Abstract
Blast overpressure has long been known to cause barotrauma to air-filled organs such as lung and middle ear. However, experience in Iraq and Afghanistan is revealing that individuals exposed to explosive munitions can also suffer traumatic brain injury (TBI) even in the absence of obvious external injury. The interaction of a blast shock wave with the brain in the intact cranial vault is extremely complex making it difficult to conclude that a blast wave interacts in a direct manner with the brain to cause injury. In an attempt to “isolate” the shock wave and test its primary effects on cells, we exposed cultured microglia to simulated blast overpressure in a barochamber. Overpressures ranging from 15–45 psi did not change microglial Cox-2 levels or TNF-α secretion nor did they cause cell damage. Microarray analysis revealed increases in expression of a number of microglial genes relating to immune function and inflammatory responses to include Saa3, Irg1, Fas and CxCl10. All changes in gene expression were dependent on pulse duration and were independent of pressure. These results indicate that microglia are mildly activated by blast overpressure and uncover a heretofore undocumented role for pulse duration in this process.
Keywords: microglia, gene expression, blast, overpressure, barochamber, primary trauma
1. Introduction
Humans exposed to detonation of explosive materials have long been known to suffer injury (i.e., barotrauma) to air-filled organs such as lung and the middle ear [10, 40]. It is also becoming apparent that blast can cause injury to the brain even in the absence of obvious external injury, referred to as blast-induced neurotrauma (BINT) [8]. Exposure to blast is common in military personnel serving in Iraq and Afghanistan and can result in traumatic brain injury (TBI) that ranges in severity from mild to fatal. Military operations in these theaters are revealing that TBI accounts for about 20–30% of all combat casualties [13, 33].
Blast causes injuries that are categorized as primary (direct effects of pressure associated with the blast wave), secondary (propelled objects make contact with a person), tertiary (person put in motion by blast and strikes solid structures), and quaternary (burns or inhalation of toxic detonation products). Injury to the brain by secondary and tertiary effects is common after blast and is similar to TBI suffered by civilians after blows to the head (e.g., fall, automobile accident). However, it is not yet known with certainty if the brain is damaged by the direct, primary effect of blast overpressure [16, 31].
Exposure of experimental animals to real or simulated blast overpressure results in a variety of insults to the brain to include axonopathy, astrocytosis, metabolic and electrophysiological changes [3, 11, 19, 27]. However, numerous factors make it very difficult to assign these effects to the direct, primary forces of the blast overpressure wave. Results from computational modeling suggest that blast can cause skull flexure even without head impact and produces loads in brain tissue that are comparable to those seen in injury-inducing impact [23]. Biomechanical testing of rodents has verified that intracranial pressures peak when skull strains are high indicating that skull flexure may play a key role in blast energy transmission to the brain [6]. It has also been discovered that bone of the skull is piezoelectric and can produce intense electromagnetic fields when traversed by a blast wave [17]. Shock waves from blast can provoke a shear response, possibly resulting in contusions as the brain strikes the hard skull [9] and regions of maximal tension are produced at countercoup sites (i.e., opposite side from the blast source; [32]). The microstructure of the brain itself leads to large differences in anisotropy with white matter showing greater ranges than gray matter, which is nearly isotropic [1, 25]. Differences in anisotropy can contribute to variations in deformation of the brain upon interaction with the blast wave [14] or after oscillatory shear forces [1]. Blast overpressure can also cause inflammation in the lungs and gastrointestinal tract and systemic responses such as this can also contribute to CNS injury [7].
In an attempt to “isolate” the overpressure wave and study its primary effects on cells without the complicating influences associated with the intact CNS discussed above, we tested homologous cells in a custom designed barochamber [18, 38]. Microglial cells were chosen for study because of their well known involvement in neurodegenerative diseases [26, 39] and in the actions of neurotoxins [5, 36, 37], and because extensive microglial activation and hypertrophy has been observed in intact animals after exposure to non-penetrating blast injury [16]. We report that microglia are not damaged by overpressure but show gene expression changes that are indicative of mild activation. Our results also uncover an interesting potential role for shock wave pulse duration in altering microglial status.
2. Materials and methods
2.1. Reagents
Lipopolysaccharide (LPS; from E. coli serotype 055:B5) was obtained from Sigma-Aldrich (St. Louis, MO). Trizol and all tissue culture reagents were purchased from Invitrogen Life Technologies (Carlsbad, CA). Illumina Sentrix MouseWG-6 v2.0 Expression Beadchip Kits were obtained from Illumina (San Diego, CA). Anti-Cox-2 antibody was purchased from Cayman Chemical (Ann Arbor, MI) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody was obtained from Amersham Biosciences (Piscataway, NJ). Western Lightning Chemiluminescence Reagent Plus was purchased from PerkinElmer Life Sciences (Waltham, MA). Quantikine M immunoassay kits for TNF-α were obtained from R&D Systems (Minneapolis, MN). Murine microglia BV-2 cells were provided by Dr. Paul Stemmer, Wayne State University, Detroit, MI.
2.2. Microglial cell culture and exposure to overpressure
Murine microglia BV-2 cells were maintained in culture as previously described [34]. Immediately prior to exposure of cells to blast overpressure, dishes were filled with fresh media and carefully covered with sterile parafilm. Dishes were then secured with Vaseline to a pedestal at the center of a barochamber and exposed to overpressure as previously described [18, 38]. The pressure wave magnitude and duration were recorded by a pressure transducer located within the barochamber using the TDAS Pro Module (DTS, Seal Beach, CA, USA) and ranged from 15–45 psi (105–314 kPa) and 7–15 msec, respectively. These simulated blast parameters are consistent with those associated with high explosives [9] and are within the range of pressure levels used in studies of the cellular responses to blast overpressure [38]. Controls were treated in the same fashion but were not exposed to the overpressure wave. At 3, 6 or 24 h after exposure to overpressure, cells were harvested, washed 3× with PBS and stored at −80°C.
2.3. Assessment of microglial activation after exposure to overpressure
Microglial activation was assessed by measuring Cox-2 protein levels in cells by immunoblotting and TNF-α in the cell culture media using an Elisa kit [35]. Both are reliable markers of microglial activation [20, 21]. As a positive control for exposure of microglia to overpressure, BV-2 cells were activated in serum-free media by treatment with LPS (100 ng/ml) for 1 hr after which the media was replaced. Cells were harvested 24 hr after treatment. Untreated cells were similarly washed and harvested as controls.
2.4. Microarray analysis of gene expression caused by overpressure
Changes in gene expression after overpressure treatment were determined by microarray analysis using Illumina gene chips that contain the entire mouse genome (> 45,000 genes) [34, 36]. Briefly, BV-2 cells were exposed to overpressure and 6 h after treatment, cells were harvested, washed and lysed in Trizol. A total of 4 independent tests were carried out in duplicate (N= 8 overpressure treated dishes) and used for microarray analysis and each duplicate set of cells exposed to overpressure had a single control associated with it (N= 4 control dishes). Genes were filtered using GeneSpring GX on expression using raw data values whereby only those genes whose expression values fell within 20–100% in all 12 gene chips were included for further investigation. This filtering provides a list of genes whose expression is considered “true” and above background. These genes obtained from the first level of filtering were further filtered on fold-change in expression of ≥ 2.
2.5. Statistics
Graph Pad Prism Software (GraphPad Software, San Diego, California, USA) was used to perform statistical analyses and graphical presentation. Experiments were reproduced at least three times. Cox-2 expression and TNF-α secretion were expressed as mean fold-change ± S.E.M. and group effects were tested for statistical significance using ANOVA followed by Dunnett's test. Results from microarray analysis of gene expression were tested for statistical significance using ANOVA followed by Tukey's test. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Effects of overpressure on microglial cells
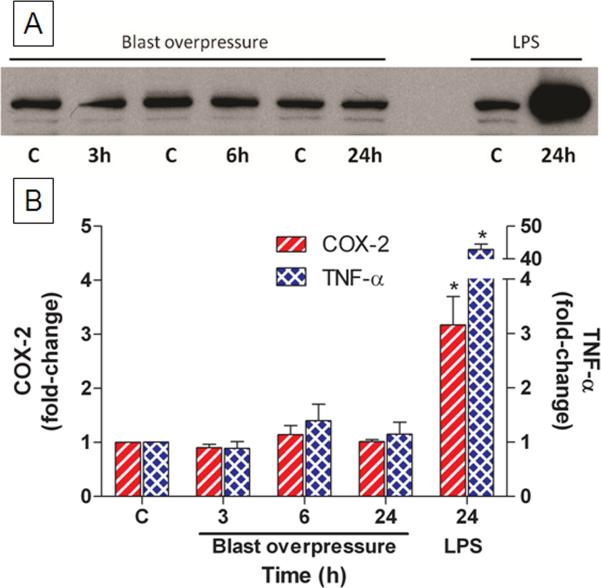
BV-2 microglial cells were exposed to overpressure and assessed for Cox-2 expression and TNF-α secretion as indices of activation. Fig. 1A shows a representative immunoblot from cells exposed to an overpressure of 20 psi. Pulse durations varied from 12.0–14.25 msec. Cox-2 levels were not changed from the respective controls at any time point. By contrast, treatment of microglia with LPS revealed an increase in Cox-2 expression of approximately 20-fold. TNF-α secretion was not changed by overpressure but was increased > 40-fold by LPS as shown in Fig. 1B.
Fig. 1.
Effects of blast overpressure on cultured microglia. Cells were exposed to simulated blast overpressure of 20 psi and assessed for activation 3, 6 or 24 h after treatment. Microglial activation was assessed by immunoblotting for cellular Cox-2 protein (A). Cox-2 levels on blots (left y-axis) were quantified via scanning and TNF-α (right y-axis) secreted into the media was quantified by Elisa (B). As a positive control, microglia were exposed to LPS and harvested for Cox-2 and TNF-α analysis 24 h after treatment. Data are presented as mean fold-change ± SEM. * p < 0.001, Dunnett's test.
3.2. Changes in microglial status by overpressure and pulse duration
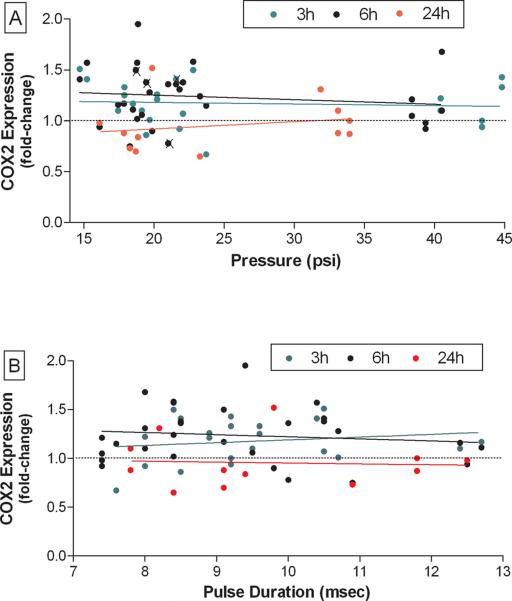
Fig. 2 shows the results from 40 independent tests where the overpressure levels were varied. It can be seen in Fig. 2A that overpressure had small and variable effects on Cox-2 expression over the range of 14– 45 psi. In general, Cox-2 was increased by less than 1.5 fold and the main effect of pressure on Cox-2 expression was not statistically significant. The results in Fig. 2B show that a correlation between pulse duration and Cox-2 expression did not exist over the range of 7.5–13 msec. TNF-α did not change over the range of overpressure levels of 14–45 psi at any pulse duration (data not shown).
Fig. 2.
Summary effects of blast overpressure on microglial activation. The effects of exposure of BV-2 microglial cells to blast overpressure Cox-2 protein levels at 3, 6 or 24 h is shown from 40 independent experiments. Cox-2 immunoblots for each experiment were scanned and normalized versus controls carried out for each separate experiment. Results (as fold-change) are presented as control versus pressure (A) or pulse duration (B). The symbol (X) indicates experiments where the protective parafilm cover on tissue culture dishes was broken during the procedure. These samples were included only when they did not show evidence of contamination.
3.3. Microarray analysis of microglial gene expression after simulated blast overpressure
In an attempt to expand the search for potential markers of overpressure-induced effects on microglial cells beyond Cox-2, we resorted to microarray analysis. Genes were filtered on expression for changes ≥ 2.0-fold by comparison to controls revealing that 17 genes were differentially expressed after treatment. A list of these genes is included in Table 1. Genes changed most in expression are related to immune-function and include Saa3 (3.7-fold increase) and Irg1 (3.7-fold increase). A number of genes related to inflammatory responses were also increased in expression to include TNF (2.2-fold), Fas (2.4-fold), Cxcl10 (2-fold), Gpr84 (2.9-fold) and Rsad (2.3-fold). No genes were significantly reduced in expression by overpressure. Genes changed in expression by overpressure (see Table 1) were subjected to principle component analysis (PCA) using GeneSpring GX and the results revealed that all changes occurred after pulse durations of 14.25 msec.
Microglial genes changed in expression by simulated blast overpressure as determined by microarray analysis
| Fold-change | p value | Gene symbol | Gene Definition | Accession # |
|---|---|---|---|---|
| 2.26 | 0.008a,b | Tyki | Thymidylate kinase family, LPS inducible | NM_020557.3 |
| 2.20 | 0.028a,b | Ehd1 | EH-domain containing 1 | NM_010119.3 |
| 2.43 | 0.008a,b | Fas | TNF receptor superfamily | NM_007987.1 |
| 2.01 | 0.015a,b | Parp14 | poly (ADP-ribose) polymerase family14 | NM_001039530.1 |
| 2.62 | 0.013a,b | Cxcl10 | Chemokine (C-X-C motif) ligand 10 | NM_021274.1 |
| 2.81 | 0.008a,b | Hp | Haptoglobin | NM_017370.1 |
| 3.70 | 0.032a | Saa3 | Serum amyloid A 3 | NM_011315 |
| 2.94 | 0.028a | Gpr84 | G protein-coupled receptor 84 | NM_030720.1 |
| 2.36 | 0.008a,b | Usp18 | ubiquitin specific peptidase 18 | NM_011909.1 |
| 3.70 | 0.008a,b | Irg1 | Immunoresponsive gene 1 | XM_127883 |
| 2.02 | 0.015a,b | Gpr109a | G protein-coupled receptor 109A | NM_030701.1 |
| 2.31 | 0.013a,b | Rsad2 | radical S-adenosyl methionine domain containing 2 | NM_021384.2 |
| 2.55 | 0.008a,b | Ifit3 | interferon-induced protein with tetratricopeptide repeats 3 | NM_010501.1 |
| 2.20 | 0.015a,b | Cd40 | CD40 antigen, transcript variant 5 | NM_170702.2 |
| 2.67 | 0.008a,b | Clecsf9 | C-type lectin domain family, member e | NM_019948.1 |
| 2.13 | 0.019a,b | Tnfrsf5 | TNF receptor superfamily, member 5 | NM_170704.1 |
| 2.16 | 0.017a,b | Tnf | Tumor necrosis factor | NM_013693 |
Significantly different (at indicated p values) from controls exposed to overpressure at a pulse duration of 14.25 sec.
Significantly different (at indicated p values) from cells exposed to overpressure at a pulse duration of 12 msec. No genes were changed in expression at a pulse duration of 12 msec by comparison to controls.
4. Discussion
Exposure to blast overpressure can result in damage to the brain in the absence of any obvious external injury. Mild brain injuries resulting from blast present significant challenges to the medical community because of difficulties in detection and treatment. Mild TBI resulting from exposure to blast can also lead to chronic injuries that require long-term rehabilitation and care [4, 10]. A growing number of military personnel serving the Iraq and Afghanistan conflicts are suffering from BINT [13, 33]. Concern over these combat injuries is extremely high and has resulted in concerted attempts to prevent or mitigate the effects of blast on the CNS [24]. Primary blast injury is generally accepted as the cause of barotrauma to air-filled organs such as the lungs and middle ear, but is far less certain with regard to BINT.
The interaction of the blast overpressure wave with the intact organism is extremely complex, particularly when considering the brain in an intact skull. Factors including skull flexure and strain [6, 23], electromagnetic forces [17] and intracranial pressure gradients [22] can result in acceleration-deceleration-induced contact of the brain with the cranium [9]. Shear and rotational forces caused by blast can also interact with the highly anisotropic brain [1, 14]. Any of these effects of the blast wave on the intact skull/brain makes it extremely difficult to attribute brain injury to the direct, primary effects of the blast wave itself. In fact, the difficulty in confidently identifying human cases of BINT in which only primary blast injury has been recognized [31].
One approach to bypass the complicating variables associated with skull-shock wave interactions would be to “isolate” the overpressure pulse using a barochamber and homologous cell lines. We have taken this approach in the current study. Microglia were of particular interest for study because of their association with numerous neurodegenerative diseases [12, 26] and neurotoxins [5, 36, 37], and because their activation can result in the synthesis and secretion of numerous reactive oxygen species and cytokines that are known to damage nervous tissue. Microglial activation has also been implicated in blast-induced injury to the brain [15, 16]. Our results revealed that isolated microglia were not damaged upon exposure to wide ranges in overpressure, and changes in Cox-2 expression and TNF-α secretion did not indicate activation. By contrast, LPS caused substantial microglial activation revealed by 20-fold increases in Cox-2 protein expression and 40-fold increases in TNF-α secretion.
In an effort to examine the broadest possible response of microglia to overpressure, we carried out microarray analysis of gene expression after treatment. Prior work in our lab has demonstrated the sensitivity and utility of this approach in mapping the microglial response to LPS and other activating species [35]. Gene expression changes were subjected to stringent filtering and revealed that 17 genes were significantly increased in expression. As expected for microglial cells (i.e., the innate immune system of the CNS), those genes increased in expression are related to innate immunity/immune function (Saa3, Irg1, Clecsf9, Ifit3, Cd40, Gpr109a) and inflammatory processes (Fas, Cxcl10, Gpr84, Rsad2). Although TNF-α protein secretion was not increased by overpressure, TNF gene expression did increase slightly (~2.2-fold) 6 h after treatment. No genes were decreased in expression after exposure to overpressure. Gene ontology analysis indicated that microglia responded to overpressure with a mild “immune system response” and “global regulation of gene expression”. These changes, both in terms of the number of genes changed and the magnitude of their changes, are smaller than seen in the response of microglia to LPS and other powerful microglial activators to include the HIV Tat neurotoxic protein and methamphetamine [35, 36]. However, increased expression of two genes that can exert defensive or protective responses to injury, haptoglobin and Usp18, may have served to limit increases seen in the immune function and inflammatory genes. Our results agree with a recent study showing that exposure of rats to simulated blast in a shock-tube also results in small changes in immune and inflammatory gene expression in brain as assessed using microarray analysis 24 h after injury [28]. These gene responses at 24 h could explain the discrepancy in our data between increased TNF gene expression without an increase in TNF-α secretion. Additional studies will be required to determine if the increase in TNF gene expression was too short-lived to allow translation into changed protein expression. Another very interesting finding emerged from the microarray analysis. PCA revealed that gene expression changes only occurred when the pulse duration was 14.25 msec. Cells exposed to pulse durations of 12 msec, with overpressure equal to that used for 14.25 msec durations (i.e., 20 psi), did not show gene expression changes by comparison to control. At present, it is difficult to regulate pulse durations in our barochamber but attempts are underway to establish better control of this aspect of the overpressure wave in light of another study that reported greater cell damage in response to overpressures at much higher pulse durations (20–30 msec) than used presently [29]. Taken together, these preliminary findings suggest the possibility that the duration of an overpressure pulse may be an important determinant of the cellular response to overpressure.
The present results are consistent with recent studies showing that C6 glioma cells do not undergo cell death after exposure to overpressure [38]. Few other studies have used a barochamber approach to study primary shock wave effects on cultured neurons or glia. Suneson [30] exposed cultured rat dorsal root ganglion cells to a pressure wave and most neurons showed extensive injury, cytoskeletal disturbances and severe dysfunction of the plasma membrane. Shepard et al. [29] used a barochamber to treat primary human glial cells with pressures varying from ~15–90 psi. Cell viability was maintained below 40 psi but decreased to < 20% at 90 psi. Although both of these studies reported severe cellular damage [29, 30], the overpressures used were much higher than those used presently and in our previous study [38]. Finally, it was reported recently that cultured NG108 and SH-SY5Y cells were mildly damaged by exposure to overpressure in a shock tube [2].
Highlights
-
▶
Microglial cells can secrete reactive species upon activation that can cause damage to neurons.
-
▶
We examined the effects of simulated blast overpressure on microglial gene expression.
-
▶
The results indicate that microglial cells are mildly activated by the primary overpressure wave.
-
▶
It appears that pulse duration may play an important role in altering microglial gene expression.
Acknowledgements
This work was supported by funding from the Department of Veterans Affairs, a VA Career Development Award (PJV) and by VA Merit and Research Career Scientist Awards (DMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arbogast KB, Margulies SS. Material characterization of the brainstem from oscillatory shear tests. J. Biomech. 1998;31:801–807. doi: 10.1016/s0021-9290(98)00068-2. [DOI] [PubMed] [Google Scholar]
- [2].Arun P, Spadaro J, John J, Gharavi RB, Bentley TB, Nambiar MP. Studies on blast traumatic brain injury using in-vitro model with shock tube. Neuroreport. 2011;22:379–384. doi: 10.1097/WNR.0b013e328346b138. [DOI] [PubMed] [Google Scholar]
- [3].Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim Y, Ritzel D, Bell R, Ecklund J, Armonda R, Bandak F, Parks S. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- [4].Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration's (VHA's) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J. Head Trauma Rehabil. 2009;24:4–13. doi: 10.1097/HTR.0b013e3181957032. [DOI] [PubMed] [Google Scholar]
- [5].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- [6].Bolander R, Mathie BA, Bir CA, Ritzel D, Vandevord PJ. Skull flexure as a contributing factor in the mechanism of injury in the rat when exposed to a shock wave. Ann. Biomed. Eng. 2011;39:2550–2559. doi: 10.1007/s10439-011-0343-0. [DOI] [PubMed] [Google Scholar]
- [7].Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front. Neurol. 2010;1:151. doi: 10.3389/fneur.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chafi MS, Karami G, Ziejewski M. Biomechanical assessment of brain dynamic responses due to blast pressure waves. Ann. Biomed. Eng. 2010;38:490–504. doi: 10.1007/s10439-009-9813-z. [DOI] [PubMed] [Google Scholar]
- [10].DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. N. Engl. J. Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- [11].Garman RH, Jenkins LW, Switzer RC, Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE, Clark RS, Bayir H, Kagan V, Jackson EK, Kochanek PM. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- [12].Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- [13].Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- [14].Hrapko M, van Dommelen JA, Peters GW, Wismans JS. The influence of test conditions on characterization of the mechanical properties of brain tissue. J. Biomech. Eng. 2008;130:031003. doi: 10.1115/1.2907746. [DOI] [PubMed] [Google Scholar]
- [15].Kaur C, Singh J, Lim MK, Ng BL, Ling EA. Macrophages/microglia as `sensors' of injury in the pineal gland of rats following a non-penetrative blast. Neurosci. Res. 1997;27:317–322. doi: 10.1016/s0168-0102(97)01164-4. [DOI] [PubMed] [Google Scholar]
- [16].Kaur C, Singh J, Lim MK, Ng BL, Yap EP, Ling EA. The response of neurons and microglia to blast injury in the rat brain, Neuropathol. Appl. Neurobiol. 1995;21:369–377. doi: 10.1111/j.1365-2990.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- [17].Lee KY, Nyein MK, Moore DF, Joannopoulos JD, Socrate S, Imholt T, Radovitzky R, Johnson SG. Blast-induced electromagnetic fields in the brain from bone piezoelectricity. Neuroimage. 2011;54(Suppl 1):S30–36. doi: 10.1016/j.neuroimage.2010.05.042. [DOI] [PubMed] [Google Scholar]
- [18].Leung LY, VandeVord PJ, Dal Cengio AL, Bir C, Yang KH, King AI. Blast related neurotrauma: a review of cellular injury. Mol. Cell. Biomech. 2008;5:155–168. [PubMed] [Google Scholar]
- [19].Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- [20].Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- [21].Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain research reviews. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- [22].Moore DF, Jerusalem A, Nyein M, Noels L, Jaffee MS, Radovitzky RA. Computational biology - modeling of primary blast effects on the central nervous system. Neuroimage. 2009;47(Suppl 2):T10–20. doi: 10.1016/j.neuroimage.2009.02.019. [DOI] [PubMed] [Google Scholar]
- [23].Moss WC, King MJ, Blackman EG. Skull flexure from blast waves: a mechanism for brain injury with implications for helmet design. Phys. Rev. Lett. 2009;103:108702. doi: 10.1103/PhysRevLett.103.108702. [DOI] [PubMed] [Google Scholar]
- [24].Nyein MK, Jason AM, Yu L, Pita CM, Joannopoulos JD, Moore DF, Radovitzky RA. In silico investigation of intracranial blast mitigation with relevance to military traumatic brain injury. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20703–20708. doi: 10.1073/pnas.1014786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prange MT, Margulies SS. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J. Biomech. Eng. 2002;124:244–252. doi: 10.1115/1.1449907. [DOI] [PubMed] [Google Scholar]
- [26].Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59:177–187. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- [27].Reneer DV, Hisel RD, Hoffman JM, Kryscio RJ, Lusk BT, Geddes JW. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma. 2011;28:95–104. doi: 10.1089/neu.2010.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, Arborelius U, Davidsson J. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54(Suppl 1):S89–97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- [29].Shepard SR, Ghajar JB, Giannuzzi R, Kupferman S, Hariri RJ. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J. Surg. Res. 1991;51:417–424. doi: 10.1016/0022-4804(91)90144-b. [DOI] [PubMed] [Google Scholar]
- [30].Suneson A, Hansson HA, Lycke E, Seeman T. Pressure wave injuries to rat dorsal root ganglion cells in culture caused by high-energy missiles. J. Trauma. 1989;29:10–18. doi: 10.1097/00005373-198901000-00003. [DOI] [PubMed] [Google Scholar]
- [31].Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: what is known? J. Neuropsychiat. Clin. Neurosci. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- [32].Taylor PA, Ford CC. Simulation of blast-induced early-time intracranial wave physics leading to traumatic brain injury. J. Biomech. Eng. 2009;131:061007. doi: 10.1115/1.3118765. [DOI] [PubMed] [Google Scholar]
- [33].Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- [34].Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J. 2006;20:515–517. doi: 10.1096/fj.05-4873fje. [DOI] [PubMed] [Google Scholar]
- [35].Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J. 2005 doi: 10.1096/fj.05-4873fje. [DOI] [PubMed] [Google Scholar]
- [36].Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment--an oligonucleotide microarray approach. J. Neurochem. 2004;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- [37].Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- [38].VandeVord PJ, Leung LY, Hardy W, Mason M, Yang KH, King AI. Up-regulation of reactivity and survival genes in astrocytes after exposure to short duration overpressure. Neurosci. Lett. 2008;434:247–252. doi: 10.1016/j.neulet.2008.01.056. [DOI] [PubMed] [Google Scholar]
- [39].von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- [40].Wightman JM, Gladish SL. Explosions and blast injuries. Ann. Emerg. Med. 2001;37:664–678. doi: 10.1067/mem.2001.114906. [DOI] [PubMed] [Google Scholar]