Abstract
BACKGROUND
Overall perceived health (OPH) is a powerful and independent predictor of negative health outcomes and low health-related quality of life. OPH is conspicuously low in patients with heart failure (HF).
OBJECTIVE
The purpose of this study was to determine the key predictors of OPH in persons with HF and explore possible mediating relationships.
METHODS
This cross-sectional predictive correlational study was a secondary analysis of an existing dataset. Individual characteristics, biophysiological variables, physical symptoms, psychological symptoms, and physical and social functioning were identified from the Wilson and Cleary Model and tested as predictors of OPH in a five-step hierarchical regression analysis.
RESULTS
The sample (n=265) was primarily male (64.2%), white (61.9%), with a mean age of 62 years, at least a high school education, and a household income enough or more than enough to meet needs. Most (69.1%) had systolic dysfunction, and 78.5% were NYHA III or IV. The final model containing 15 predictors explained 39.2% of the variance in OPH. Six variables were significant independent predictors of OPH: perceived sufficiency of income, social functioning, comorbid burden, symptom stability, race, and the interaction of gender and social functioning, the last indicating social functioning as a stronger predictor for males than for females. In a multiple mediation analysis, the effects of shortness of breath and fatigue on OPH were mediated by physical and social functioning. Gender moderated the effect of fatigue through social functioning.
CONCLUSIONS
These variables explained a significant portion of the variance in OPH and can be used to target individuals at risk for low OPH and to tailor interventions. If OPH is low, a focus on patient symptoms and ability to participate in life activities is appropriate, with particular attention to social functioning in men.
Keywords: perceived health, self-rated health, heart failure, health status, health-related quality of life
Heart failure (HF) is a progressive and complex clinical syndrome with no cure. HF affects 5.7 million people in the United States1 with an age-adjusted 5-year mortality estimate of 48%.2 For surviving HF patients, the need for rehospitalization is frequent3 and HRQOL is poor.4 These negative health outcomes share one common independent predictor, low overall perceived health (OPH).
OPH is a subjective, individualized self-assessment of the current overall state of personal health that is typically measured by a single question asking for a rating of current general health status. OPH is an independent and unique variable that is sensitive to physical, psychological, and social changes.5–7 Importantly, OPH influences subsequent health behaviors. Persons with poor OPH are less likely to participate in physical activity and to adhere to prescribed medical therapy.8–9 Yet, no research has been conducted that offers clinically useful information to guide the use of OPH to improve negative health outcomes in persons with HF. Therefore, the purpose of this study was to examine the variables that significantly influence OPH in chronic HF patients and explore possible mediating relationships.
Background and Significance
OPH is notably lower in HF patients than in the general population, healthy age-peers, and patients with other chronic conditions.10–11 Commonly misconstrued as a proxy for HRQOL, OPH is a distinct construct that directly influences and significantly mediates the influence of other factors on HRQOL.12–13 OPH has also been verified to be more than solely a personality trait14 or enduring self-concept.5
In patients with HF, OPH has been demonstrated to be a powerful predictor of worsening health,15 need for hospitalization,15–17 mortality,15–16,18–19 and low HRQOL12 even after controlling for demographic characteristics; 12,15–16,18–19 comorbid conditions;12,17–19 New York Heart Association (NYHA) class; 12,16,19 exercise tolerance;15 clinical measures such as ejection fraction (EF),16,19 estimated glomerular filtration rate,18 hemoglobin, 18 or B-type natriuretic peptide; 19 and treatment with medication or revascularization.16 Because of the impressive prognostic value of OPH, experts have suggested the institution of routine monitoring of this variable in the clinical arena.17,19–20 However, no research has been completed offering direction for how clinicians might effectively respond when low OPH values are detected in patients with HF. Studies are needed to provide clinicians with an understanding of the factors associated with OPH in HF patients.
Variables found in other populations to be associated with OPH include age,6,21–22 gender,21,23–25 race/ethnicity,23,25 education, 21,23,26 income, 21,23–24 comorbid conditions, 5,23–24,26 physical symptoms,6–7,21 depressive symptoms,22,24,27 and social5,13,24,28 and physical functioning.6,21,23–24,28 An interaction effect between age and both comorbidity and physical functioning has also been found with these factors having a stronger negative association with OPH in younger individuals.22 Only 3 studies have focused on factors associated with OPH in patients with HF.29–31 Predictors of OPH included age, health beliefs, exertional dyspnea, fatigue, emotional distress, physical functioning, and the ability to work outside the home. 29–31 In one additional study focused on HRQOL, shortness of breath (SOB) and fatigue predicted OPH.12 Interestingly in this analysis, physical functioning was not related to OPH and the relationship between physical symptoms and OPH was not mediated through physical functioning.12 This important finding led us to explore how physical functioning influences the relationship between symptoms and OPH.
In spite of the low levels of OPH and its predictive validity for poor health outcomes in persons with chronic HF, few studies have focused on this construct in this population and the findings from these have limited generalizability. The samples tested have been limited to predominantly males with only systolic dysfunction, only patients with advanced HF with potential for cardiac transplantation or hospitalized for an acute HF exacerbation, or urbanites with low education and limited resources. No study of the key factors known to predict OPH in other populations has been conducted in a typical community-dwelling, general sample of patients living with HF. This study was designed to determine the key variables associated with OPH in persons with chronic HF and to evaluate the amount of variability in OPH attributable to each using a hierarchical regression analysis.
The Wilson and Cleary Model (WCM) of patient outcomes refined by Ferrans et al.32 was used as a conceptual framework for this analysis. This model postulates five levels of increasing integration and complexity: biophysiological factors, symptoms, functioning, general health perceptions, and overall quality of life, with individual and environmental characteristics postulated to influence each. Because OPH is the core domain of general health perceptions,33 the major portion of the model offers a framework of factors thought to influence OPH. Therefore, the specific aims of this study were twofold. First we assessed the extent to which OPH in persons with HF is uniquely predicted by individual characteristics (age, gender, race/ethnicity, education, income), biophysiological factors (number of chronic illnesses, comorbid burden, diabetes, atrial fibrillation), physical symptoms (fatigue, shortness of breath, symptom stability), psychological symptoms (depression), and functional status (physical functioning, social functioning). Second, we tested whether functional status mediates the relationship between physical symptoms and OPH.
Methods
Design and Samples
A cross-sectional descriptive and predictive design was used to conduct a secondary analysis of baseline data from patients with HF recruited between 2007 and 2009 for a prospective study examining the influence of excessive daytime sleepiness (EDS) on self-care, HRQOL, and unplanned hospitalization (conducted by BR). For the parent study, subjects were identified from outpatient settings in Philadelphia, PA and Newark, DE. One setting was a university referral center for HF and transplantation. Another was a Veterans Affairs hospital where subjects were enrolled from various cardiology and medical clinics. The third was a community hospital where subjects were enrolled from the practices of any physician treating patients with HF. Inclusion criteria included Stage C chronic HF confirmed by echocardiography and clinical evaluation, ability to speak and read English, adequate literacy, hearing adequate to engage in dialogue, and living in a setting amenable to performing self-care. Patients were excluded if they had major depression, severe dementia or significant cognitive impairment at the time of screening, renal failure requiring dialysis, night shift work, terminal illness, planned relocation, or heavy and regular drug or alcohol abuse within the past year. More detailed information about the sampling and recruitment of subjects in the parent study has been published elsewhere.34 Medical record reviews were conducted in clinic offices and self-report data were collected during home visits. For this secondary analysis all data were obtained without identifying information and the study was approved by the University of Nebraska Medical Center institutional review board.
A power analysis was performed using GPower 3.010. A sample of 97 subjects was required to explain 40% of the variance in the outcome with 14 predictors. A sample size of 120 was required for a hierarchical regression analysis with an R2 increase of 5% for the final step with a power of .80 and individual regression coefficient alpha = .05. For the mediation analysis, at least 162 subjects were needed if the path coefficients involved in the mediation effect were both small to medium in size.35 The available sample exceeded all estimates.
In all, 280 subjects were enrolled in the parent study. The 15 patients who did not have data needed for our study had significantly better NYHA functional class, fewer symptoms, and better physical functioning. Our final sample consisted of 265 patients with symptomatic HF.
Measures
OPH was measured with the commonly used first item in the SF-36 (v2) often referred to as self-rated health. This item states “In general, would you say your health is:” with response choices of excellent, very good, good, fair, or poor. The Medical Outcomes Study scoring algorithm was used to recalibrate the response scale and convert scores to a 0–100 scale with 0 indicating “poor” and 100 indicating “excellent” health.36 The self-rated health item has been used extensively in healthcare research as a subjective assessment of overall health.6,11–12,25,30,33 Construct and discriminant validity of this measure have been established.37–38
Age, gender, race/ethnicity, education, and family income were measured with a sociodemographic questionnaire designed specifically for the parent study. Type, severity and duration of HF were collected from the medical record and used only to describe the sample. Chronic illness was measured with four distinct variables identified in the literature as related to OPH: (a) number of chronic illnesses, (b) comorbidity burden operationalized as an index of number and severity of chronic illnesses, (c) diagnosis of diabetes, and (d) diagnosis of chronic atrial fibrillation. Chronic illness data were collected from medical record review. Comorbid burden was measured with the Charlson Comorbidity Index (CCI) total score. Construct validity of the CCI has been confirmed in the HF population.39
Physical symptoms and physical and social functioning were measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), a HF-specific health status measure.40 Scores on the KCCQ range from 0 to 100 with higher values indicating better health status. Content and construct validity of the KCCQ as well as internal consistency and test-retest reliabilities for each of the subscales have been established.40 Internal consistency reliability of the physical and social functioning subscales in this study sample was adequate with Cronbach’s alpha values of .85 and .86 respectively. Individual scores for fatigue and SOB were computed from each set of 2 items measuring frequency and severity of each symptom. The internal consistency reliability of the fatigue and SOB symptom scales in this sample were .88 and .89 respectively.
Depression was measured with the 9-item Patient Health Questionnaire (PHQ-9), which offers a list of depressive symptoms and directions to indicate the frequency of each over the past month. Response values are summed to create a range from 0 to 27. Higher scores indicate greater depression severity. Content and construct validity have been established.41 Internal consistency reliability of the PHQ-9 has been demonstrated in primary care patients and in women with Cronbach’s alpha coefficients of .89 and .86 respectively.41 However, in this sample, internal consistency reliability of this measure was only .68, presumably because major depression was an exclusion criterion.
Statistical Analysis
Descriptive statistics were used to describe the sample and predictor and outcome variables. Bivariate correlational analyses of all variables were conducted to verify expected relationships and identify potential multicollinearity. Relationships between categorical predictors and OPH were assessed by statistical testing of differences between groups with independent t-tests or ANOVA. To achieve aim 1, a hierarchical multiple regression analysis using five steps was performed with all new variables at each step entered simultaneously as a block. Interaction terms of age with each comorbidity variable and each functioning variable were included in the appropriate blocks. Models were fit with each measure of comorbidity to select the one that explained the most variance. Continuous variables were centered about the mean prior to creating the interaction terms to reduce potential collinearity. All assumptions of linear ordinary least-squares regression were assessed and no violations were found.
To achieve aim 2, a multiple mediator analysis was conducted to assess the indirect effects of physical symptoms on OPH via one or both of the functioning variables. The bias-corrected bootstrapping method for multiple mediator analysis was used to test total and indirect effects in the model.42 This method reduces potential bias due to omitted variables and offers the advantage of determining the total indirect effect of symptoms on OPH as well as the unique indirect effects of each mediator. Bootstrapping was done by nonparametric resampling with replacement 1,000 times. Based on finding a gender influence on the relationship between social functioning and OPH in the regression analysis, a moderated mediation analysis was done to assess whether the mediation of SOB or fatigue by social functioning differed by gender. Conditional indirect effects were computed in males and females.43 Significance level for all tests was set at P = .05. Data were analyzed using SPSS 17.0 software (SPSS, Inc, Chicago, Illinois).
Results
Patient Characteristics
The sample was predominately older white males (Table 1). Only 25 participants (9.4%) were without comorbid conditions. The vast majority (76.2%) had a total of 3 or more chronic illnesses and over one-third (37.0%) had 5 or more.
Table 1.
Sample Characteristics (N = 265)
| Variable | Mean (SD) or Number (%) |
|---|---|
| Age (range 24 to 89) | 61.89 (12.40) |
| Male | 170 (64.2) |
| Race/ethnicity | |
| White | 164 (61.9) |
| Black | 92 (34.7) |
| Other | 9 (3.4) |
| Education level | |
| Less than high school | 26 (9.8) |
| High school | 95 (35.8) |
| Business school, some college, or associate degree | 73 (27.5) |
| Bachelor’s degree | 43 (16.2) |
| Graduate degree | 28 (10.5) |
| Household income | |
| Not enough | 44 (16.6) |
| Enough | 127 (47.9) |
| More than enough | 94 (35.5) |
| Heart failure type | |
| Systolic | 183 (69.1) |
| Diastolic | 50 (18.9) |
| Mixed | 32 (12.1) |
| Ejection fraction (n = 264) | 35.25 (17.05) |
| Preserved ejection fraction (50% or greater) (n = 264) | 62 (23.5) |
| Ischemic HF | 98 (37.0) |
| Mean duration of HF in months | 74.71 (72.29) |
| New York Heart Association Class | |
| I | 12 (4.5) |
| II | 45 (17.0) |
| III | 158 (59.6) |
| IV | 50 (18.9) |
| Comorbid conditions | |
| Hypertension | 169 (63.8) |
| Diabetes | 103 (38.9) |
| CAD (history of myocardial infarction) | 98 (37.0) |
| Atrial fibrillation | 86 (32.5) |
| Renal disease | 70 (26.4) |
| Chronic obstructive pulmonary disease | 58 (21.9) |
| Pulmonary hypertension | 54 (20.4) |
| Anemia | 49 (18.5) |
| Cerebrovascular disease | 39 (14.7) |
| Peripheral vascular disease | 31 (11.7) |
| Cancer | 19 (7.2) |
| Connective tissue disease | 14 (5.3) |
| Liver disease | 3 (1.1) |
| AIDS | 1 (0.4) |
Note. Data given as mean (SD) or number (%). CAD = coronary artery disease.
More than half (55.1%) of the participants rated their OPH as fair or poor and only 11.3% rated their OPH as very good or excellent (Table 2). Most patients reported being symptomatic with fatigue (61.5%) or SOB (50.9%) at least weekly. Only 11.3% of the sample experienced a worsening in symptoms during the previous month. Most (64.2%) subjects reported no difficulty with life activities because of depressive symptoms. The vast majority of participants reported limitations in physical functioning (88.3%) and social functioning (82.6%) due to HF.
Table 2.
Descriptive Statistics for Continuous Variables (N = 265)
| Variable | Range | Mean (SD) |
|---|---|---|
| Number of chronic illnesses | 1–10 | 4.00 (1.88) |
| Charlson Comorbidity Index | 1–11 | 2.77 (1.66) |
| Fatigue symptom Scorea | 0–100 | 61.89 (29.55) |
| Shortness of breath symptom scorea | 0–100 | 67.56 (29.64) |
| Symptom stability scorea | 0–100 | 57.26 (22.33) |
| Depressive symptoms score | 0–18 | 4.48 (3.66) |
| Physical functioning scorea | 0–100 | 68.93 (23.55) |
| Social functioning scorea | 0–100 | 65.79 (28.16) |
| Overall perceived healtha | 0–100 | 40.68 (26.68) |
higher score indicates better health.
Bivariate Relationships
Correlational relationships between predictor variables and OPH are shown in Table 3. A strong association was evident between physical functioning and OPH while other predictors had weak to moderate correlations with OPH. Age was not correlated with OPH.
Table 3.
Intercorrelations between Overall Perceived Health and Predictor Variables
| Age | Education | Income | # CI | CCI | Fatigue | SOB | Symptom Stability | Depressive Symptoms | Physical Function | Social Function | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPH | .07 | .25*** | .39*** | −.27*** | −.27*** | .38*** | .45*** | .18** | −.32*** | .51*** | .49*** |
| Age | - | −.11 | .18** | .34*** | .25*** | .22*** | .08 | .08 | −.18** | −.01 | .12 |
| Education | - | .28*** | −.16** | −.10 | .04 | .18** | −.00 | −.12* | .21** | .13* | |
| Income | - | −.08 | −.02 | .28*** | .33*** | −.04 | −.17** | .38*** | .37*** | ||
| # CI | - | .71*** | −.00 | −.16** | .03 | .01 | −.28*** | −.16** | |||
| CCI | - | −.05 | −.16* | .04 | .04 | −.22*** | −.16* | ||||
| Fatigue | - | .60*** | .15* | −.54*** | .54*** | .68*** | |||||
| SOB | - | .22*** | −.40*** | .68*** | .65*** | ||||||
| Symptom Stability | - | −.14* | .16** | .14* | |||||||
| Depressive Symptoms | - | −.38*** | −.42*** | ||||||||
| Physical Function | - | .71*** |
Note. Values for education and income are reported as Spearman’s rho; all other values reported as Pearson’s r correlation coefficient. # CI = number chronic illnesses; CCI = Charlson Comorbidity Index; Fib = fibrillation; OPH = overall perceived health; SOB = shortness of breath.
p < .05.
p < .01.
p < .01
Hierarchical Regression Analysis
The results of the hierarchical regression analysis are summarized in Table 4. Age, gender, race/ethnicity, education, and income accounted for 20.5% of the variance in OPH after adjustment for the number of variables (F(6,258) = 12.38, p <.001). Income, gender, and race/ethnicity were significant predictors of OPH in this model with higher income and female gender predicting better OPH and black compared to white race predicting worse OPH. All variables were retained because of theoretical importance and collective contribution to variance explained.
Table 4.
Coefficient values for each predictor from hierarchical regression analysis
| Step
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
|
| ||||||||||
| Predictor | B | β | B | β | B | β | B | β | B | β |
| Age | .06 | .03 | .21 | .10 | .10 | .04 | .07 | .03 | .12 | .05 |
| Gender | 7.08 | .13* | 5.07 | .09 | 5.82 | .11* | 6.36 | .12* | 5.70 | .10 |
| Black versus white | −11.02 | −.20** | −8.30 | −.15* | −6.92 | −.12* | −7.22 | −.13* | −6.39 | −.11* |
| Other race/ethnicity versus white | −10.45 | −.07 | −9.08 | −.06 | −5.03 | −.03 | −5.48 | −.04 | −4.48 | −.03 |
| Education | 2.42 | .10 | 2.45 | .11 | 2.17 | .09 | 1.84 | .08 | 1.67 | .07 |
| Income | 11.52 | .30*** | 11.85 | .31*** | 9.09 | .24*** | 9.28 | .24*** | 7.87 | .21*** |
| Comorbid burden | −4.16 | −.26*** | −3.47 | −.22*** | −3.44 | −.21*** | −2.99 | −.19** | ||
| Age x comorbid burden | .17 | .12* | .14 | .10* | .15 | .11* | .10 | .07 | ||
| Fatiguea | .14 | .15* | .08 | .09 | .02 | .02 | ||||
| SOBa | .15 | .17* | .14 | .16* | .04 | .05 | ||||
| Symptom stabilitya | .17 | .14* | .16 | .14* | .15 | .12* | ||||
| Depressive symptoms | −9.10 | −.12* | −.83 | −.11 | ||||||
| Physical functioninga | .15 | .14 | ||||||||
| Social functioninga | .18 | .20* | ||||||||
| Gender x social functioning | −.20 | −.12* | ||||||||
| Adjusted R2 | .20*** | .26*** | .36*** | .37*** | .39*** | |||||
| ΔR2 | .06*** | .10*** | .01* | .03** | ||||||
Note. Gender coding was 0 for males and 1 for females. Continuous variables were centered about the mean to create interaction terms. β = standardized regression coefficient; SOB = shortness of breath; Δ= change.
Higher score indicates better health.
p < .05.
p < .01.
p < .01
Several variables were not retained in the sequential models because they offered little or no significant information. These included number of chronic illnesses, atrial fibrillation, diabetes, and all interaction terms involving age, with the exception of age by comorbid burden. When physical and social functioning were added in the last step of the regression analysis, each was significant only when the other was removed. Based on changes in coefficient values for gender and black versus white race when social functioning was added or removed, interaction terms for social functioning with each of these variables were tested. With the gender by social functioning interaction term included, social functioning and the interaction term were both significant independent predictors of OPH in the final model. The interaction term indicated that social functioning differed by gender. Although the coefficient for physical functioning was not significant (t = 1.68, P =.09) in this final step, the variable was retained because of theoretical importance.
When controlling for individual characteristics, comorbid burden and its interaction with age indicating a stronger negative association in younger patients, uniquely accounted for 6% of the variance in OPH. Adjusting for individual characteristics and comorbid burden, fatigue, SOB, and symptom stability were each significant independent predictors and explained another 10.5% of the variance in OPH. Depressive symptoms, when controlling for previously entered variables, was a significant independent predictor of OPH, but explained only an additional 1% of the variance. Finally, physical functioning, social functioning, and a gender by social functioning interaction term explained an additional 3% of the variance in OPH beyond what was explained by the other variables.
The final model containing 15 variables explained 39.2% of the variance in OPH after adjustment for the number of variables. Six variables were significant independent predictors of OPH: perceived sufficiency of income, social functioning, comorbid burden, symptom stability, black compared to white race, and the interaction of gender and social functioning indicating that social functioning was a stronger predictor for males than for females. Higher OPH was predicted by higher income, symptom stabilization or improvement, and, in males only, higher social functioning. Lower OPH was predicted by higher comorbid burden and black compared to white race. The other variables collectively contributed to the explanation of the variance in OPH, significantly increasing the R2 value, but none offered significant unique contributions. These included higher age, female gender, more education, lower fatigue burden, lower SOB burden, and higher physical functioning predicting higher OPH and depressive symptoms and other race/ethnicity compared to white predicting lower OPH.
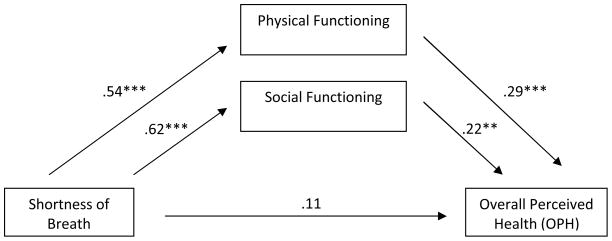
As depicted in Figures 1 and 2, physical and social functioning mediated the effect of SOB (F(3, 261) = 37.42, P < .001) and fatigue on OPH (F(3, 261) = 36.33, P < .001). In both cases the total effect of symptoms on OPH was significant, but the direct effect was not. This suggests that the effect of the physical symptom on OPH is entirely transmitted through its effect on the mediating variables. Also in both models, the two indirect effects did not significantly differ from each other indicating that physical functioning and social functioning equally mediate the effect of the symptom on OPH. Moderated mediation was identified in one model. Specifically, the effect of fatigue on OPH mediated by social functioning was stronger for males (B = .30, P < .001, 95% CI [0.20, 0.40]) than for females (B = .15, P =.02, 95%CI [0.02, 0.28]).
Figure 1.
Multiple mediation model of shortness of breath on OPH through physical and social functioning with coefficients indicated for each path. Total effect = .41 (P = < .001). Indirect effect for physical functioning = .16 (95% CI, .05–.26) and for social functioning = .14 (95% CI, .03–.23). Contrast in indirect effects was 0.03 (95% CI, −.15 to .20). R2 = .30; Adjusted R2 = .29 (F3,261 = 37.42, P < .001).
** < .01. *** < .001.
Figure 2.
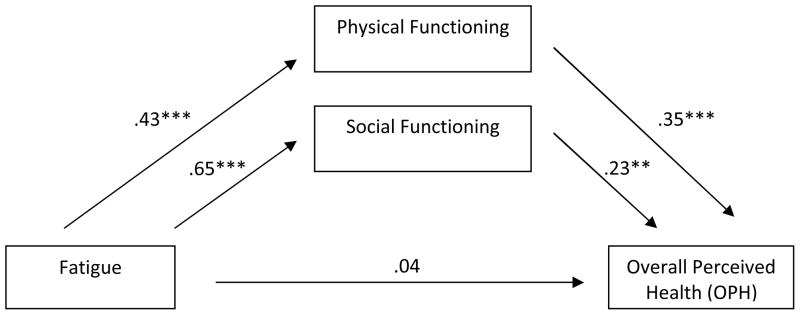
Multiple mediation model of fatigue on OPH through physical and social functioning with coefficients indicated for each path. Total effect = .34 (P = < .001). Indirect effect for physical functioning = .15 (95% CI, .07–.23) and for social functioning = .15 (95% CI, .04–.25). Contrast in indirect effects was 0.002 (95% CI, −.16 to .17). R2 = .29; Adjusted R2 = .29 (F3,261 = 36.33, P < .001).
** < .01. *** < .001.
Discussion
This study was the first to determine the key variables associated with OPH in community-dwelling persons with chronic HF and to evaluate the amount of variability in OPH attributable to each. Our finding that the selected variables accounted for 39.2% of the variance in OPH is comparable to the 29% to 38% range reported in large populations studies.5,27,44 In a study of older patients with a combination of comorbid conditions24 and one study of HF patients,31 44.7% and 46% of the variance in OPH were explained respectively with similar predictors. The variables that predicted OPH in our study are also consistent with those found in population studies.5,21–22,27 The contribution of symptoms and social functioning to OPH in our study is consistent with other studies of chronically ill patients in which OPH was measured in basically the same manner.24,44–46 Our results offer validation that the amount of variance and the general factors that account for variance in OPH in patients with HF are similar to those that have been identified in theoretical models, the general population, and others with chronic illness.
Our finding that each group of variables uniquely explained a small, but significant portion of the variance over and above those already added to the model is consistent with theoretical modeling of the integrative nature of this concept,32 yet highlights the additional and distinct information each offers. This finding is also consistent with results from the sole population study using a similar analysis with similar predictors, but in a sample of older adults.27 Ours are the first such results in patients with HF.
We found that unmodifiable factors accounted for a sizable portion of the explained variance in OPH, as others have found. 27 of the sociodemographic characteristics we included are known to affect health either biologically or due to influences on lifestyle choices or healthcare use. However, all such personal attributes also form the framework in which self-evaluations of personal health are made, the viewpoint within which other factors are interpreted.47
We found a particularly strong positive association between income and OPH in our sample. This predictor remained significant through all steps of the hierarchical regression and had the largest standardized coefficient in the final model. Interestingly, 83.4% of our subjects reported having an income that was enough or more than enough to meet household needs. Chronic illness is expensive, creates disability, and requires life changes. An adequate financial income offers the resources to obtain medical care, drug therapy, and assistance with lifestyle modification or personal obligations in order to maintain health. It also offers protection for OPH from the stress, lack of treatment, and barriers to self-care created by inadequate resources. Our measure of income as a perception of adequacy likely provided a more relevant perspective for OPH than that gained by measures using dollar amounts. This finding illustrates the importance of evaluating perceived manageability in patients with HF from the standpoint of personal financial resources.
Race was an additional unmodifiable factor explaining OPH in our sample. Our finding that black race is predictive of lower OPH is consistent with population studies, but a new finding in patients with HF. Only one other study of OPH in HF patients had sufficient black subjects to analyze race and no association was found.29 The difference in results may lie in the fact that black respondents in that study were likely to be female, who typically have higher OPH values,21,23–24 while in our sample most black subjects were male. Health disparities between black and white races are well-known. Cultural differences in OPH related to ethnicity are also well documented and numerous reasons have been postulated, including differences in what constitutes health and the factors considered during self-evaluation of health status.23,47 Our findings suggest that an assessment of OPH may be particularly important in HF patients of black race.
Comorbidity is another nonmodifiable significant predictor of OPH that has been identified in multiple population studies 21,23,27,44,48 and a study of chronically ill subjects.24 However, this finding is in contrast to the one study in HF patients that found no relationship between comorbidity and OPH, likely because billing codes were used to measure comorbid burden.29 Although it was already known that a HF diagnosis alone is associated with lower OPH, our results illustrate that additional chronic illnesses further worsen subjective health perception. Multiple chronic illnesses, through the awareness of medical diagnoses as well as the need to manage multiple medical prescriptive therapies, negatively influence self-assessment of overall health as obvious indicators of the antithesis of health. Sensitivity to the cumulative burden of multiple comorbidities is necessary in care management and patient education to reduce the often overwhelming self-care expectations and deflation of OPH.
The significant modifiable predictors of OPH in our sample were symptom stability, and, in men only, social functioning. Although the physical symptoms included in our model, fatigue and SOB, have been identified as predictors of OPH in two other studies of HF patients,12,31 only one other study included a measure of symptom stability. In residents of retirement communities, Winter et al.7 found that changes in symptoms, beyond level of symptoms, contributed independently to OPH on a daily basis. Considering how symptomatic our sample was and most patients with HF typically are, it is not surprising that a change in symptoms is a more powerful predictor of OPH than the symptoms themselves. Living with symptoms becomes commonplace for many HF patients. Our findings add to the empirical support for the importance of symptom change to OPH and highlight the need to immediately assess for such a change when OPH values are found to be low.
In men, social functioning was a significant independent predictor of OPH, stronger than physical functioning. This finding was unexpected and, to our knowledge, only one small study has reported such a relationship.13 Smith et al.13 found similar results in a sample of 89 HIV positive men. In all other studies and a meta-analysis of predictors of OPH, physical functioning is a far stronger predictor of OPH.11,13,24,44 A decrease in the ability to perform life activities is the most apparent indicator of worsening health to patients and those around them. However some restrictions may have more inherent meaning than others. Our results suggest that, in men with HF, a restriction in social functioning is a more compelling indicator of poor health, consistent with Jylha’s47 hypothesis that personal expectations influence health ratings. It should be noted that the measure we used contains items conceptualized by others as both social and role functioning.49 It may be a more inclusive measure and possibly more sensitive to the influence of ill health. One item, in particular, assessed limitation with intimate relationships and the males in our sample reported being significantly more limited than the females (P < .01). Our findings suggest that this may be a critical area for assessment in males with low OPH.
We are the first to find that, in a sample of HF patients, physical and social functioning mediated the effects of SOB and fatigue on OPH and that the direct effect of physical symptoms was not significant when functional status was in the model. Our results are in contrast to that of Heo et al.7 who found no mediation effect of functional status in the relationship between symptoms and OPH. This difference is likely due to striking differences in the study samples as their subjects were hospitalized for acute decompensated HF. Also, NYHA was used to measure functional status in Heo’s study and no measure of social functioning was included. Using both the physical and social functioning KCCQ subscales may provide a more inclusive, and possibly more sensitive, measure of functioning. Our findings are consistent with the WCM model which postulates that symptoms influence OPH through their effect on functioning and help explain the mechanism of the relationship between symptoms and OPH.
Limitations
Because we conducted a secondary analysis, it was not possible to consider other potential variables that may influence OPH in HF patients. Two factors identified in multiple previous studies that were not available for this study were physical activity and a broader construct of mental health encompassing not only depression, but also affect and emotional distress. Selection bias was possible because patients with low levels of OPH may have been more likely to refuse to participate when informed of the longitudinal nature and testing requirements of the parent study. In this sample, internal consistency reliability of the PHQ-9 was low. The low alpha coefficient appeared to be related to the homogeneity of the sample with respect to depression (due to exclusion of patients with major depression) and low variability of responses on items related to fatigue and sleep, common physical symptoms of HF. Finally, the use of cross-sectional data precludes establishment of a causal relationship between variables and may produce misleading estimates of longitudinal meditational processes.
Conclusions and Implications
These results offer useful information for targeting vulnerable populations at risk for adverse outcomes and for guiding further assessment and tailoring interventions when OPH ratings are low. A guided assessment based on our results can be used to facilitate appraisal and identify unrecognized or unreported worsening symptoms or functioning. Specifically, an increase in social functioning limitations in men, particularly, may be associated with worsening or profound fatigue, a symptom that may not be thought noteworthy by a patient but is associated with worsening HF and need for rehospitalization in patients with chronic HF.50
Further research is necessary to identify other factors associated with the large amount of unexplained variance in OPH in this population and to further explore gender differences, as well as age, ethnic, and cultural differences. Longitudinal studies are needed to capture changes in OPH and its predictors over time in order to establish the temporal relationships required in casual modeling and mediation analyses. We recommend the use of the CCI rather than a count of the number of chronic illnesses in future research because this measure was a stronger predictor of OPH even as other variables were added to the model.
Numerous efforts have been made to improve outcomes for HF patients. Although progress is being made, the prognosis for those with HF remains dismal and clinicians are continually challenged to find ways to improve the lives of these patients. OPH is a powerful predictor of adverse health outcomes. Armed with an understanding of the factors that are associated with OPH, clinicians can target vulnerable populations for intervention.
Contributor Information
Carlson Beverly, School of Nursing, San Diego State University, San Diego, CA.
Bunny Pozehl, College of Nursing, University of Nebraska Medical Center, Omaha, NE.
Melody Hertzog, College of Nursing, University of Nebraska Medical Center, Omaha, NE.
Lani Zimmerman, College of Nursing, University of Nebraska Medical Center, Omaha, NE.
Barbara Riegel, School of Nursing, University of Pennsylvania, Philadelphia, PA.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. Retrieved January 30, 2011, from http://www.circ.ahajournals.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. Retrieved on August 21, 2009 from http://circ.ahajournals.org/cgi/content/full/119/14/e391. [DOI] [PubMed] [Google Scholar]
- 4.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7(2):243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Soc Sci Med. 2003;56(2):203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Benyamini Y, Leventhal EA, Leventhal H. Elderly people’s ratings of the importance of health-related factors to their self-assessments of health. Soc Sci Med. 2003;56(8):1661–1667. doi: 10.1016/s0277-9536(02)00175-2. [DOI] [PubMed] [Google Scholar]
- 7.Winter L, Lawton MP, Langston CA, Ruckdeschel K, Sando R. Symptoms, affects, and self-rated health: Evidence for a subjective trajectory of health. J Aging Health. 2007;19(3):453–469. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
- 8.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: A meta-analysis. Med Care. 2007;45(6):521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann E, Ekholm O, Grønbæk M, Curtis T. Predictors of changes in physical activity in a prospective cohort study of the Danish adult population. Scand J Public Health. 2008;36(3):235–241. doi: 10.1177/1403494808086982. [DOI] [PubMed] [Google Scholar]
- 10.Riedinger MS, Dracup KA, Brecht M for the SOLVD Investigators. Quality of life in women with heart failure, normative groups, and patients with other chronic conditions. Am J Crit Care. 2002;11(3):211–219. [PubMed] [Google Scholar]
- 11.Heo S, Moser DK, Lennie TA, Zambroski SH, Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart Lung. 2007;36(1):16–24. doi: 10.1016/j.hrtlng.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing a published model of health-related quality of life in HF. J of Card Fail. 2005;11(5):372–379. doi: 10.1016/j.cardfail.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: A meta-analysis. Qual Life Res. 1999;8(5):447–459. doi: 10.1023/a:1008928518577. [DOI] [PubMed] [Google Scholar]
- 14.McCullough ME, Laurenceau J. Religiousness and the trajectory of self-rated health across adulthood. Pers Soc Psychol Bull. 2005;31(4):560–573. doi: 10.1177/0146167204271657. [DOI] [PubMed] [Google Scholar]
- 15.Havranek EP, Lapuerta P, Simon TA, L’Italien G, Block AJ, Rouleau JL. A Health Perception score predicts cardiac events in patients with HF: Results from the IMPRESS trial. J of Card Fail. 2001;7(2):153–157. doi: 10.1054/jcaf.2001.24121. [DOI] [PubMed] [Google Scholar]
- 16.Konstam V, Salem D, Pouleur J, Kostis J, Gorkin L, Shumaker S, et al. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. Am J Cardiol. 1996;78(8):890–895. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 17.Roe-Prior P. Variables predictive of poor postdischarge outcomes for hospitalized elders in heart failure. West J Nurs Res. 2004;26(5):533–546. doi: 10.1177/0193945904265684. [DOI] [PubMed] [Google Scholar]
- 18.Farkas J, Nabb S, Zaletel-Kragelj L, Cleland JGF, Lainscak M. Self-rated health and mortality in patients with chronic heart failure. Eur J Heart Fail. 2009;11(5):518–524. doi: 10.1093/eurjhf/hfp038. [DOI] [PubMed] [Google Scholar]
- 19.Johansson P, Brostrom A, Dahlstrom U, Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. The Eur J Heart Fail. 2008;10(10):1040–1047. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question: A meta-analysis. J Gen Intern Med. 2005;20(1):267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shooshtari S, Menec V, Tate R. Comparing predictors of positive and negative self-rated health between younger (25–54) and older (55+) Canadian adults. Research on Aging. 2007;29(6):512–554. [Google Scholar]
- 22.Schnittker J. When mental health becomes health: Age and the shifting meaning of self-evaluations of general health. Milbank Quarterly. 2005;83(3):397–423. doi: 10.1111/j.1468-0009.2005.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menec VH, Shooshtari S, Lambert P. Ethnic differences in self-rated health among older adults: A cross-sectional and longitudinal analysis. J Aging Health. 2007;19(1):62–84. doi: 10.1177/0898264306296397. [DOI] [PubMed] [Google Scholar]
- 24.Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities. Ann Fam Med. 2007;5(5):395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diehr P, Williamson J, Patrick DL, Bild DE, Burke GL. Patterns of self-rated health in older adults before and after sentinel health events. J Am Geriatr Soc. 2001;49(1):36–44. doi: 10.1046/j.1532-5415.2001.49007.x. [DOI] [PubMed] [Google Scholar]
- 26.Jylha M, Colpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population study. J Clin Epidemiol. 2006;59(5):465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Bryant LL, Beck A, Fairclough DL. Factors that contribute to positive perceived health in an older population. J Aging Health. 2000;12(2):169–192. doi: 10.1177/089826430001200202. [DOI] [PubMed] [Google Scholar]
- 28.Katz P, Morris A, Gregorich S, Yazdany J, Eisner M, Yelin E, Blanc P. Valued life activity disability played a significant role in self-rated health among adults with chronic health conditions. J Clin Epidemiol. 2008:e-9. doi: 10.1016/j.jclinepi.2008.06.002. Retrieved September 14, 2008, from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark DO, Tu W, Weiner M, Murray MD. Correlates of health-related quality of life among lower-income, urban adults with congestive heart failure. Heart Lung. 2005;32(6):391–401. doi: 10.1016/j.hrtlng.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Rosen RC, Contrada RJ, Gorkin L, Kostis JB. Determinants of perceived health in patients with left ventricular dysfunction: A structured modeling analysis. Psychosom Med. 1997;59(2):193–200. doi: 10.1097/00006842-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Sulllivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: Relationship to clinical variables and outcome. J Card Fail. 2007;13(7):560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health- related quality of life. J Nurs Scholarsh. 2005;37(4):336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 33.Davies AR, Ware JE. Measuring health perceptions in the health insurance experiment (Document number R-2711-HHS) 1981 Retrieved April 30, 2008 from http://www.rand.org/pubs/reports/2007/R2711.pdf.
- 34.Riegel B, Moelter ST, Ratcliffe SJ, Pressler SJ, De Geest S, Potashnik S, et al. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. doi: 10.1016/j.cardfail.2010.11.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36R health survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 37.DeSalvo KB, Fisher WP, Tran K, Bloser N, Merrill W, Peabody J. Assessing measurement properties of two single-item general health measures. Qual Life Res. 2006;15(2):191–201. doi: 10.1007/s11136-005-0887-2. [DOI] [PubMed] [Google Scholar]
- 38.McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(3):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79(12):1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 40.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 43.Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- 44.Lewis LM, Riegel BJ. Determinants of perceived health in older adults with hypertension. Heart Lung. 2010;39(1):41–49. doi: 10.1016/j.hrtlng.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen JQ, Donesky-Cueno D, Kohlman V. Associations between symptoms, functioning, and perceptions of mastery with global self-rated health in patients with COPD: A cross-sectional study. Int J Nurs Stud. 2008;45(9):1355–1365. doi: 10.1016/j.ijnurstu.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walke LM, Byers AL, Gallo WT, Endrass J, Fried TR. The association of symptoms with health outcomes in chronically ill adults. J Pain Symptom Manage. 2007;33(1):58–66. doi: 10.1016/j.jpainsymman.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jylha M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Kosloski K, Stull DE, Kercher K, Van Dussen DJ. Longitudinal analysis of the reciprocal effects of self-assessed global health and depressive symptoms. J Gerontol B Psychol Sci Soc Sci. 2005;60B(6):P296–P303. doi: 10.1093/geronb/60.6.p296. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Sherbourne CD. The MOS 36-item short-form survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 50.Albert N, Trochelman K, Li J, Lin S. Signs and symptoms of heart failure: Are you asking the right questions? Am J Crit Care. 2010;19(5):443–453. doi: 10.4037/ajcc2009314. [DOI] [PubMed] [Google Scholar]