Abstract
Arteriolar hyalinosis is a common histological finding in renal transplant recipients treated with the calcineurin inhibitor tacrolimus; however, the pathophysiologic mechanisms remain unknown. In addition to increasing transforming growth factor (TGF)-β levels, tacrolimus inhibits calcineurin by binding to FK506 binding protein 12 (FKBP12). FKBP12 alone also inhibits TGF-β receptor activation. Here we tested whether tacrolimus binding to FKBP12 removes an inhibition of the TGF-β receptor, allowing ligand binding, ultimately leading to receptor activation and arteriolar hyalinosis. We found that specific deletion of FKBP12 from endothelial cells was sufficient to activate endothelial TGF-β receptors and induce renal arteriolar hyalinosis in these knockout mice, similar to that induced by tacrolimus. Tacrolimus-treated and knockout mice exhibited significantly increased levels of aortic TGF-β receptor activation as evidenced by SMAD2/3 phosphorylation, along with increased collagen and fibronectin expression compared to controls. Treatment of isolated mouse aortas with tacrolimus increased TGF-β receptor activation, collagen and fibronectin expression. These effects were independent of calcineurin, absent in endothelial denuded aortic rings, and could be prevented by the small molecule TGF-β receptor inhibitor SB-505124. Thus endothelial cell TGF-β receptor activation is sufficient to cause vascular remodeling and renal arteriolar hyalinosis.
Keywords: tacrolimus, TGF-β, collagen, fibronectin, SMAD2/3, FK506 binding protein 12
INTRODUCTION
Renal arteriolar hyalinosis is a cardinal feature of calcineurin inhibitor toxicity, which is one of the leading causes of chronic allograft nephropathy in transplant recipients. Clinical studies have demonstrated a significant correlation between degree of arteriolar hyalinosis and dosage of the calcineurin inhibitors tacrolimus (TAC) and ciclosporin as well as duration of exposure.1,2 By 10 years post-transplant, 100% of renal and renal-pancreas allograft recipients exhibit arteriolar hyalinosis.2,3 Evidence of this vasculopathy may indicate progression towards chronic allograft nephropathy and has been suggested to be more important than tubular atrophy or interstitial fibrosis in the progression towards renal injury.4 Although an association between severity of hyalinosis and graft loss has not been demonstrated,5 arteriolar hyalinosis is often associated with renal dysfunction and the development of glomerulosclerosis.1–4,6 Despite the almost universal presence and predictive nature of this arteriolopathy in allograft recipients, little is known about how arteriolar hyalinosis develops during calcineurin inhibitor therapy.
Arteriolar hyalinosis consists of the deposition of hyaline into the vascular wall coupled with matrix protein synthesis and is evident in other diseases including hypertension and diabetes. Vascular matrix proteins such as collagen type I and IV and fibronectin are increased in patients and animals exhibiting arteriolar hyalinosis and likely play a major pathogenetic role.7,8 Arteriolar hyalinization alone may lead to a conduit vessel-like structure resulting in reduced smooth muscle contractility and loss of autoregulation.4 Experimental and clinical studies have implicated transforming growth factor (TGF)-β1 as the primary initiator of arteriolar hyalinosis with angiotensin II also playing a role.6–10 TGF-β1 is a pleiotropic cytokine and primarily functions as an anti-inflammatory and pro-fibrotic molecule. Calcineurin inhibitors markedly increase TGF-β1 levels in humans and animals and neutralizing antibodies against TGF-β1 reduce the degree of arteriolar hyalinosis and collagen expression in kidneys from ciclosporin-treated rats.8,11–13 However, TGF-β1 exerts both receptor-dependent as well as receptor-independent effects. Whether or not the TGF-β receptor plays a role and the vascular cell type involved in calcineurin inhibitor-induced renal arteriolar hyalinosis has not been examined.
The TGF-β receptor consists of two subunits (I and II) exhibiting a high affinity for one another and TGF-β1 binding leads to receptor trans-phosphorylation and gene transcription via the SMAD2/3-SMAD4 complex. The immunophilins FK506 binding protein 12 (FKBP12) and its related isoform 12.6 (FKBP12.6) bind the TGF-β1 receptor subunit I and prevent subunit phosphorylation in the absence of a ligand.14 FKBP12/12.6 is then displaced upon ligand binding to the receptor allowing subunit interaction/phosphorylation and downstream signaling to occur.15 FKBP12 and 12.6 are also the intracellular targets of TAC and we have shown that modulation of FKBP12/12.6 alters endothelial function whereas direct inhibition of calcineurin, the downstream target inhibited by the TAC/FKBP12 complex, had no acute vascular effect.16–18
Given the role of FKBP12 in TGF-β receptor-mediated signaling as well as TGF-β1 in the development of arteriolar hyalinosis, we hypothesized that the TAC-mediated activation of TGF-β receptors in endothelial cells causes renal arteriolar hyalinosis by increasing matrix protein synthesis. Since both TAC and TGF-β1 have numerous other cellular effects, we also used a genetic approach in mice to eliminate the contribution of these other effects. We generated mice lacking FKBP12 only in endothelial cells (FK12EC KO) to conditionally activate TGF-β receptors in an effort to determine whether endothelial cell TGF-β receptor activation is responsible for the development of renal arteriolar hyalinosis.
RESULTS
TGF-β receptor activation in TAC-treated mice and FK12EC KO mice
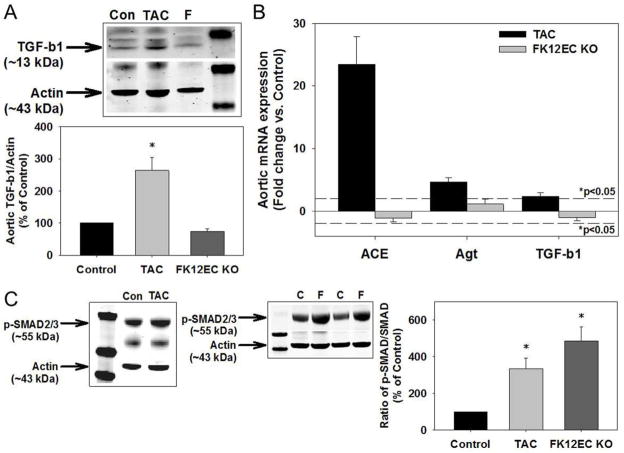
Mice treated for 1 week with TAC (10 mg/kg/day) exhibited a significant increase in aortic TGF-β1 protein expression as well as aortic mRNA expression of angiotensin converting enzyme, angiotensinogen, and TGF-β1 (Figures 1A and 1B). These increases were associated with TGF-β receptor activation as demonstrated by increased SMAD2/3 phosphorylation (Figure 1C). Aortic SMAD2/3 phosphorylation was also increased in mice treated with a lower concentration of TAC (1 mg/kg/day; Supplemental Figure 1). In contrast, FK12EC KO mice did not exhibit an increase in aortic TGF-β protein expression or angiotensin converting enzyme, angiotensinogen, or TGF-β1 mRNA expression (Figures 1A and 1B). However, due to the lack of inhibition by FKBP12, aortic TGF-β receptor activation was significantly increased in FK12EC KO mice compared to controls (Figure 1C). To examine whether FK12EC KO mice, which still have endothelial FKBP12.6 (Supplemental Figure 2A), might exhibit alterations in circulating levels of TGF-β or angiotensin II which can also activate SMAD2/3,19 we measured serum levels by ELISA. FK12EC KO mice did not exhibit significant changes in serum levels of TGF-β or angiotensin II compared to control mice (Supplemental Figures 2B and 2C). Additionally, there were no differences in aortic calcineurin protein expression or activity in FK12EC KO mice compared to controls (Supplemental Figures 3A and 3B).
Figure 1.
Tacrolimus (TAC) treatment or genetic deletion of FKBP12 from endothelial cells increases SMAD2/3 activation. (A) Mice treated with TAC (10 mg/kg/day, 1 week) had increased aortic expression of TGF-β1 compared to controls, however FK12EC KO mice did not exhibit any changes in TGF-β1 expression. (B) Aortic mRNA levels of angiotensin converting enzyme (ACE), angiotensinogen (Agt), and TGF-β1 were increased significantly in TAC-treated mice but were not changed in FK12EC KO mice. Dotted line represents *p<0.05 vs. control. (C) SMAD2/3 phosphorylation was increased in aortas from mice treated with TAC (10 mg/kg/day) and FK12EC KO mice. Densitometry of TGF-β1 to actin or p-SMAD2/3 to SMAD2/3 as a % of control; *p<0.05 vs. control. n=8–11 mice/experimental group. C=Controls, F=FK12EC KO.
TGF-β receptor activation leads to renal arteriolar hyalinosis
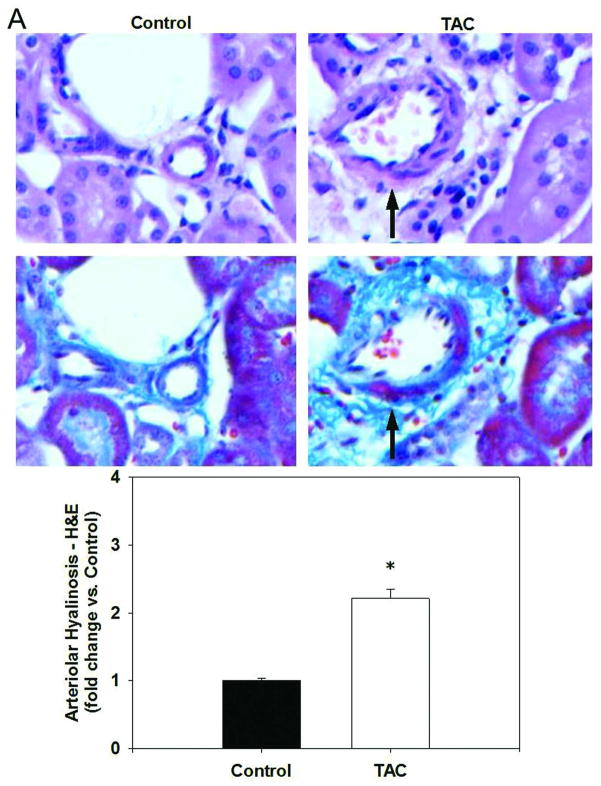
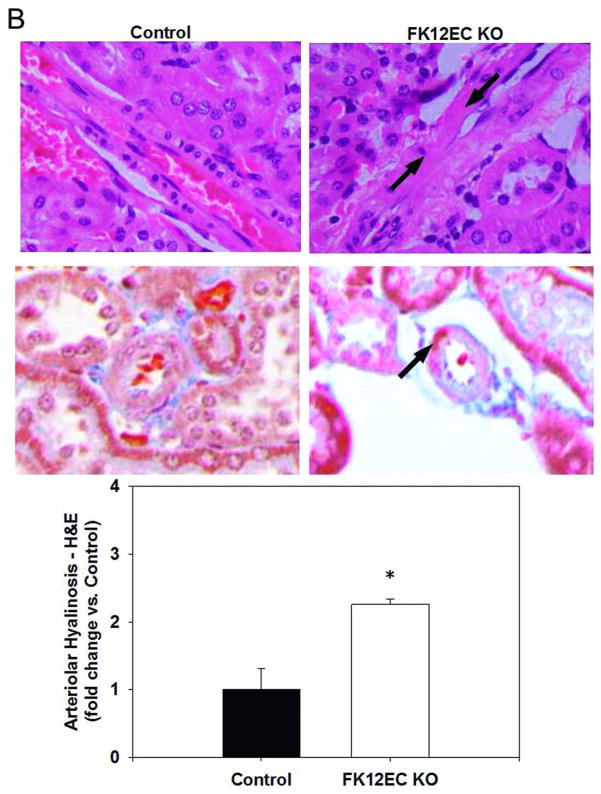
Renal arteriolar hyalinosis appears as a pink, glassy area encompassing the vascular wall in longitudinal sections of histological examinations and can be either focal, where only certain parts of the blood vessel are affected, or concentric, which affects the entire cross section of the blood vessel. TAC-treated mice exhibited a mild, but significant increase in renal arteriolar hyalinosis determined by both H&E and Masson’s trichrome staining (Figure 2A). A significant increase in renal arteriolar hyalinosis was also evident in FK12EC KO mice as young as 12 weeks of age (Figure 2B). In both models, the hyalinosis was focal in nature which is similar to that seen in renal allograft recipients treated with TAC.
Figure 2.
Tacrolimus (TAC) treatment or genetic deletion of FKBP12 from endothelial cells caused renal arteriolar hyalinosis. H&E staining (top panels) and Masson’s trichrome staining (bottom panels) reveal hyalinosis seen as the pink, glassy area indicated by the arrows in (A) TAC-treated mice (10 mg/kg/day; 1 week) and in (B) FK12EC KO mice compared to controls. Images at 400x final magnification. Quantification of arteriolar hyalinosis was determined using a scoring system previously described.7 n=6 mice/experimental group; *p<0.05 vs. control.
TGF-β receptor activation increases vascular matrix protein expression
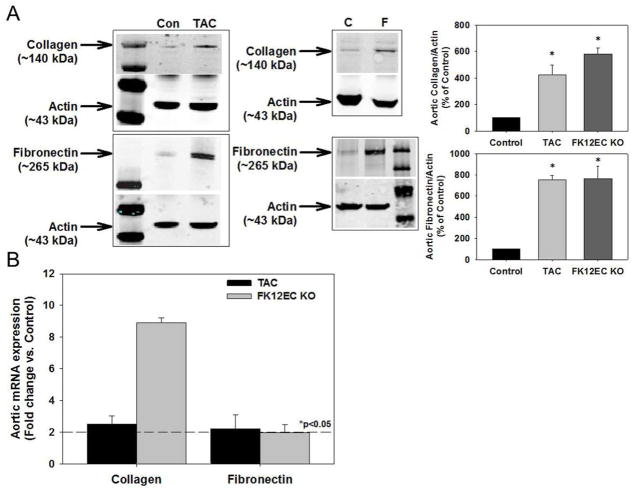
To examine whether the TGF-β receptor activation and renal arteriolar hyalinosis was associated with increased production of vascular matrix proteins, we measured collagen and fibronectin expression in aortas of TAC-treated mice as well as FK12EC KO mice. Figure 3A demonstrates that TAC significantly increased aortic collagen and fibronectin expression, which were also increased in FK12EC KO mice compared to controls. TAC at 1 mg/kg/day for 1 week also increased aortic collagen and fibronectin expression (Supplemental Figure 1). Additionally, mRNA levels of collagen and fibronectin were increased significantly in both TAC-treated mice as well as FK12EC KO mice compared to controls (Figure 3B).
Figure 3.
Tacrolimus (TAC) treatment or genetic deletion of FKBP12 from endothelial cells increased vascular matrix protein production. (A) Increased protein levels of collagen and fibronectin in aortas from mice treated with TAC (10 mg/kg/day) and FK12EC KO mice. Densitometry of collagen and fibronectin to actin as a % of control; *p<0.05 vs. control. (B) Increased mRNA levels of collagen and fibronectin in aortas from mice treated with TAC (10 mg/kg/day) and FK12EC KO mice. n=8–11 mice in each group. Dotted line represents *p<0.05 vs. control.
Detrimental vascular effects of TAC are direct, independent of calcineurin inhibition, and endothelium-dependent
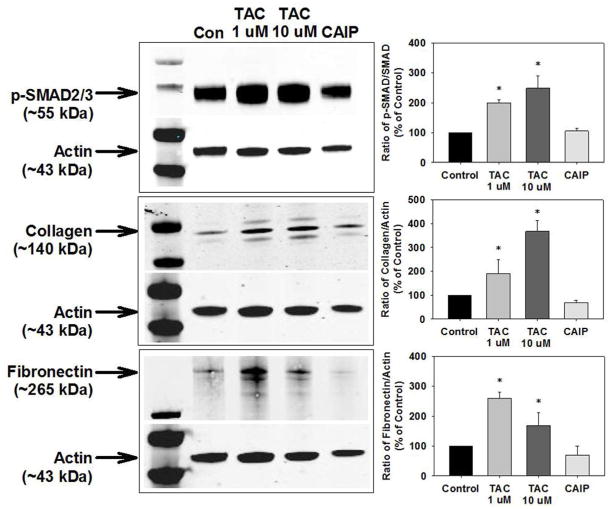
We next determined whether the TAC-induced changes were a direct vascular effect by treating isolated aortas from control mice with either vehicle, low-dose (1 μM) TAC, high-dose (10 μM) TAC, or the calcineurin autoinhibitory peptide (CAIP, 10 μM) for 24 hours. Both 1 μM and 10 μM TAC treatment significantly increased SMAD2/3 phosphorylation as well as collagen and fibronectin expression (Figure 4). However, CAIP, used at a concentration that inhibits calcineurin activity equal to that of TAC (Supplemental Figure 4), had no effects on SMAD2/3 phosphorylation, collagen expression, or fibronectin expression (Figure 4).
Figure 4.
Tacrolimus (TAC) treatment, but not calcineurin inhibition, increased SMAD2/3 activation and vascular matrix protein production in isolated aortas. TAC increased SMAD2/3 phosphorylation and protein levels of collagen and fibronectin in aortas isolated from control mice whereas the calcineurin autoinhibitory peptide (CAIP) had no effect. n=8–11 mice/experimental group. Densitometry of p-SMAD2/3 to SMAD2/3 or collagen and fibronectin to actin as a % of control; *p<0.05 vs. control.
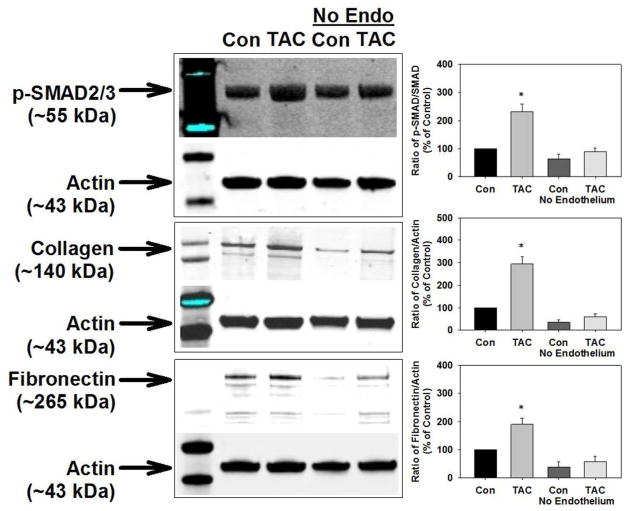
To determine the vascular cell type crucial for the TAC-induced SMAD2/3 signaling and matrix protein synthesis, we removed the endothelium of isolated aortas and treated them with TAC as above. Although it did not reach statistical significance, endothelium removal tended to decrease vascular collagen and fibronectin expression suggesting that the endothelium is a source of these proteins (Figure 5). Denudation of control vessels did not increase SMAD2/3 phosphorylation and collagen and fibronectin expression. Importantly, TAC treatment of endothelium-denuded vessels also did not increase SMAD2/3 phosphorylation, collagen expression, or fibronectin expression (Figure 5). Together, these results demonstrate that TAC, independent of calcineurin inhibition, directly activates endothelial cell TGF-β receptors which causes collagen and fibronectin production.
Figure 5.
Removal of endothelium prevented the tacrolimus (TAC)-induced increase in SMAD2/3 activation and vascular matrix protein production in isolated aortas. Denuding of endothelial cells prevented the TAC-induced increase in SMAD2/3 phosphorylation and protein levels of collagen and fibronectin in aortas isolated from control mice. n=8–11 mice/experimental group. Densitometry of p-SMAD2/3 to SMAD2/3 or collagen and fibronectin to actin as a % of control.
Inhibition of TGF-β receptor activation prevents vascular matrix protein expression
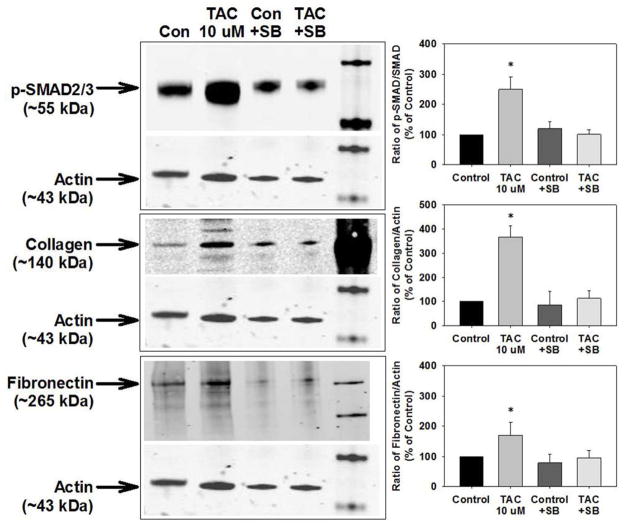
To further examine whether TGF-β receptor activation mediates the increase in vascular collagen and fibronectin, we co-treated isolated aortas with TAC and the TGF-β receptor inhibitor SB-505124.20 SB-505124 prevented the increase in SMAD2/3 phosphorylation and collagen and fibronectin expression induced by TAC (Figure 6).
Figure 6.
Inhibition of TGF-β receptor activation prevented the tacrolimus (TAC)-induced increase in SMAD2/3 activation and vascular matrix protein production in isolated aortas. TAC increased SMAD2/3 phosphorylation and protein levels of collagen and fibronectin in aortas isolated from control mice but this was prevented by the TGF-β receptor inhibitor SB-505124. n=8–11 mice/experimental group. Densitometry of p-SMAD2/3 to SMAD2/3 or collagen and fibronectin to actin as a % of control; *p<0.05 vs. control.
DISCUSSION
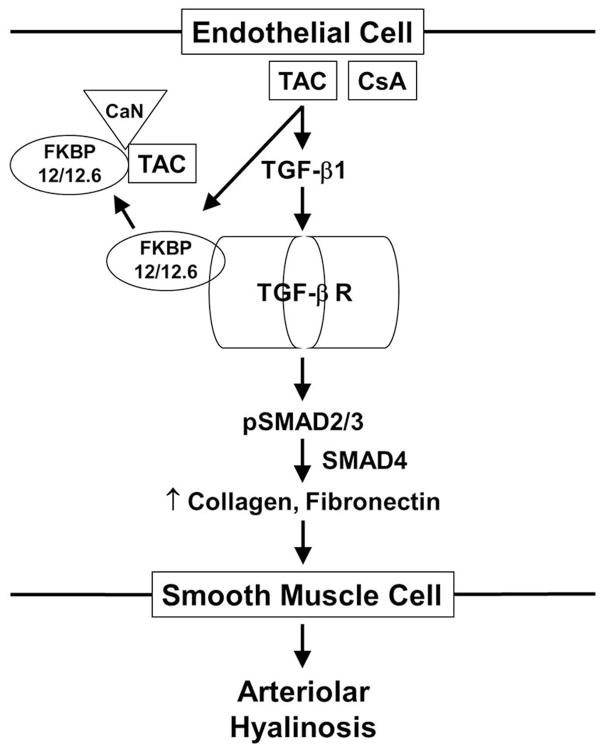
Although most renal transplant recipients exhibit renal arteriolar hyalinosis, the molecular mechanisms by which this develops are unknown. To test the hypothesis that endothelial cell TGF-β receptor activation plays a central role in the development of calcineurin inhibitor-induced renal arteriolar hyalinosis, we compared findings in TAC-treated mice with mice that we generated which lack FKBP12 in endothelial cells resulting in constitutive TGF-β receptor activation without increased TGF-β or angiotensin II levels. Our findings reveal that TAC, through its known effects of increasing TGF-β1 levels,11–13 increased SMAD2/3 activation, vascular matrix protein production, and renal arteriolar hyalinosis (Figure 7). The TAC-induced increase in SMAD2/3 activation and matrix protein production was calcineurin-independent but did depend on the endothelium and TGF-β receptor activation. In FK12EC KO mice, circulating TGF-β or angiotensin II levels were not increased, however these mice exhibited a similar increase in SMAD2/3 activation, vascular matrix protein production, and renal arteriolar hyalinosis. Although the extent of hyalinosis in the renal arterioles of both models was relatively mild and the lumen diameter was not compromised, the presence of this arteriolopathy after 1 week of TAC treatment and in young FK12EC KO mice likely represents the early stages of this progressive disease. Nonetheless, the similar findings suggest that endothelial TGF-β receptor activation is sufficient to induce vascular matrix protein synthesis and renal arteriolar hyalinosis.
Figure 7.
Diagram of molecular mechanisms involved in the development of renal arteriolar hyalinosis. Increased TGF-β receptor activation leads to SMAD2/3 activation and increased production of collagen and fibronectin which are secreted to the smooth muscle cell causing injury and hyalinosis. TAC=tacrolimus, CsA=cyclosporine, TGF-β=transforming growth factor beta, FKBP=FK506 binding protein, CaN=calcineurin.
Animal models of calcineurin inhibitor toxicity that exhibit renal arteriolar hyalinosis include rats treated with ciclosporin or TAC, as well as sodium-depleted mice administered these calcineurin inhibitors.7,8,11,21–23 TGF-β1 and angiotensin II were found to be crucial for hyalinosis development in these models as inhibition of TGF-β1, sodium repletion, or blockade of the angiotensin II type 1 receptor prevented the development of arteriolar hyalinosis. Additionally, lowering of blood pressure with hydralazine/furosemide alone had no effect on hyalinosis. Angiotensin II has been shown to increase TGF-β1, SMAD2/3 phosphorylation, and collagen I mRNA levels and these effects were mediated by both the TGF-β receptor as well as the angiotensin II type 1 receptor.6,10 The convergence of these 2 pathways on SMAD2/3, plus another report showing that knockdown of SMAD3 prevents the induction of collagen I mRNA,10 suggests that SMAD3 activation is important in the development of arteriolar hyalinosis. These findings were supported in our TAC-treated mice as these mice exhibited increased TGF-β1 and angiotensin II, TGF-β receptor activation, collagen and fibronectin production, and renal arteriolar hyalinosis. Mice treated with TAC at 1 mg/kg/day exhibited increased vascular SMAD2/3 phosphorylation and collagen and fibronectin expression. Even though this dose in mice is ~10 times higher than doses administered to patients, it achieves plasma levels comparable to that of treated patients.24 Treatment of mice with 10 mg/kg/day, which represents a nephrotoxic dose likely resulting in whole blood and plasma levels ~5–10 times higher than those seen clinically,24,25 exacerbated these effects. The TAC-induced increases in SMAD2/3 activation and collagen and fibronectin production were a direct vascular effect as these same effects were observed in isolated blood vessels treated with TAC. The in vitro concentrations of 1 μM and 10 μM TAC used in our study correspond to ~800 ηg/mL and ~8,000 ηg/mL, respectively, and are much higher than the ideal whole blood levels of 10–30 ηg/mL in patients. Although these doses were shown to inhibit T cell proliferation and cytokine production in immune cells in vitro and are within the range of effective concentrations (1 ηM - 25 μM) for in vitro use, results from our in vitro studies may not reflect what is occurring in vivo.26–28 Nonetheless, the detrimental vascular effects could be prevented by removing the endothelium or inhibiting TGF-β receptor activation.
Despite convincing evidence that TGF-β1 and angiotensin II play important roles in the development of arteriolar hyalinosis in these experimental models, our FK12EC KO mice did not exhibit alterations in serum or vascular mRNA levels of either TGF-β1 or angiotensin II. However, complete deletion of endothelial cell FKBP12 resulting in constitutive activation of TGF-β receptors and arteriolar hyalinosis suggests that SMAD2/3 activation plays a critical and sufficient role. Other potential mediators of arteriolar hyalinosis include osteopontin and PAI-1.6,8,22 Both TAC and ciclosporin treatment increase osteopontin gene expression in mice as well as human proximal tubular epithelial cells, and osteopontin expression is increased early in the hyalinosis/fibrotic process.6,22,29 Additionally, osteopontin deficient mice exhibit reduced arteriolar hyalinosis and interstitial collagen deposition in response to low sodium plus ciclosporin treatment.30 However, the induction of osteopontin and PAI-1 expression by calcineurin inhibitors are mediated by increases in TGF-β1 signaling which supports our hypothesis that TGF-β receptor activation mediates the elevation of these fibrogenic factors.8,24
The immunosuppressive drug sirolimus also increases TGF-β1 levels, binds FKBP12, and increases SMAD2/3 activation,31 however studies have shown that nephrotoxicity is reduced and the progression of chronic allograft lesions is decreased in renal allograft recipients.32 Like TAC, sirolimus binds FKBP12/12.6 and leads to TGF-β receptor activation, however the sirolimus/FKBP12 complex inhibits the kinase mammalian target of rapamycin (mTOR) instead of the phosphatase calcineurin. mTOR plays a major role in cell proliferation, inhibits apoptosis, and may contribute to vascular matrix protein synthesis. Interestingly, TAC increases mTOR in vascular smooth muscle cells and this is associated with increased vascular collagen I expression.33 Thus, inhibition of mTOR, in addition to TGF-β receptors, may prevent the development of arteriolar hyalinosis in TAC-treated allograft recipients.
Since ciclosporin and TAC both increase TGF-β1 and angiotensin II levels, inhibit calcineurin, and cause renal arteriolar hyalinosis, it remained unknown whether SMAD2/3 activation and/or calcineurin inhibition is the crucial mediator. If calcineurin inhibition is the pathogenetic mechanism, then one would expect calcineurin KO mice to exhibit renal arteriolar hyalinosis. Gooch and colleagues reported that calcineurin Aα KO mice exhibit increased renal expression of fibronectin and renal arteriolar hyalinosis.34 However, the degree of arteriolar hyalinosis was much lower than that seen in ciclosporin-treated mice and a major confounding factor is that TGF-β1 levels were increased significantly in calcineurin Aα KO mice. Calcineurin Aβ KO mice, which do not exhibit renal arteriolar hyalinosis, did not have increased levels of TGF-β1 compared to control mice. We addressed the role of calcineurin using a pharmacological approach and hypothesized that if calcineurin inhibition is responsible for the increased matrix protein synthesis then we would expect CAIP to increase collagen and fibronectin expression in isolated vessels. However, the peptide had no effect. Thus, calcineurin inhibitor-induced activation of TGF-β receptors mediates the increased matrix protein production and the development of renal arteriolar hyalinosis independent of calcineurin inhibition. Unlike TAC, ciclosporin does not bind FKBP12 however, like TAC, increases TGF-β1 and angiotensin II which would lead to TGF-β receptor activation and displacement of FKBP12 resulting in SMAD2/3 phosphorylation and collagen and fibronectin production. Whether this pathway is responsible for the development of ciclosporin-induced arteriolar hyalinosis remains to be determined.
Lastly, the vascular cell type that initiates the process of hyalinization remained unknown. Previous studies have shown that endothelial cells can produce and secrete collagen and fibronectin which would diffuse to and injure the interstitium and smooth muscle cells.35–39 This selective injury of medial smooth muscle cells is then replaced by hyaline deposits resulting in a focal pattern consistent with that observed in calcineurin inhibitor toxicity. In contrast, hypertension and diabetes result in arteriolar hyalinosis that is concentric in nature which may result from increased vascular permeability leading to subendothelial hyaline deposition. Our findings that endothelial cell-specific TGF-β receptor activation (FK12EC KO mice) leads to vascular matrix protein production and arteriolar hyalinosis, and that removal of the endothelium prevents the tacrolimus-induced increase in SMAD2/3 activation and matrix protein production supports the notion that endothelial cells initiate the hyalinization process induced by TAC.
In conclusion, these are the first data to demonstrate that endothelial cell TGF-β receptor activation is sufficient to cause renal arteriolar hyalinosis. Although some of these studies were performed in mouse aortas, it is likely that these signaling pathways and mechanisms also occur in renal arterioles. Nonetheless, endothelial cell SMAD2/3 activation appears to be the major initiator in the pathogenesis of renal arteriolar hyalinosis and future immunosuppressive drugs that do not increase TGF-β1 levels or lead to TGF-β receptor activation should be developed for renal allograft recipients.
METHODS
Animals
Wild-type male C57Bl/6J mice (The Jackson Laboratory) were treated with daily i.p. injections of either low-dose TAC (1 mg/kg/day), high-dose TAC (10 mg/kg/day), or vehicle (DMSO, <1% final concentration) for 1 week. FK12EC KO mice were generated by crossing Tie2-Cre C57Bl/6J mice (The Jackson Laboratory, Bar Harbor, ME) with mice containing lox P sites flanking either side of FKBP12 (generously provided by Dr. Susan Hamilton, Baylor College of Medicine).40 Wild-type male C57Bl/6J mice served as controls and all animals were studied at 10–12 weeks of age. All procedures were approved by the Texas A&M Health Science Center/Scott & White Memorial Hospital Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Confirmation of FK12EC KO
Cardiac endothelial cells were isolated from control and FK12EC KO mice and protein levels of FKBP12 and FKBP12.6 were measured using a LI-COR Odyssey (see below) and an anti-FKBP12/12.6 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) with β-actin serving as a loading control.
ELISAs
Plasma (50 μL) was used in the ELISAs for both TGF-β1 and angiotensin II per the manufacturer’s protocol (Phoenix Pharmaceuticals, Inc., Burlingame, CA).
Histology
Control and FK12EC KO mice were anesthetized by isoflurane and euthanized by exsanguination. Sections (3–5 μm thick) from isolated kidneys were obtained on a microtome, deparaffinized, and stained with either hematoxylin and eosin or Masson’s trichrome. Scoring for hyalinosis was performed as described previously.30 In brief, a blinded reviewer scored 25 fields per section for both the left and right kidneys of each mouse using a 1–4 scale (1 = hyalinosis evident in <10% of the field, 2 = 10–25%, 3 = 25–50%, and 4 = >50%).
qRT-PCR
Quantitative real-time PCR was performed to analyze mRNA expression in endothelium-intact aortas from control and FK12EC KO mice. Gene expression was measured using a Mouse Endothelial Cell Biology RT2 Profiler™ PCR Array and was performed according to the manufacturer’s protocol (SABiosciences, Frederick, MD). Results are expressed as fold change compared to control with an increase or decrease of 2-fold considered statistically significant.
Immunoblotting
Endothelium-intact and endothelium-denuded aortas were processed and imaged as described previously.16–18 Some aortas isolated from control mice were denuded of endothelial cells by repeatedly injecting air through the aorta, treated with CAIP (10 μM, 30 minutes; Calbiochem), or treated with the TGF-β receptor inhibitor SB-505124 (10 μM, 30 minutes; Sigma) followed by treatment with TAC (1 or 10 μM, 24 hours). Primary antibodies for TGF-β1, phospho-SMAD2/3, SMAD2/3, collagen type I, fibronectin, calcineurin, and β-actin were used followed by secondary antibodies consisting of anti-mouse, anti-rabbit, or anti-goat IgGs conjugated to either Alexa-Fluor 680 or IR800Dye (LI-COR Biosciences; Lincoln, NE).
Calcineurin Activity Assay
Isolated aortas from FK12EC KO and control mice as well as isolated control aortas treated with TAC or CAIP were homogenized in the presence of protease and phosphatase inhibitors. Vascular homogenates (250 μg of protein) were used to measure calcineurin activity which was performed using a Calcineurin Assay Kit according to the manufacturer’s protocol (Calbiochem).
Statistical Analyses
Results are presented as mean ± SEM. For serum TGF-β1 and angiotensin II levels the values obtained from the ELISA were compared to the mean of the controls in each trial and expressed as a % of controls. For multiple comparisons between TAC-treated and control mice, an analysis of variance was used followed by the Student’s-Newman-Keuls post hoc test. The two-tailed Student’s t-test was used to compare variables between FK12EC KO and controls. The significance level was set at 0.05.
Supplementary Material
Acknowledgments
This work was supported by NIH grant HL084299 (BMM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
DISCLOSURE
There are no financial conflicts of interest to disclose.
Supplementary information is available at Kidney International’s website.
References
- 1.Falkenhain ME, Cosio FG, Sedmak DD. Progressive histologic injury in kidneys from heart and liver transplant recipients receiving cyclosporine. Transplantation. 1996;62:364–370. doi: 10.1097/00007890-199608150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CLS, et al. Calcineurin inhibitor toxicity: Longitudinal assessment by protocol histology. Transplantation. 2004;78:557–565. doi: 10.1097/01.tp.0000128636.70499.6e. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Borrows RJ, Fung CLS, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 4.Kambham N, Nagarajan S, Shah S, et al. A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol. 2007;2:135–142. doi: 10.2215/CJN.01320406. [DOI] [PubMed] [Google Scholar]
- 5.Takeda A, Uchida K, Haba T, et al. Chronic cyclosporin nephropathy: Long-term effects of cyclosporin on renal allografts. Clin Transplantation. 2001;15:22–29. doi: 10.1034/j.1399-0012.2001.0150s5022.x. [DOI] [PubMed] [Google Scholar]
- 6.Andoh TF, Bennett WM. Chronic cyclosporine nephrotoxicity. Curr Opin Nephrol Hypertens. 1996;7:265–270. doi: 10.1097/00041552-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Shihab FS, Yi H, Bennett WM, et al. Effect of nitric oxide modulation on TGF-beta1 and matrix proteins in chronic cyclosporine nephrotoxicity. Kidney Int. 2000;58:1174–1185. doi: 10.1046/j.1523-1755.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.Islam M, Burke JF, McGowan TA, et al. Effect of anti-transforming growth factor-β antibodies in cyclosporine-induced renal dysfunction. Kidney Int. 2001;59:498–506. doi: 10.1046/j.1523-1755.2001.059002498.x. [DOI] [PubMed] [Google Scholar]
- 9.Saurina A, Campistol JM, Lario S, et al. Conversion from calcineurin inhibitors to sirolimus in kidney transplant patients reduces the urinary transforming growth factor-beta1 concentration. Transplant Proc. 2007;39:2138–2141. doi: 10.1016/j.transproceed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Chung ACK, Huang XR, et al. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and –indpendent Smad pathways. The role of Smad3 Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 11.Bing P, Maode L, Li F, Sheng H. Comparison of expression of TGF-β1, its receptors TGFβ1R-I and TGFβ1R-II in rat kidneys during chronic nephropathy induced by cyclosporine and tacrolimus. Transplant Proc. 2006;38:2180–218. doi: 10.1016/j.transproceed.2006.06.102. [DOI] [PubMed] [Google Scholar]
- 12.Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-β1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 13.Khanna A, Cairns V, Hosenpud JD. Tacrolimus induces increased expression of transforming growth factor-[beta]1 in mammalian lymphoid as well as nonlymphoid cells. Transplantation. 1999;67:614–619. doi: 10.1097/00007890-199902270-00021. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Li BY, Danielson PD, et al. The immunophilin FKBP12 functions as a common inhibitor of the TGFβ family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen YG, Liu F, Massague J. Mechanism of TGFβ receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook LG, Chiasson VL, Long C, et al. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int. 2009;75:719–726. doi: 10.1038/ki.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long C, Cook LG, Hamilton SL, et al. FK506 binding protein 12/12. 6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–576. doi: 10.1161/01.HYP.0000257914.80918.72. [DOI] [PubMed] [Google Scholar]
- 18.Long C, Cook LG, Wu GY, et al. Removal of FKBP12/12. 6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1580–158. doi: 10.1161/ATVBAHA.107.144808. [DOI] [PubMed] [Google Scholar]
- 19.Akool ES, Doller A, Babelova A, et al. Molecular mechanisms of TGFβ receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporine A and FK506. J Immunol. 2008;181:2831–2845. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 20.DaCosta Byfield S, Major C, Laping NJ, et al. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 21.Andoh TF, Lam TT, Lindsley J, et al. Enhancement of chronic cyclosporine nephrotoxicity by sodium depletion in an experimental mouse model. Nephrology. 1997;3:471–478. [Google Scholar]
- 22.Pichler RH, Franceschini N, Young BA, et al. Pathogenesis of cyclosporine nephropathy: Roles of angiotensin II and osteopontin. J Am Soc Nephrol. 1995;6:1186–1196. doi: 10.1681/ASN.V641186. [DOI] [PubMed] [Google Scholar]
- 23.Hoorn EJ, Walsh SB, McCormick JA, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki K, Gangappa S, Dillehay DL, et al. Pathogenic virus-specific T cells cause disease during treatment with the calcineurin inhibitor FK506: implications for transplantation. J Exp Med. 2010;207:2355–2367. doi: 10.1084/jem.20100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deniz H, Ogutmen B, Cakalagaoglu F, et al. Inhibition of the renin angiotensin system decreases fibrogenic cytokine expression in tacrolimus nephrotoxicity in rats. Transplant Proc. 2006;38:483–486. doi: 10.1016/j.transproceed.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 26.Andersson J, Nagy S, Groth CG, et al. Effects of FK 506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunology. 1992;75:136–142. [PMC free article] [PubMed] [Google Scholar]
- 27.Goto T, Kino T, Hatanaka H, et al. FK 506: historical perspectives. Transplant Proc. 1991;23:2713–2717. [PubMed] [Google Scholar]
- 28.Dumont FJ, Staruch MJ, Flattery A, et al. Up-regulation of gene expression by FK 506. Transplant Proc. 1991;23:2870–2872. [PubMed] [Google Scholar]
- 29.Khanna A. Tacrolimus and cyclosporine in vitro and in vivo induce osteopontin mRNA and protein expression in renal tissues. Nephron Exp Nephrol. 2005;101:e119–e126. doi: 10.1159/000087438. [DOI] [PubMed] [Google Scholar]
- 30.Mazzali M, Hughes J, Dantas M, et al. Effects of cyclosporine in osteopontin null mice. Kidney Int. 2002;62:78–8. doi: 10.1046/j.1523-1755.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 31.Kreis H, Oberbauer R, Campistol JM, et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809–817. doi: 10.1097/01.asn.0000113248.59077.76. [DOI] [PubMed] [Google Scholar]
- 32.Osman B, Doller A, Akool el-S, et al. Rapamycin induces the TGFbeta1/Smad signaling cascade in renal mesangial cells upstream of mTOR. Cell Signal. 2009;21:1806–1817. doi: 10.1016/j.cellsig.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Giordano A, Romano S, Mallardo M, et al. FK506 can activate transforming growth factor-β signaling in vascular smooth muscle cells and promote proliferation. Cardiovasc Res. 2008;79:519–526. doi: 10.1093/cvr/cvn079. [DOI] [PubMed] [Google Scholar]
- 34.Gooch JL, Roberts BR, Cobbs SL, et al. Loss of the α-isoform of calcineurin is sufficient to induce nephrotoxicity and altered expression of transforming growth factor-β. Transplantation. 2007;83:439–447. doi: 10.1097/01.tp.0000251423.78124.51. [DOI] [PubMed] [Google Scholar]
- 35.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–964. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahalak SM, Lin WL, Essner E, Shichi H. Increased immunoreactivity of collagen types I, II, and V, fibronectin and TGF-β in retinal vessels of rats with experimental autoimmune uveoretinitis. Curr Eye Res. 1991;10:1059–1063. doi: 10.3109/02713689109020344. [DOI] [PubMed] [Google Scholar]
- 37.Myers JC. Differential expression of type I collagen and cellular fibronectin isoforms in endothelial cell variants. Kidney Int. 1993;43:45–52. doi: 10.1038/ki.1993.9. [DOI] [PubMed] [Google Scholar]
- 38.Sage H, Crouch E, Bornstein P. Collagen synthesis by bovine aortic endothelial cells in culture. Biochemistry. 1979;18:5433–5442. doi: 10.1021/bi00591a028. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe EA, Mosher DF. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978;147:1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang W, Ingalls CP, Durham WJ, et al. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. FASEB J. 2004;18:1597–1599. doi: 10.1096/fj.04-1587fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.