAy mice (Fig. 4, bottom). The increase in p21 mRNA expression was not significantly inhibited in Val-Val15 and Val- Val50. Val-Val+Ali normalized the p21 mRNA expression in the kidney. Furthermore, Ali-mono and Ali- Ali+Val significantly reduced the p21 mRNA expression levels in the kidney (1.15 ± 0.45-fold and 0.89 ± 0.11-fold, respectively).
Fig. 4.
Senescence-associated β galactosidase (SABG) staining and renal p21 mRNA expression. The SABG-positive areas (dark staining) are significantly greater in the tubular cells in the vehicle-treated mice than in C57BL mice. Val-Val50 show a comparable reduction in the SABG-positive area to the vehicle group. The SABG-positive areas are almost abolished in Val-Val+Ali. The p21 mRNA expression is significantly increased in the kidney of the vehicle group. The increase in p21 mRNA expression is not inhibited by Val-Val15 and Val-Val50. On the other hand, Val-Val+Ali shows almost normalized p21 mRNA expression in the kidney. *P < 0.05 vs. C57BL mice, #P < 0.05 vs. vehicle group. Val-Val15: 15 mg/kg per day of valsartan for 10 weeks, Val-Val50: treatment with 15 mg/kg per day of valsartan for the first 4 weeks and treatment with 50 mg/kg per day of valsartan from weeks 4 to 10, Val-Val+Ali: treatment with 15 mg/kg per day of valsartan for 10 weeks and additional treatment with 25 mg/kg per day of aliskiren from weeks 4 to 10.
Discussion
Although RAS inhibitors, such as ACE inhibitors and AT1-receptor antagonists, delay the onset and progression of albuminuria in diabetic patients (9), many patients still suffer from a decline in renal function even with ACE inhibitor / AT1-receptor antagonist treatment. Thus, there is no doubt that identification of new possible therapeutic tools in addition to ACE inhibitors / AT1-receptor antagonists is required. In the present study, we have demonstrated that additional treatment with aliskiren in mice that had already received AT1-receptor antagonist treatment induced a significant anti-albuminuric effect, which was accompanied by podocyte-protective and anti-senescence effects. Importantly, even single treatment with aliskiren showed strong renoprotective effect, whereas a simple increase in the dosage of valsartan showed no anti-albuminuric effects in KKAy mice. Therefore, the present results suggest that treatment with aliskiren will be a possible therapeutic choice for type 2 diabetic kidney disease patients who cannot get effective renoprotection using an AT1-receptor antagonist.
Our study supports the findings in clinical trials showing the additional effect of aliskiren on RAS-inhibitor treatment in type 2 diabetic hypertensive patients (10, 21). One question that has arisen from these clinical studies is whether the RAS inhibitor dosage, such as 100 mg/day of losartan in the AVOID study, was sufficient for RAS suppression. In other words, it is unclear whether the benefit induced by aliskiren was dependent on more strict RAS inhibition compared with monotherapy or other unknown pharmacological effects of aliskiren. Several studies have shown that a combination of RAS inhibitors or an increase in the RAS inhibitor dosage, which are expected to suppress RAS more effectively, induces better therapeutic renal outcomes (22 – 24). In fact, clinical studies have shown that additional treatment with aliskiren for treatment with 80 or 160 mg of valsartan showed more efficient suppression of RAS (plasma renin activity, plasma AngI and II levels, and urinary aldosterone excretion) than increasing the dosage of valsartan to 160 or 320 mg (25, 26). Animal studies capable of using greater dosages to inhibit RAS more strictly have revealed that olmesartan dose-dependently (10 – 100 mg/kg per day) ameliorated the renal injury in aldosterone-salt hypertensive rats (27) and that aliskiren treatment in addition to valsartan protected the kidney in a unilateral ureteral obstruction model (valsartan: 15 mg/kg per day, aliskiren: 10 mg/kg per day) (28) and type 2 diabetic db/db mice (valsartan: 5 or 10 mg/kg per day, aliskiren: 3 mg/kg per day) (29). However, no studies have compared the efficacy between the combination therapy with aliskiren and a mild dosage of AT1-receptor antagonist and monotherapy with high dosage of AT1-receptor antagonist on the kidney injury in type 2 diabetic animals. We unexpectedly found that the KKAy mice were somehow resistant to the anti-albuminuric effect of valsartan in the present study. In addition, the present study has shown that aliskiren treatment either with or without valsartan treatment elicited strong renoprotection in the KKAy mice. Although aliskiren combination therapy and a high dosage of valsartan showed similar renal AngII reductions, the reflexive increase in renin mRNA was greater in the aliskiren group than in the valsartan group. As we did not measure any angiotensin peptides other than AngII in this study, further studies are required to clarify whether the greater renoprotection by the combination therapy was attributable to the renin inhibition–independent pharmacology of aliskiren or changes in RAS induced by aliskiren that were not reflected in the renal AngII level.
Aliskiren in addition to valsartan suppressed the increases in p21 expression and SABG staining in the kidney of KKAy mice, indicating suppression of renal cell senescence in the diabetic kidney (20). Renal cell senescence, a stress-induced response, is defined as a termination of cell mitosis (30). Our SABG staining revealed that the tubular cells were senescent, although it was difficult to identify which segments in the nephron showed cell senescence because there was a limitation associated with the counterstaining of renal sections for SABG, in that the organic solvents used during hematoxylin staining flushed out the blue indigo derivatives of SABG staining. A previous study showed that the proximal tubules were stained most strongly for SABG in the diabetic kidney (20), and we previously reported that there was strong SABG staining in the proximal tubules of hypertensive animals (15), suggesting that, at least, the proximal tubules could show cell senescence in vivo. Functional changes in the senescent proximal tubules have not been apparent, and they may not be involved in the albumin retrieval ability at proximal tubules in KKAy mice, since either valsartan (high dose) or aliskiren attenuated the accumulation of SABG-positive senescent cells in the kidney but only aliskiren-based treatment suppressed the albuminuria. Although the expression of p21 was only significantly suppressed in the aliskiren-treated group, it is hard to speculate on whether the effect on p21 expression could induce the difference in albuminuria between the valsartan-treated and aliskiren-treated mice because the aliskiren treatment did not significantly suppress p21 expression compared with the high dosage of valsartan. Aliskiren suppressed the blood glucose level, but this effect is also unlikely to result from the anti-senescence effect for the same reason described above.
It was reported that the renal collecting duct expresses renin and that the expression was augmented in the diabetic kidney (31). Either aliskiren or valsartan administered orally is mainly excreted in the feces, with only 6% of orally administered aliskiren recovered in the urine (32) and only 20% of valsartan is excreted in the urine even when it is intravenously administered (33, 34). Therefore, it remains unclear whether the amount of aliskiren that can reach the renal collecting duct is sufficient to inhibit the renin in the renal collecting duct and induce the difference in pharmacology between these two drugs. Measurement of the renin activity in the renal collecting duct, which has already been performed in situ (35), in these animals is required to clarify this point.
The reason why valsartan, even at a dosage that can suppress the renal AngII level, did not attenuate the albuminuria in KKAy mice is not clear in the present study. Although the high dosage of valsartan lowered the blood pressure and attenuated the podocyte injury, the increase in urinary albumin excretion could have occurred by either an increase in albumin permeability from the glomerular filtration barrier or a reduction in albumin uptake in proximal tubules (36 – 38). Thus, the result that valsartan did not affect albuminuria may be caused by damage to the other glomerular filtration barrier components, such as the endothelium (39) or glomerular basement membrane (40), or a reduction in albumin uptake in the proximal tubules. Another possibility is that the albuminuria at baseline (approximately 1 mg/mg of the UACR) in KKAy mice might already have been at an irreversible level for treatment with valsartan, in comparison with studies that have demonstrated beneficial effects of valsartan on the development of albuminuria (29, 41, 42). In any case, from our results it cannot be simply interpreted that treatment with a low dose of AT1 antagonist does not protect the kidney in type 2 diabetes because several studies have shown that AT1-receptor antagonists prevented the development of albuminuria in other models of type 2 diabetes (19, 29, 41 – 44), although there are no reports on KKAy mice.
Aliskiren treatment, in either the group starting the treatment at week 4 or the group treated from week 0, suppressed the postprandial blood glucose level at week 10 of treatment. The mechanisms by which aliskiren showed its blood sugar-lowering effects are unclear in the present study. We checked that this dosage of aliskiren did not affect food intake in a preliminary experiment (data not shown). Aliskiren treatment started at week 0 showed smaller BW compared with the other treatments; however, aliskiren treatment started at week 4 did not affect BW at week 10. A previous study reported that aliskiren alleviated insulin resistance and increased the pancreatic β cell number in KKAy mice (45). Thus, the blood glucose reduction in the present study might have resulted from improvements in insulin resistance and pancreatic function. We cannot eliminate the possibility that the decreases in blood glucose improved the development of albuminuria in the present study.
More recently, the interim review of the data from the recent clinical trial (Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints: ALTITUDE) suggested no benefit (and more side effects) of add-on aliskiren in type 2 diabetic patients treated with an ACE inhibitor or an AT1-receptor antagonist. Since no detailed information has been reported yet, we cannot provide precise explanations for why the results of the present study are different from the ALTITUDE study except for the baseline renal function: more than two-thirds of the patients had estimated glomerular filtration rate (eGFR) below 60 mL/min per 1.73 m2 in the ALTITUDE study (46), but the eGFR of patients in the AVOID study were within the normal range (10), and there was no difference in the serum creatinine level between non-diabetic control C57BL and diabetic KKAy mice at the age used in our study. More detailed analyses of the ALTITUDE study may suggest possible explanations.
In conclusion, treatment with aliskiren in addition to valsartan induced a stronger anti-albuminuric effect than an increased dosage of valsartan in type 2 diabetic KKAy mice. Although the detailed mechanism by which aliskiren elicits the additional renoprotective effect has not been elucidated, the strong effect may be a useful therapeutic tool against type 2 diabetic nephropathy, especially in the subjects who are resistant to AT1-receptor antagonist therapy.
Acknowledgments
We are grateful to Novartis Pharma K.K. for supplying the aliskiren and valsartan. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Akira Nishiyama (20590303).
References
- 1.Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, et al. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659–668. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano D, Diah S, Kitada K, Hitomi H, Mori H, Masaki T, et al. Short-term calorie restriction in early life attenuates the development of proteinuria but not glucose intolerance in type 2 diabetic oletf rats. ISRN Endocrinology. doi: 10.5402/2011/768637. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boor P, Floege J. Chronic kidney disease growth factors in renal fibrosis. Clin Exp Pharmacol Physiol. 2011;38:391–400. doi: 10.1111/j.1440-1681.2011.05487.x. [DOI] [PubMed] [Google Scholar]
- 5.Ihara G, Kiyomoto H, Kobori H, Nagai Y, Ohashi N, Hitomi H, et al. Regression of superficial glomerular podocyte injury in type 2 diabetic rats with overt albuminuria: effect of angiotensin II blockade. J Hypertens. 2010;28:2289–2298. doi: 10.1097/HJH.0b013e32833dfcda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toba H, Mitani T, Takahashi T, Imai N, Serizawa R, Wang J, et al. Inhibition of the renal renin-angiotensin system and renoprotection by pitavastatin in type 1 diabetes. Clin Exp Pharmacol Physiol. 2010;37:1064–1070. doi: 10.1111/j.1440-1681.2010.05436.x. [DOI] [PubMed] [Google Scholar]
- 7.Menne J, Chatzikyrkou C, Haller H. Microalbuminuria as a risk factor: the influence of renin-angiotensin system blockade. J Hypertens. 2010;28:1983–1994. doi: 10.1097/HJH.0b013e32833c206d. [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Fassi A, Ilieva AP, Iliev IP, Chiurchiu C, Rubis N, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29:207–216. doi: 10.1097/hjh.0b013e32834069bd. [DOI] [PubMed] [Google Scholar]
- 9.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 11.Ina K, Kitamura H, Okeda T, Nagai K, Liu ZY, Matsuda M, et al. Vascular cell adhesion molecule-1 expression in the renal interstitium of diabetic KKAy mice. Diabetes Res Clin Pract. 1999;44:1–8. doi: 10.1016/s0168-8227(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Uehara S, Ohta H, Taguchi K, Kemi M, Nishikibe M, et al. Losartan ameliorates progression of glomerular structural changes in diabetic KKAy mice. Life Sci. 2004;75:869–880. doi: 10.1016/j.lfs.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Fan YY, Hitomi H, Kiyomoto H, Kimura S, Kong CZ, et al. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am J Physiol Renal Physiol. 2009;297:F802–F808. doi: 10.1152/ajprenal.00197.2009. [DOI] [PubMed] [Google Scholar]
- 14.Rafiq K, Nakano D, Ihara G, Hitomi H, Fujisawa Y, Ohashi N, et al. Effects of mineralocorticoid receptor blockade on glucocorticoid-induced renal injury in adrenalectomized rats. J Hypertens. 2011;29:290–298. doi: 10.1097/hjh.0b013e32834103a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, et al. Aldosterone/mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–688. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- 16.Nakano D, Lei B, Kitada K, Hitomi H, Kobori H, Mori H, et al. Aldosterone does not contribute to renal p21 expression during the development of angiotensin II-induced hypertension in mice. Am J Hypertens. 2012;25:354–358. doi: 10.1038/ajh.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–2212. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1563–F1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa S, Nako K, Okamura M, Senda M, Mori T, Ito S. Aliskiren reduces albuminuria and oxidative stress, and elevates glomerular filtration rates in Japanese patients with advanced diabetic nephropathy. Hypertens Res. 2011;34:400–401. doi: 10.1038/hr.2010.250. [DOI] [PubMed] [Google Scholar]
- 22.Campbell R, Sangalli F, Perticucci E, Aros C, Viscarra C, Perna A, et al. Effects of combined ace inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003;63:1094–1103. doi: 10.1046/j.1523-1755.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 23.Rossing K, Schjoedt KJ, Jensen BR, Boomsma F, Parving HH. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 2005;68:1190–1198. doi: 10.1111/j.1523-1755.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg NK, Parving HH, Viberti G, Remuzzi G, Ritter S, Zelenkofske S, et al. Albuminuria response to very high-dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25:1921–1926. doi: 10.1097/HJH.0b013e328277596e. [DOI] [PubMed] [Google Scholar]
- 25.Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Riviere A, Vaidyanathan S, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–3133. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- 26.Azizi M, Menard J, Bissery A, Guyene TT, Bura-Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium-replete normotensive individuals. Clin J Am Soc Nephrol. 2007;2:947–955. doi: 10.2215/CJN.00360107. [DOI] [PubMed] [Google Scholar]
- 27.Fan YY, Baba R, Nagai Y, Miyatake A, Hosomi N, Kimura S, et al. Augmentation of intrarenal angiotensin II levels in uninephrectomized aldosterone/salt-treated hypertensive rats; renoprotective effects of an ultrahigh dose of olmesartan. Hypertens Res. 2006;29:169–178. doi: 10.1291/hypres.29.169. [DOI] [PubMed] [Google Scholar]
- 28.Wu WP, Chang CH, Chiu YT, Ku CL, Wen MC, Shu KH, et al. A reduction of unilateral ureteral obstruction-induced renal fibrosis by a therapy combining valsartan with aliskiren. Am J Physiol Renal Physiol. 2010;299:F929–F941. doi: 10.1152/ajprenal.00192.2010. [DOI] [PubMed] [Google Scholar]
- 29.Dong YF, Liu L, Lai ZF, Yamamoto E, Kataoka K, Nakamura T, et al. Aliskiren enhances protective effects of valsartan against type 2 diabetic nephropathy in mice. J Hypertens. 2010;28:1554–1565. doi: 10.1097/HJH.0b013e328338bb11. [DOI] [PubMed] [Google Scholar]
- 30.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 31.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldmeier F, Glaenzel U, Wirz B, Oberer L, Schmid D, Seiberling M, et al. Absorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteers. Drug Metab Dispos. 2007;35:1418–1428. doi: 10.1124/dmd.106.013797. [DOI] [PubMed] [Google Scholar]
- 33.Brookman LJ, Rolan PE, Benjamin IS, Palmer KR, Wyld PJ, Lloyd P, et al. Pharmacokinetics of valsartan in patients with liver disease. Clin Pharmacol Ther. 1997;62:272–278. doi: 10.1016/S0009-9236(97)90029-1. [DOI] [PubMed] [Google Scholar]
- 34.Iqbal M, Khuroo A, Batolar LS, Tandon M, Monif T, Sharma PL. Pharmacokinetics and bioequivalence study of three oral formulations of valsartan 160 mg: a single-dose, randomized, open-label, three-period crossover comparison in healthy indian male volunteers. Clin Ther. 2010;32:588–596. doi: 10.1016/j.clinthera.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Prokai A, Peti-Peterdi J. Recent advances in tissue (pro)renin imaging. Front Biosci (Elite Ed) 2010;2:1227–1233. doi: 10.2741/e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangalli F, Carrara F, Gaspari F, Corna D, Zoja C, Botti L, et al. Effect of ace inhibition on glomerular permselectivity and tubular albumin concentration in the renal ablation model. Am J Physiol Renal Physiol. 2011;300:F1291–F1300. doi: 10.1152/ajprenal.00656.2010. [DOI] [PubMed] [Google Scholar]
- 39.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and altered microvascular permeability. J Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng F, Zeng YJ, Plati AR, Elliot SJ, Berho M, Potier M, et al. Combined age inhibition and acei decreases the progression of established diabetic nephropathy in B6 db/db mice. Kidney Int. 2006;70:507–514. doi: 10.1038/sj.ki.5001578. [DOI] [PubMed] [Google Scholar]
- 42.Gao Q, Shen W, Qin W, Zheng C, Zhang M, Zeng C, et al. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 43.Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, et al. Enhanced effect of combined treatment with smp-534 (antifibrotic agent) and losartan in diabetic nephropathy. Am J Nephrol. 2006;26:50–58. doi: 10.1159/000091786. [DOI] [PubMed] [Google Scholar]
- 44.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwai M, Kanno H, Tomono Y, Inaba S, Senba I, Furuno M, et al. Direct renin inhibition improved insulin resistance and adipose tissue dysfunction in type 2 diabetic KK-A(y) mice. J Hypertens. 2010;28:1471–1481. doi: 10.1097/HJH.0b013e32833bc420. [DOI] [PubMed] [Google Scholar]
- 46.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) J Renin Angiotensin Aldosterone Syst. doi: 10.1177/1470320311434818. In press. [DOI] [PubMed] [Google Scholar]