Abstract
Purpose
The anti-proliferative effects of 1α,25-dihydroxyvitamin D3 (1,25-D3, calcitriol, the active form of vitamin D) are mediated by the nuclear vitamin D receptor (VDR). In the present study, we characterized VDR expression in lung adenocarcinoma (AC).
Experimental Design
We examined VDR mRNA expression using a quantitative real-time PCR (qRT-PCR) in 100 patients who underwent surgery for lung AC. In a subset of these patients (n = 89), we examined VDR protein expression using immunohistochemistry. We also examined the association of VDR protein expression with circulating serum levels of 25-hydroxyvitamin D3 (25-D3) and 1,25-D3. The antiproliferative effects and cell cycle arrest of 1,25-D3 were examined using lung cancer cell lines with high (SKLU-1) as well as low (A549) expression of VDR mRNA.
Results
Higher VDR expression correlates with longer survival after adjusting for age, sex, disease stage and tumor grade (HR 0.73, 95% CI 0.58–0.91). In addition, there was a positive correlation (r = 0.38) between serum 1,25-D3 and tumor VDR protein expression. A greater anti-proliferative effect of 1,25-D3 was observed in high compared to low VDR-expressing cell lines; these effects corresponded to G1 cell cycle arrest; this was associated with a decline in cyclin D1, S-phase kinase protein 2 (Skp2), retinoblastoma (Rb) and minichromosome maintenance 2 (MCM2) proteins involved in S-phase entry.
Conclusions
Increased VDR expression in lung AC is associated with improved survival. This may relate to a lower proliferative status and G1 arrest in high VDR-expressing tumors.
Keywords: VDR; Vitamin D; 1,25-D3; Lung Adenocarcinoma; Survival
1. Introduction
The nuclear vitamin D receptor (VDR) is a member of the steroid-thyroid-retinoid receptor gene superfamily. 1α,25-Dihydroxyvitamin D3 (1,25-D3) binds to VDR and upon ligand binding, dimerizes with the retinoic acid X receptor [1]. This complex binds to vitamin D-responsive elements (VDRE) within the promoter regions of vitamin D-responsive genes [2]. Transcriptional activation is enhanced by nuclear receptor coactivator proteins [3] and vitamin D-receptor-interacting proteins (DRIP) [4], that are recruited after the VDR complex binds to the VDRE. The strongest ligand for VDR is 1,25-D3 [5]. There is an up-regulation of VDR upon exposure to 1,25-D3 [6, 7]. The anti-proliferative and pro-differentiating effects in cancer are mostly mediated through the nuclear VDR. Non-genomic actions of 1,25-D3 may indirectly affect gene transcription via regulation of intracellular signaling pathways that target transcription factors [8]. Genomic effects through nuclear VDR have been studied in various solid organ tumors, including colon [9], breast [10] and prostate [11]. In colorectal [12], breast [13] and prostate [14] cancers, decreased VDR expression has been found in advanced neoplasms, suggesting that loss of VDR may contribute to cancer progression. In lung squamous cell carcinoma, Menezes et al [15] demonstrated a differential expression of VDR (nuclear/cytoplasm) in progression of normal to invasive squamous cell carcinoma. Srinivasan et al [16] reported increased VDR protein expression in lung adenocarcinoma (AC) correlated with improved prognosis.
In the present study, we investigated VDR mRNA and protein expression in a well-characterized set of 100 patients undergoing surgery for lung AC. We also evaluated the associations between serum 25-hydroxyvitamin D3 (25-D3) and 1,25-D3 levels and VDR expression. Additionally, using high/low VDR expressing cell lines, we demonstrated that anti-proliferative effects of 1,25-D3 are due to G1 arrest and proportional to VDR mRNA expression.
2. Patients and Methods
2.1. Human samples
Lung tumor and associated serum samples were obtained from patients undergoing surgery for lung cancer between February 1992 and November 2007 without preoperative radiation or chemotherapy, as previously described [17]. Tissue specimens were banked with informed consent after approval from University of Michigan Institutional Review Board and Ethics Committee, frozen in liquid nitrogen and stored in −80°C. Regions containing a minimum of 70% tumor cellularity were utilized for RNA isolation.
2.2. Clinical and follow-up data
Patients that underwent resection were identified. Patient charts were abstracted and reviewed for demographics, smoking history, disease stage, histology, tumor grade and follow-up for death. Dates of death were obtained from the registry at the University of Michigan Hospitals. Patients lost to follow-up in 5 years were treated as censored and patients were also censored at 5 years. Follow-up for mortality was through December 2009.
2.3. Cell culture
Human lung AC cancer cell lines including A549 and SKLU-1 were obtained from American Type Culture Collection (ATCC) and cultured with DMEM/F12 or DMEM medium with 10% FBS at 37°C in a humid atmosphere consisting of 5% CO2/95% air.
2.4. RNA extraction and cDNA synthesis
Total RNA was isolated from tissue samples and cell lines followed by column purification using RNeasy Mini kit (Qiagen) according to the manufacturers' protocols. RNA was eluted from the spin column using RNase-free dH2O. cDNA was prepared from RNA samples using High Capacity cDNA Reverse Transcription kit (Applied Biosytems) according to manufacturer's instructions.
2.5. Quantitative real-time PCR (qRT-PCR)
The qRT-PCR reaction was prepared using Power SYBR Green PCR Master Mix (Applied Biosystems) and performed with StepOne Real-Time PCR System (Applied Biosystems). Each sample had a final volume of 15 μL containing approximately 100 ng of cDNA. The primers for VDR (203 bp PCR product) were as follows: 5’-GCCCACCATAAGACCTACGA-3’ (forward) and 5’-AGATTGGAGAAGCTGGACGA-3’ (reverse). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was used to standardize the VDR qRT-PCR results. Relative mRNA levels of VDR were assessed using the 2− ΔΔCt method.
2.6. Immunohistochemistry and tissue microarray (TMA)
TMAs were constructed, as previously described [17], with formalin-fixed, paraffin-embedded tissues from 89 out of 100 patients. Immunohistochemical (IHC) staining was done on the DAKO Autostainer using DAKO LSAB+. Antigen retrieval was achieved with preheated 10 mmol/L (pH 6) citrate buffer for 20 min to 95°C. Deparaffinized and rehydrated sections of the TMA at 4-μm thickness were labeled with VDR antibody (Abcam, rat polyclonal antibody, 1:200 dilution). Staining was visualized with 3,3′-diaminobenzidine and sections were lightly counterstained with hematoxylin. Each sample was scored independently by two readers using a scale of 0 (< 10% cells staining), 1+ (10–25% cells staining), 2+ (25–60% cells staining) or 3+ (≥ 60% cells staining).
2.7. Protein isolation and immunoblot analysis
Cells were plated and grown until 80% confluent. Total and nuclear proteins were extracted from cells using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to the manufacturers’ instruction. Protein was quantified using Bio-Rad protein assay kit (Bio-Rad Laboratories). Proteins (20 μg) were resolved on 10% tris-glycine gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore). The blots were probed with antibodies against VDR (Abcam), cyclin D1 (Cell Signaling Biotechnology), retinoblastoma (Rb, Cell Signaling Biotechnology), phospho-Rb (Cell Signaling Biotechnology), minichromosome maintenance 2 (MCM2, Cell Signaling Biotechnology), S-phase kinase protein 2 (Skp2, Cell Signaling Biotechnology) diluted 1:1,000, or β-actin (Abcam) diluted 1:10,000. Each band was normalized by β-actin. Arbitrary units in the figures represent the ratio between the normalized band density of the specific protein treated with 1,25-D3 and the corresponding untreated protein. This was repeated three times independently.
2.8. Serum 25-D3 and 1,25-D3 assay
Serum 25-D3 and 1,25-D3 concentrations were determined by radioimmunoassay (RIA) using radioiodinated tracer as described previously [18, 19]. The meancoefficient of variations calculated from blinded quality control samples were 12.5% for 1,25-D3 and 16.3% for 25-D3, respectively.
2.9. Cell proliferation assays
The effect of 1,25-D3 on proliferation of A549 and SKLU-1 cells was measured using WST-1 cell proliferation reagent (Roche). Cellswere plated at 3×103 (day 3) and 1× 103 (day 6) cells per well in a 96 well micro-titer plates (Corning), incubated overnight and treated with 0, 1, 10 and 100 nmol/L of 1,25-D3 (5–6 wells per each treatment). At day 3 and 6, cells were treated with WST-1 reagent according to manufacturer’s instructions. Cell proliferation was estimated by dividing the mean absorbance of the treatment group divided by the mean absorbance of the vehicle-treated control × 100%.
2.10. Flow cytometry
A549 and SKLU-1 cells were treated with 100 nmol/L of 1,25-D3 for 72 h, harvested in 0.25% trypsin/0.1% EDTA, centrifuged for 5 min at 500g and 4 C, washed and fixed with 75% ethanol and then resuspended in 1 mL of propidium iodine staining solution (i.e. 50 μg/mL of propidium iodide, 100 U/mL of ribonuclease A and 0.1% glucose) for 1 h. Cell cycle distribution was determined by analyzing 10,000 to 20,000 cells using a FACS caliber flow cytometer and Cell Quest software (Becton Dickinson). Red fluorescence (measured at 585/542 nm), indicative of propidium iodide uptake by damaged cells, was measured by use of logarithmic amplification and electronic compensation for spectral overlap. The cell cycle experiments were performed in triplicate and repeated three times.1,25-D3 was added once for up to 72-h incubation. For more than 72-h incubation, the media was replaced and 1,25-D3 was added.
2.11. Statistical analysis
Survival curves were generated using the Kaplan-Meier method. Log-rank test and Cox proportional hazards model were used to compare the overall survival and disease-free survival. The relationship between the VDR mRNA and protein was evaluated using Spearman’s correlation coefficient (r). For continuous variables, the groups were compared by analysis of variance (ANOVA) or t-tests. For categorical variables, Chi-square tests were used to make comparisons. All reported P values are two-sided with a type I error rate of 5%. All data represent mean ± standard deviation (SD).
3. Results
In our cohort of 100 patients, there were 48 male and 52 female; the average age was 67.1 years (Table 1). There were 87 former and current smokers and 13 non-smokers. Serum 25-D3 and 1,25-D3 levels were obtained at the time of surgery. None of the patients received preoperative chemotherapy or radiation. The information regarding adjuvant chemotherapy or radiation was previously reported [17, 20]. Among 68 patients with stage 1B or higher, 38 patients received adjuvant chemotherapy or radiation therapy but no survival differences between patients receiving adjuvant therapy and those not receiving adjuvant therapy were observed (log-rank test, P = 0.6).
Table 1.
Characteristics of 100 lung adenocarcinoma patients by VDR mRNA and protein expression
| Characteristics | All patients(n=100) |
VDR mRNA expression
|
P | All patients (n=89) | VDR protein expression
|
P | ||
|---|---|---|---|---|---|---|---|---|
| High (n=28) | Low (n=72) | High (n=28) | Low (n=61) | |||||
| Age at diagnosis of lung AC | 0.465 | 0.0933 | ||||||
| Mean ± SD | 67.1 ± 9.68 | 66.0 ± 9.30 | 67.5 ± 9.86 | 0.0129 | 67.6 ± 9.68 | 65.1 ± 9.15 | 68.8 ± 9.77 | |
| Gender | 0.492 | |||||||
| Female | 52 (52.0)* | 9 (32.1) | 43 (59.7) | 46 (51.9) | 16 (57.1) | 30 (49.2) | ||
| Male | 48 (48.0) | 19 (67.9) | 29 (40.3) | 43 (48.3) | 12 (42.9) | 31 (50.8) | ||
| Smoking status | 0.419 | 0.582 | ||||||
| Never smokers | 13 (13.0) | 5 (17.9) | 8 (11.1) | 13 (14.6) | 5 (17.9) | 8 (13.1) | ||
| Former and current smokers | 87 (87.0) | 23 (82.1) | 64 (88.9) | 76 (85.4) | 23 (82.1) | 53 (86.9) | ||
| Tumor grade | 0.366 | 0.528 | ||||||
| Well differentiated | 27 (27.0) | 9 (32.1) | 18 (25.0) | 23 (25.8) | 6 (21.4) | 17 (27.9) | ||
| Moderately differentiated | 38 (38.0) | 11 (39.3) | 27 (37.5) | 34 (38.2) | 11 (39.3) | 23 (37.7) | ||
| Poorly differentiated | 35 (35.0) | 8 (28.6) | 27 (37.5) | 32 (36.0) | 11 (39.3) | 21 (34.4) | ||
| Disease stage | 0.140 | 0.394 | ||||||
| I | 59 (59.0) | 20 (71.4) | 39 (54.2) | 55 (61.8) | 16 (57.1) | 39 (63.9) | ||
| II | 16 (16.0) | 3 (10.7) | 13 (18.1) | 12 (13.5) | 3 (10.7) | 9 (14.8) | ||
| III | 25 (25.0) | 5 (17.9) | 20 (27.8) | 22 (24.7) | 9 (32.1) | 13 (21.3) | ||
| Status censored at 5-year | 0.0467 | 0.472 | ||||||
| Alive | 56 (56.0) | 20 (71.4) | 36 (50.0) | 49 (55.1) | 17 (60.7) | 32 (52.5) | ||
| Deceased | 44 (44.0) | 8 (28.6) | 36 (50.0) | 40 (44.9) | 11 (39.2) | 29 (47.5) | ||
VDR: Vitamin D receptor.
The number in parenthesis is percentage (%).
We performed qRT-PCR in lung tumors (n = 100) to assess VDR mRNA expression. VDR mRNA is differentially expressed in lung AC (Fig. 1A). Higher expression of VDR correlates with longer survival based on continuous VDR expression (HR 0.80, 95% CI 0.65–0.99) in Table 2 and dichotomized VDR expression (HR 0.495, 95% CI 0.264–0.928) as shown in Fig. 1B. In the multivariate analysis, VDR expression also remains significant after adjusting for age, sex, disease stage and tumor grade (HR 0.73, 95% CI 0.58–0.91, Table 2). Moreover, VDR expression is also significantly related to disease-free survival after adjusting for age, sex, disease stage and tumor grade (HR 0.76, 95% CI 0.61–0.95, Table 2). Among the 44 dead, there were 19/59 stage 1, 10/16 stage 2 and 15/25 stage 3 disease. The median survival periods were 48.6, 23.0 and 27.5 months and mortality was 32.2, 62.5 and 60.0% for stage 1, 2 and 3, respectively.
Fig. 1.
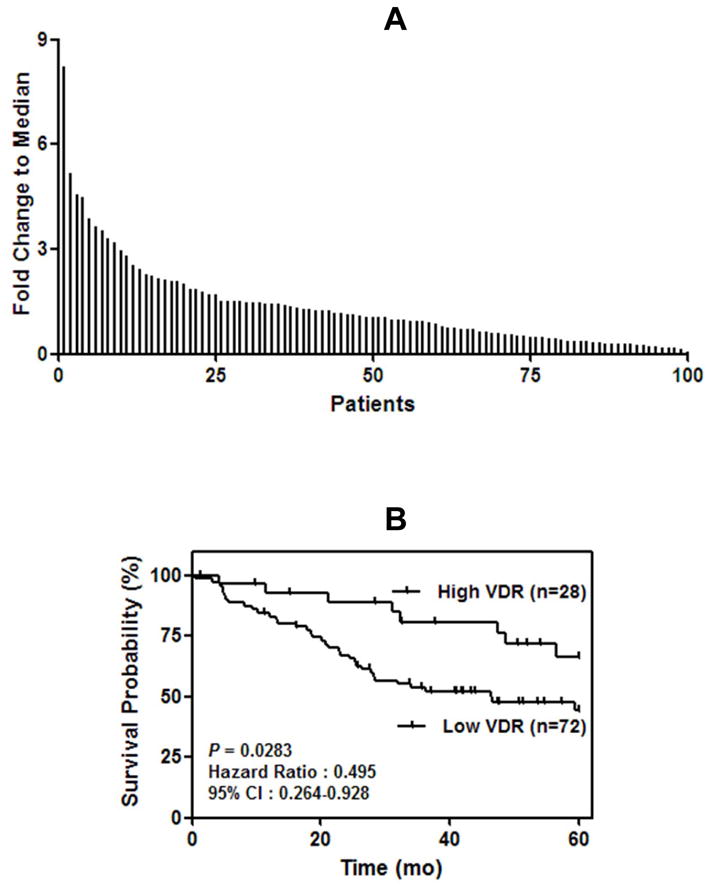
Vitamin D receptor (VDR) mRNA expression in 100 lung adenocarcinoma (AC) patients. A, using quantitative real time-PCR (qRT-PCR) there was a differential expression of VDR mRNA in 100 resected lung AC patients. 30% of the patients had high VDR mRNA levels (mRNA expression > 1.5 fold). B, Kaplan-Meier survival curve showing poor survival in patients with lung AC expressing low VDR mRNA. Surgically resected patients with lung AC was divided into high (>1.5 fold, N = 28) or low to medium (< 1.5 fold, N = 72).
Table 2.
Cox model results of VDR mRNA and overall/disease-free survival with continuous value in lung adenocarcinoma patients
| Factor | Overall Survival
|
Disease-free Survival
|
|||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | ||
| Univariate | n=100 | n=98 | |||
| VDR | 0.80 (0.65–0.99) | 0.03* | 0.86 (0.71–1.05) | 0.86* | |
| Multivariate | N=100 | N=98 | |||
| VDR | 0.73 (0.58–0.91) | 0.006 | 0.76 (0.61–0.95) | 0.01 | |
| Age | 1.09 (0.93–1.28) | 0.29 | 1.02 (0.88–1.18) | 0.82 | |
| Sex | 0.28 (0.13–0.56) | 0.0004 | 0.36 (0.19–0.67) | 0.001 | |
| Disease stage | 0.0007 | <.0001 | |||
| II vs I | 0.29 (0.13–0.68) | 0.35 (0.17–0.75) | |||
| III vs I | 0.25 (0.11–0.55) | 0.24 (0.12–0.49) | |||
| Tumor grade | 0.08 | 0.25 | |||
| Moderately vs Well | 0.38 (0.14–0.94) | 0.52 (0.23–1.13) | |||
| Poorly vs Well | 0.35 (0.12–0.87) | 0.58 (0.25–1.26) | |||
VDR: Vitamin D receptor.
Likelihood ratio test.
TMAs were constructed for IHC to identify the relationship between VDR mRNA and protein levels. We had 89 evaluable tumors to obtain a TMA score (0–3). VDR mRNA expression did not correlate with TMA score (r = −0.02). We then measured serum 25-D3 and 1,25-D3 levels using RIA from 86 lung AC patients. TMA score was moderately correlated with serum 25-D3 or 1,25-D3 levels (r = 0.27 and 0.38 for 25-D3 and 1,25-D3, respectively), the mean values of 25-D3 and 1,25-D3 levels both increase with increasing TMA scores (Table 3). Serum 25-D3 and 1,25-D3 levels were measured in 15 patients with benign lung conditions and compared to the levels in 86 lung AC patients. Serum 25-D3 levels were not significantly different (21.2 vs 19.6 nmol/L, P = 0.73) but 1,25-D3 serum levels were significantly higher in patients with benign conditions than those in patients with lung AC (35.2 vs 25.7 pmol/L, P = 0.003).
Table 3.
Characteristics of VDR mRNA expression and serum level of 25-D3 and 1,25-D3 categorized by patient status at 5-year follow-up
| Variables | VDR | P | 1,25-D3 (pmol/L) | P | 25-D3 (nmol/L) | P |
|---|---|---|---|---|---|---|
| Disease state | 0.232 | 0.704 | 0.438 | |||
| I | 1.49 ± 1.40 | 25.4 ± 8.94 | 19.5 ± 8.16 | |||
| II | 0.893 ± 0.609 | 27.5 ± 5.14 | 22.2 ± 5.89 | |||
| III | 1.28 ± 1.14 | 25.5 ± 8.64 | 18.9 ± 7.33 | |||
| Tumor grade | 0.528 | 0.779 | 0.927 | |||
| Well differentiated | 1.51 ± 1.16 | 24.9 ± 6.99 | 19.3 ± 7.30 | |||
| Moderately differentiated | 1.39 ± 1.50 | 26.4 ± 9.07 | 20.1 ± 7.64 | |||
| Poorly differentiated | 1.16 ± 1.01 | 25.6 ± 8.59 | 19.8 ± 8.06 | |||
| TMA | 0.528 | 0.0197 | 0.127 | |||
| 0 | 1.21 ± 0.876 | 22.4 ± 6.35 | 16.8 ± 5.73 | |||
| 1 | 1.57 ± 1.69 | 24.9 ± 8.58 | 19.2 ± 7.72 | |||
| 2 | 1.09 ± 0.986 | 28.0 ± 10.4 | 20.9 ± 9.40 | |||
| 3 | 1.54 ± 1.41 | 31.5 ± 5.73 | 22.9 ± 5.98 |
VDR: vitamin D receptor, 25-D3: 25-hydroxyvitamin D3, 1,25-D3: 1,25-dihydroxyvitamin D3, TMA: tissue microarray.
All data are expressed as mean ± standard deviation (SD).
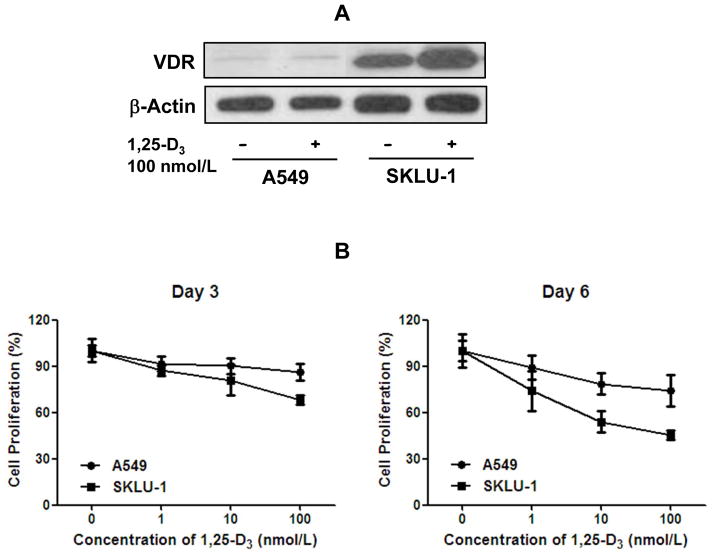
Because higher expression of VDR is associated with improved survival in lung AC patients (Fig. 1 and Table 2), we hypothesized that VDR expression determines the anti-proliferative effects of 1,25-D3 on lung cancer cells. We examined the VDR mRNA levels in 14 lung cancer cell lines [20]. Among 14 human lung cancer cell lines, we chose two representative cell lines, A549 (low VDR) and SKLU-1 (high VDR). The VDR protein levels were confirmed by immunoblot analysis. The VDR protein was highly expressed in SKLU-1 cells, whereas it was low in A549 cells (Fig. 2A). To assess the functional consequences of VDR expression, we performed cell proliferation assays using both cell lines in the presence of varying doses of 1,25-D3 for 3 and 6 days. On both day 3 and day 6, A549 cells were more resistant to the anti-proliferative effect of 1,25-D3 when compared with SKLU-1 cells using the WST-1 assays (Fig.2B). Similar anti-proliferative effects of 1,25-D3 were also obtained in another set of lung cancer cell lines, H1935 (high VDR) and A427 (low VDR) cells (data not shown).
Fig. 2.
Protein expression of vitamin D receptor (VDR) and the effect of 1,25-dihydroxyvitamin D3 (1,25-D3) in human lung cancer cell lines. A, immunoblot analysis of VDR protein in high (SKLU-1) and low (A549) VDR mRNA expressing lung adenocarcinoma (AC) cell lines.β-actin was used as a loading control. The protein expression of VDR was significantly higher in SKLU-1 cells. B, the effect of 1,25-D3 on cell proliferation in A549 and SKLU-1 cell lines by WST-1 assay. SKLU-1 demonstrated more marked decrease in cell proliferation in response to 1,25-D3 compared to A549 at both days 3 and 6. Protein expression and cell proliferation experiments were performed at least three times independently.
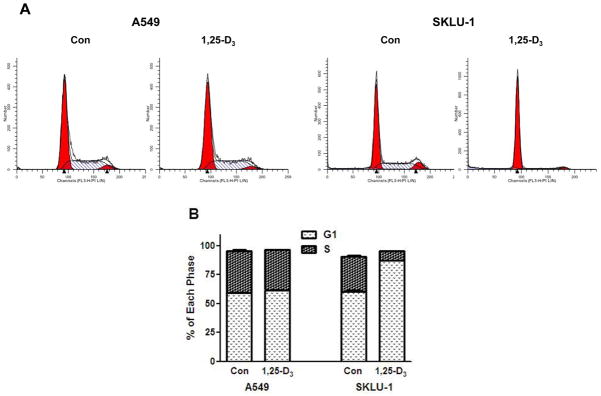
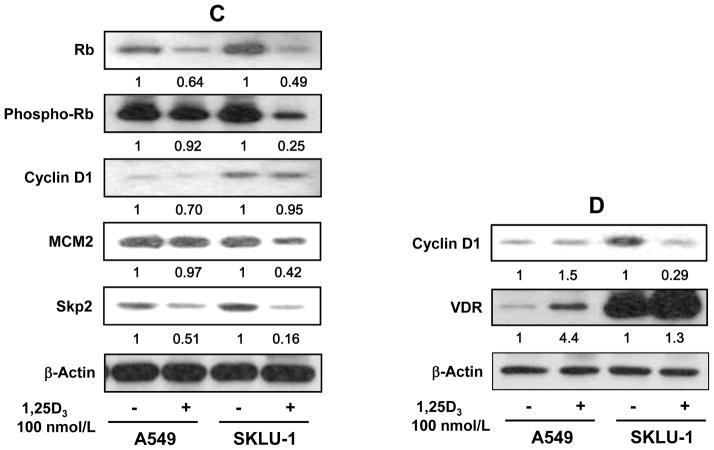
To validate anti-proliferative effect of 1,25-D3 mechanistically, we performed cell cycle analysis in these representative cell lines, A549 and SKLU-1, using FACScaliber flow cytometer. SKLU-1 cells showed a significantly strong G1 arrest (44.6% increase in G1 phase) when treated with 100 nmol/L 1,25-D3 for-72 h compared to that of untreated (87.5% vs 60.5%, P < 0.001), but there were no significant changes in G1 (59.4% vs 61.7%) using A549 cells treated with 100 nmol/L 1,25-D3 for 72-h (Fig. 3A and B). This was also supported by protein expression of Rb, phospho-Rb, MCM2 and Skp2, which are involved in G1/S cell cycle regulation, using immunoblot analysis (Fig. 3C). Strong inhibition of Rb, phospho-Rb, MCM2 and Skp2 from total protein was found in SKLU-1 cells when cells were treated with 100 nmol/L 1,25-D3 at 72-h compared to A549 cells but minimal changes in cyclin D1 expression was detected from total protein in both A549 and SKLU-1 cells (Fig. 3C). We isolated nuclear protein from these two cells and confirmed the VDR and cyclin D1 levels (Fig. 3D). As expected, nuclear cyclin D1 was strongly inhibited by 1,25-D3 in SKLU-1 cells with the induction of VDR, whereas minor changes of nuclear cyclin D1 was found in A549 cell even though VDR was induced by 1,25-D3 (Fig. 3D). These results indicate that exogenous 1,25-D3 mediated through VDR has an antiproliferative effect reflected in decreased cell numbers. This is associated with cell cycle arrest in G1 and inhibition of proteins cyclin D1, Rb, phospho-Rb, MCM2 and Skp2.
Fig. 3.
FACScan analysis and immunoblot analysis in human lung cancer cell lines. A549 and SKLU-1 cells were treated with 100 nmol/L of 1,25-dihydroxyvitamin D3 (1,25-D3) for 72 h. A, representative flow cytometry data showed 1,25-D3 induced G1/S arrest in SKLU-1 cell. B, changes in the distribution of cells in G1 and S phase following treatment with 1,25-D3. Results are expressed as mean ± standard deviation (SD) of 3 replicates of the percentage of cells in the different phases of the cell cycle. This was repeated three times. C, total protein expression of retinoblastoma (Rb), phospho-Rb, cyclin D1, S-phase kinase protein 2 (Skp2) and minichromosome maintenance 2 (MCM2), markers of cells entering S-phase, in high (SKLU-1) and low (A549) vitamin D receptor (VDR) mRNA expressing lung adenocarcinoma (AC) cell lines. The total protein expression of Rb, phospho-Rb, Skp2 and MCM2 significantly decreased by 1,25-D3 in SKLU-1 cells compared to A549 cells. Minimal changes of cyclin D1 from total protein were shown in both A549 and SKLU-1 cells. D, nuclear protein expression of cyclin D1 and VDR in A549 and SKLU-1 cells. Nuclear protein expression of cyclin D1 was significantly reduced by 1,25-D3 in SKLU-1 cells but no changes were observed in A549 cell with the treatment of 1,25-D3. Each band was normalized by β-actin. Arbitrary units in the figures represent the ratio between the normalized band density of the specific protein treated with 1,25-D3 and the corresponding untreated protein. This was repeated three times independently.
4. Discussion
VDR has been found in many cell types including the classical target tissues that mediate calcium and mineral homeostasis. This has led to the realization that vitamin D has diverse biological functions, including anti-proliferative and pro-differentiating effects in cancer [8, 21]. Pulmonary diseases associated with aberrant pulmonary VDR expression include pulmonary fibrosis [22], asthma [23], chronic obstructive pulmonary disease (COPD) [24] and lung cancer [25]. Our laboratory has been studying the antiproliferative effects of 1,25-D3 in lung cancer. The cellular effects of 1,25-D3 in lung AC are different from other cancers. In prostate cancer, 1,25-D3 affects both G1 and G2 phase and results in apoptosis [26]. We do not observe apoptosis in lung AC; instead we find a decrease in cell number as a cytostatic effect. For various reasons, including post translational modification of CYP24A1 by miR125 in breast cancer [27], the determinants of vitamin D metabolism are different in the lung.
We have previously reportedthat increased CYP24A1 expression was associated with a reduced anti-proliferative capability of 1,25-D3 in lung cancer [20]. In the present study, we noted a differential expression of VDR mRNA in lung AC with 30% of patients showing a greater than 1.5 fold increase in mRNA levels. Poorly differentiated cancers had a lower VDR mRNA expression. Using available TMAs in 89 patients, we characterized VDR protein expression. Consistent with the literature, we noted a nuclear pattern of VDR expression [16]. Although VDR mRNA did not correlate with protein levels, we noted that patients with higher nuclear VDR expression had a trend toward improved survival (data not shown). The fact that there was no correlation between mRNA and protein levels is not surprising. Similar disparate results between VDR mRNA and protein have been described by others [28–30]. We found that serum 1,25-D3 levels and VDR protein expression were statistically correlated as an increase in ligand corresponded with an increase in protein. Our study demonstrates that 1,25-D3 is associated with greater VDR expression and that VDR is inducible by 1,25-D3.
Increasing concentrations of 1,25-D3 were proportional to the anti-proliferative effects. These effects were more pronounced in cell lines with higher compared to lower VDR mRNA. Moreover, the reduction in cell proliferation was due to decreased number of cells in the S-phase of the cell cycle. We noted a decrease in cyclin D1, Rb, phospho-Rb and Skp2, markers of reduced entry of cells into S-phase. Others have reported similar mechanisms involved in 1,25-D3 mediated G1 arrest [31, 32].
These experiments demonstrate that VDR can be induced by 1,25-D3 and mediate anti-proliferative effects. Studies have demonstrated a correlation between the VDR expression and sensitivity to growth inhibition by vitamin D compounds [33, 34]. However, the antiproliferative effects may not be entirely due to differential VDR expression. The ligand bound effects of 1,25-D3 via VDR and subsequent transcriptional regulation of G1/S phase genes may be one explanation. Others have demonstrated that the antiproliferative effects, specifically on G1 phase of the cell cycle was noted in VDR+/+ cells and not VDR−/− cells [35]. While siRNA experiments may be performed, they can be confounding as other transcriptional mediators of VDR may affect anti-proliferative effects. An et al [36] recently demonstrated that ligand bound VDR regulates the posttranslational modification and function of FoxO proteins.
It is generally regarded that 25-D3 levels in the blood is a reflection of the vitamin D status in the human body. Our patients had very low levels of 25-D3 (Table 3). In an independent study of veterans without cancer, nearly 70% had serum 25-D3 lower 25 nmol/L (data unpublished). We believe that the latitude of residence may have contributed to low levels. Unfortunately we do not have dietary or genotype informationto correlate with observed 25-D3 levels.
The anti-proliferative effects on cancer are mediated by 1,25-D3, the ligand for VDR and may be more relevant compared with 25-D3 in cancer. There is also a question of whether one can safely achieve anti-proliferative levels of 1,25-D3 in humans. Preclinical data indicate that maximal antitumor effects are seen with pharmacological doses of 1,25-D3 and that such exposure can be safely achieved in animals using an intermittent schedule. The area under the plasma concentration-time curve (AUC) and maximum concentration (Cmax) of 1,25-D3 are 32 ng h/mL and 9.2 ng/mL are associated with striking antitumor effects in a murine squamous cell carcinoma model [37].
A recent phase I clinical trial demonstrates that the maximum tolerated dose (MTD) of intravenous 1,25-D3 is 74 μg per week. The AUC of 1,25-D3 at the MTD was 35.65 ± 8.01 ng h/mL [38]. Therefore, it is possible to achieve biologically relevant anti-proliferative levels in the blood. However, more pre-clinical studies are needed to investigate if systemic 1,25-D3 levels are predictive of local tissue levels. Pulmonary epithelial cells are capable of generating 1,25-D3 locally due to the presence of CYP27B1, the enzyme metabolizing 25-D3 to 1,25-D3 [39]. Whether we increase systemic 1,25-D3 levels or 25-D3 substrate levels to increase local production of 1,25-D3 needs study. An inhaled aerosol form of 25-D3 could take advantage of alveolar epithelial CYP27B1 to increase local production of 1,25-D3 and tissue VDR levels. These approaches may, in the future allow a personalized dosing of 1,25-D3 to reduce the risk of lung cancer for both primary as well as in the secondary prevention setting.
Acknowledgments
Grant Support
NIH R21CA128193-01-A1 and VA Merit I01CX000333-02.
Footnotes
Disclosure of Potential conflicts of Interest
No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li XY, Xiao JH, Feng X, Qin L, Voorhees JJ. Retinoid X receptor-specific ligands synergistically upregulate 1,25-dihydroxyvitamin D3-dependent transcription in epidermal keratinocytes in vitro and in vivo. J Invest Dermatol. 1997;108:506–12. doi: 10.1111/1523-1747.ep12289733. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 4.Rachez C, Lemon BD, sulden Z, Bromleigh V, Gamble M, Näär AM, et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–8. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 5.Molnar F, Perakyla M, Carlberg C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J Biol Chem. 2006;281:10516–26. doi: 10.1074/jbc.M513609200. [DOI] [PubMed] [Google Scholar]
- 6.Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3. A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest. 1998;101:2223–32. doi: 10.1172/JCI1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davoodi F, Brenner RV, Evans SR, Schumaker LM, Shabahang M, Mauta RJ, et al. Modulation of vitamin D receptor and estrogen receptor by 1,25(OH)2-vitamin D3 in T-47D human breast cancer cells. J Steroid Biochem Mol Biol. 1995;54:147–53. doi: 10.1016/0960-0760(95)00128-m. [DOI] [PubMed] [Google Scholar]
- 8.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 9.Shabahang M, Buras RR, Davoodl F, Schumaker LM, Nauta RJ, Uskokovic MR, et al. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res. 1994;54:4057–64. [PubMed] [Google Scholar]
- 10.Elstner E, Linker-Israeli M, Said J, Umiel T, de Vos S, Shintaku IP, et al. 20-epi-vitamin D3 analogues: a novel class of potent inhibitors of proliferation and inducers of differentiation of human breast cancer cell lines. Cancer Res. 1995;55:2822–30. [PubMed] [Google Scholar]
- 11.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–10. [PubMed] [Google Scholar]
- 12.Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2765–72. doi: 10.1158/1055-9965.EPI-09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80(6 Suppl):1721S–4S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29:2378–85. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes RJ, Cheney RT, Husain A, Tretiakova M, Loewen G, Johnson CS, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008;17:1104–10. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan M, Parwani AV, Hershberger PA, Lenzner DE, Weissfeld JL. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J Steroid Biochem Mol Biol. 2011;123:30–6. doi: 10.1016/j.jsbmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Kim S, Taylor JM, Wang Z, Lee O, Ramnath N, et al. Development and validation of a quantitative real-time polymerase chain reaction classfier for lung cancer prognosis. J Thorac Oncol. 2011;6:1481–7. doi: 10.1097/JTO.0b013e31822918bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydrosyvitamin D by radioimmunoassay using radioiodinated tracers. Methos Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 19.Peters U, Hayes RB, Chatterjee N, Shao W, Schoen RE, Pinsky P, et al. Circulating vitamin D metabolites, polymorphism in vitamin D receptor and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2004;13:546–52. [PubMed] [Google Scholar]
- 20.Chen G, Kim SH, King AN, Zhao L, Simpson RU, Christensen PJ, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin Cancer Res. 2010;17:817–26. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wali RK, Kong J, Sitrin MD, Bissonnette M, Li YC. Vitamin D receptor is not required for the rapid actions of 1,25-dihydroxyvitamin D3 to increase intracellular calcium and activate protein kinase C in mouse osteoblasts. J Cell Biochem. 2003;88:794–801. doi: 10.1002/jcb.10432. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zügel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118:142–50. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–6. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser U, Schilli M, Wegmann B, Barth P, Wedel S, Hofmann J, Havemann K. Expression of vitamin D receptor in lung cancer. J Cancer Res Clin Oncol. 1996;122(6):356–9. doi: 10.1007/BF01220803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee SW, Campbell MJ, Simons C. Inhibition of Vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. J Steroid Biochem Mol Biol. 2006;98:228–35. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol. 2009;76:702–9. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- 28.Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51:2294–300. doi: 10.2337/diabetes.51.7.2294. [DOI] [PubMed] [Google Scholar]
- 29.Crofts LA, Hancock MS, Morrison NA, Eisman JA. Multiple promoters direct the tissue-specific expression of novel N-terminal variant human vitamin D receptor gene transcripts. Proc Natl Acad Sci USA. 1998;95:10529–34. doi: 10.1073/pnas.95.18.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992;267:20082–6. [PubMed] [Google Scholar]
- 31.Huang YC, Hung WC. 1,25-dihydroxyvitamin D3 transcriptionally represses p45Skp2 expression via the Sp1 sites in human prostate cancer cells. J Cell Physiol. 2006;209:363–9. doi: 10.1002/jcp.20741. [DOI] [PubMed] [Google Scholar]
- 32.Li P, Li C, Zhao X, Zhang X, Nicosia SV, Bai W. p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J Biol Chem. 2004;279:25260–7. doi: 10.1074/jbc.M311052200. [DOI] [PubMed] [Google Scholar]
- 33.Hedlund TE, Moffatt KA, Miller GJ. Stable expression of the nuclear vitamin D receptor in the human prostatic carcinoma cell line JCA-1: evidence that the antiproliferative effects of 1 alpha, 25-dihydroxyvitamin D3 are mediated exclusively through the genomic signaling pathway. Endocrinology. 1996;137:1554–61. doi: 10.1210/endo.137.5.8612485. [DOI] [PubMed] [Google Scholar]
- 34.Hedlund TE, Moffatt KA, Miller GJ. Vitamin D receptor expression is required for growth modulation by 1 alpha,25-dihydroxyvitamin D3 in the human prostatic carcinoma cell line ALVA-31. J Steroid Biochem Mol Biol. 1996;58:277–88. doi: 10.1016/0960-0760(96)00030-1. [DOI] [PubMed] [Google Scholar]
- 35.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya R. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–6. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 36.An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26:2551–6. [PubMed] [Google Scholar]
- 38.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:1216–23. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 39.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]