Abstract
Rhes, the Ras Homolog Enriched in Striatum, is a GTP-binding protein whose gene was discovered during a screen for mRNAs preferentially expressed in rodent striatum. This 266 amino acid protein is intermediate in size between small Ras-like GTP-binding proteins and α-subunits of heterotrimeric G proteins. It is most closely related to another Ras-like GTP-binding protein termed Dexras1 or AGS1. Although subsequent studies have shown that the rhes gene is expressed in other brain areas in addition to striatum, the striatal expression level is relatively high, and Rhes protein is likely to play a vital role in striatal physiology and pathology. Indeed, it has recently been shown to interact with the Huntingtin protein and play a pivotal role in the selective vulnerability of striatum in Huntington’s disease (HD). Not surprisingly, Rhes can interact with multiple proteins to affect striatal physiology at multiple levels. Functional studies have indicated that Rhes plays a role in signaling by striatal G protein-coupled receptors (GPCR), although the details of the mechanism remain to be determined. Rhes has been shown to bind to both α- and β-subunits of heterotrimeric G proteins and to affect signaling by both Gi/o- and Gs/olf-coupled receptors. In this context, Rhes can be classified as a member of the family of accessory proteins to GPCR signaling. With documented effects in dopamine- and opioid-mediated behaviors, an interaction with thyroid hormone systems and a role in HD pathology, Rhes is emerging as an important protein in striatal physiology and pathology.
Keywords: Striatum, Dopamine, cAMP, Huntington’s disease, Ras, GTP
Introduction
The striatum is responsible for integrating a large glutamatergic input from cerebral cortex and a dopaminergic input from the substantia nigra. This brain area is involved in pathologies such as Huntington’s disease (HD), Parkinson’s disease, schizophrenia, and drug addiction (Han et al. 2010; Koob and Volkow 2010; Obeso et al. 2008; Perez-Costas et al. 2010). Several striatal-specific or striatal-enriched proteins have been described to date, with many of them [e.g., DARPP-32, adenylyl cyclase 5 (AC 5), Gαolf, Gγ7] involved in aspects of signal transduction (Cali et al. 1992; Glatt and Snyder 1993; Herve et al. 1993; Walaas et al. 1983; Watson et al. 1994). One such protein, Rhes, or Ras Homolog Enriched in Striatum, plays a role in diverse cellular functions in striatum. Early work on Rhes described its role in signaling by G protein-coupled receptors (GPCRs), whereas more recent work has demonstrated its critical role in the mechanism of striatal-specific cell death in HD. This brief review will focus on the discovery of Rhes and what is known about its regulation, its role in behavior, and its cellular mechanism of action.
Discovery of Rhes
A subtractive hybridization procedure with cDNAs from rat striatum and cerebellum was employed by Usui et al. (1994) to estimate the number of striatally enriched genes. They estimated that at least 300 mRNAs are enriched in striatum, with approximately 100 of these showing striatal-specific expressions. One of their clones, termed SE6C, was from the 3′-untranslated region of a previously unidentified gene. When SE6C was used as a probe to screen a rat brain cDNA library, a full-length cDNA was obtained that contains a 798-bp open reading frame, thus encoding a 266 amino acid protein. The conceptually translated protein showed similarity to the Ras family from residues 17–205. Due to its enrichment in striatum and its homology with Ras proteins, it was termed the Ras Homolog Enriched in Striatum, or Rhes. Like Ras proteins, Rhes contains sequences for binding the phosphate and guanine nucleotide moieties of GDP and GTP. Furthermore, it contains conserved sequences for farnesylation and palmitoylation, suggesting membrane localization (Falk et al. 1999).
Rhes shares closest homology with the mouse Dexras1 protein, showing 62 % amino acid identity and 67 % identity in the open reading frames of their genes (Falk et al. 1999). Dexras1 is a GTP-binding protein that was first identified as a dexamethasone-inducible protein in AtT-20 cells, as well as in mouse heart, brain, liver, and kidney (Kemppainen and Behrend 1998). The human homolog of Dexras1 was identified in a screen of liver cDNA for gene-encoding proteins that could activate G proteins independently of a receptor; it was thus termed AGS1, or Activator of G protein Signaling 1(Cismowski et al. 2000). Rhes and AGS1/Dexras1 are most similar to the Rap2 subfamily (Falk et al. 1999), but they form a novel subfamily of the Ras superfamily due to their homology and extended C-termini (Graham et al. 2001). However, the two proteins show the lowest homology in the 60 C-terminal amino acids (39 % identity and 59 % similarity; Falk et al. 1999), suggesting that this part may subserve divergent functions in the two proteins. Like the Rad/Gem/Kir/Rem Ras subfamily with extended C-termini (Finlan and Andres 1997; Maguire et al. 1994), Rhes and Dexras1 transcription is regulated physiologically (Falk et al. 1999; see below).
A human ortholog of Rhes was identified at chromosome 22q13.1. Upon removal of a presumed intron of 4,432 nucleotides, it encodes a protein 95 % identical to Rhes (Falk et al. 1999). The human gene is termed rasd2, with AGS1/Dexras1 termed rasd1. In a separate line of investigation, Rhes was identified as one of a family of tumor endothelial markers and named TEM2 (Tumor Endothelial Marker 2; St. Croix et al. 2000). Figure 1 shows the sequence alignment of Rhes and related proteins.
Fig. 1.
Sequence alignment of several forms of Rhes and related proteins. The following sequences were aligned by using ClustralW2: human Dexras1 (EAW55705.1), mouse Dexras1 (CAJ18583.1), mouse Rhes (NP_083458.1), a longer form of mouse Rhes (AAH36988.1), human Rhes (NP_055125.2), and human H-Ras (P01112.1). Asterisk all residues in a column identical; colon conserved substitutions present; dot semi-conserved substitutions present
Anatomical Localization of Rhes
As the name implies, Rhes expression is enriched in striatum, and indeed this was the mechanism for its identification. However, subsequent studies have shown that in addition to this striatal enrichment, rhes mRNA is also expressed in other brain areas and tissues, albeit at a relatively lower level. Northern blot studies showed that rhes mRNA is localized in brain and thyroid gland, but not in liver, lung, adrenal, muscle, kidney, testis, or thymus (Vargiu et al. 2004). However, RT-PCR indicated low levels in kidney, thyroid, lung, heart, and testis, but no signal in liver (Spano et al. 2004). Furthermore, it was detected in rat and human pancreatic islets (Chan et al. 2002), as well as at low levels in rat adrenal and stomach (Taylor et al. 2006). Within brain, rhes mRNA has been shown to be localized to several areas in addition to the initially described striatal localization: layers 2/3 and 5 of cerebral cortex, piriform cortex, olfactory tubercle and bulb, subiculum, hippocampus (pyramidal and granular layers), anterior thalamic nucleus, cerebellum (granular layer), inferior colliculus, and nucleus accumbens (Vargiu et al. 2004). Within nucleus accumbens, there is differential distribution, with shell showing much higher expression than core. Dorsal striatum displays a medial-to-lateral gradient of increasing expression (Harrison and LaHoste 2006; Harrison et al. 2008). This pattern is reminiscent of dopamine receptor binding and mRNA expression, with D2 expression showing strong medial-to-lateral gradients of increasing expression (Altar et al. 1984; Joyce et al. 1985; Mansour et al. 1990) and D1 expression showing highest levels in ventrolateral striatum (Mansour et al. 1992). It should be noted that despite the extra-striatal distribution, the striatal expression appears primary, as we have always found it to be much higher than expression in other areas of adult brain. The limited brain distribution of Rhes contrasts with AGS1/Dexras1, which has a less restricted distribution in brain, having been detected by in situ hybridization at relatively high levels in mouse cortex, accessory olfactory nucleus, supraoptic nucleus, Purkinje layer of cerebellum, dentate gyrus, CA1 and CA2 regions of hippocampus, and in lower amounts in CA3 of hippocampus, thalamus, diagonal band of Broca, substantia nigra pars compacta, pontine nucleus, trapezoid body, pedunculopontine tegmental nucleus, and nucleus solitarius (Fang et al. 2000). Peripherally, AGS1/Dexras1 is found in liver (Cismowski et al. 2000), and in rat testes and lung (Fang et al. 2000), as well as kidney and heart of mouse (Kemppainen and Behrend 1998).
Developmentally, rhes mRNA expression appears to be predominantly postnatal. Northern blot indicated low levels from E16–P10, with a large increase between P10 and P15, followed by a decline in adulthood (Falk et al. 1999). However, RT-PCR showed some signal beginning at Day E13.5. This signal was considerably lower than adult levels (Spano et al. 2004). The postnatal expression of rhes mRNA was also examined with in situ hybridization in sagittal and coronal sections of rat brain to get greater detail of anatomical localization of signal. The developmental time course agreed with Northern blot results, with an increase in expression up to Day 15 and a decrease in adulthood. The medial-to-lateral gradient of increasing striatal signal that was previously described in adult rats was evident as early as Day 4. Some features were unique to neonatal, as opposed to adult, expression. For example, the signal in hippocampus and cerebellum was actually higher than that in striatum in neonates. Furthermore, in hippocampus, signal shifted from CA3 and dentate gyrus on Day 6 to CA1 and CA2 on days 15 and 24. A striking signal was apparent in anterior thalamic nucleus on Day 4 that peaked on Day 10 and dissipated by the late postnatal period (Harrison et al. 2008).
Overall, the distribution of rhes mRNA in adult rat brain suggests that the striatal localization is primary and thus that Rhes protein is likely to play an important role in striatal physiology and pathology. This localization has thus guided functional studies of Rhes protein, with an initial emphasis on dopamine systems and thyroid hormone systems. It should be noted that there has not yet been a characterization of Rhes protein throughout brain, due to the lack of an antibody suitable for immunohistochemical studies.
As the striatal expression of rhes mRNA is predominant, characteristics of its localization within this brain area have been examined in more detail by in situ hybridization. Expression was found in both patch and matrix, as determined with combined calbindin immunohistochemistry. In addition, an interesting pattern of distribution of mRNA within the cell has been observed. Vargiu et al. reported that rhes mRNA, visualized with a digoxigenin-labeled probe, was observed both over clearly delineated cell bodies as well as scattered over areas not associated with cell bodies. This pattern differed from their observations with another striatally enriched mRNA, rc3, and the authors suggested possible dendritic localization of the rhes mRNA (Vargiu et al. 2001). We have made similar observations with both digoxigenin- and 35S-labeled rhes mRNA, in hybridization experiments in which a radiolabeled preproenkephalin probe clearly labeled cell bodies (unpublished observations).
Finally, within cell lines, rhes mRNA was detected in undifferentiated PC12 cells, the rat thyroid cell line FRTL-5, and the mouse ES cell line R1, but not in N2A, C6 glioma, or GT1-7 cells (Spano et al. 2004; Vargiu et al. 2004). Rhes mRNA was amplified by RT-PCR from α- and β-pancreatic cell lines (αTC1-9, RINm5F, BRIN-BD11, MIN6, and INS-1) and from AtT20 and CHO-K1 cells (Chan et al. 2002; Taylor et al. 2006). However, we do not detect any endogenous Rhes protein in CHO cells by Western blotting (Harrison et al. 2008).
Regulation of Rhes Gene and Protein
During the initial characterization of the rhes gene, it was determined that unlike AGS1/Dexras1, it is not regulated by dexamethasone in adult mice (Falk et al. 1999; Kemppainen and Behrend 1998). The ability of thyroid hormones to regulate its expression was investigated due the well-known role of thyroid hormones in striatal development (Bernal and Nunez 1995; Falk et al. 1999; Porterfield and Hendrich 1993), whereas other investigations have focused on dopaminergic regulation of rhes based on the high expression of dopamine receptors in striatum. Most recently, NMDA receptor regulation of Rhes protein was investigated in the context of HD. Overall, as detailed below, the results indicated that Rhes expression is indeed physiologically regulated.
Neonatal hypothyroidism causes a dramatic decrease in rhes mRNA, measured by Northern blot, which is reversed by repeated T4 administration (Falk et al. 1999). Further studies with in situ hybridization showed that hypothyroid rat pups show normal striatal rhes mRNA expression at Day P5, but fail to show the dramatic increase in expression by P10 that control rats show. A single injection of T3 was able to restore striatal rhes mRNA expression in hypothyroid neonates by 8 h. When hypothyroidism was induced in adult rats, rhes mRNA expression was unaffected (Vargiu et al. 2001). In other studies, however, 3 weeks of a low iodine diet in adult mice, combined with 0.15 % propylthiouracil and 0.02 % methimazole in the drinking water, induced a significant decrease in striatal rhes mRNA expression that was not reversed with T3 treatment (Vallortigara et al. 2008). The reason for the discrepancy in the two studies is unclear. Also, there is some debate as to which thyroid hormone receptor subtype mediates the effects on rhes expression. In neonates, thyroid hormone receptor β is involved in the induction of rhes mRNA in response to T3, as the β-specific agonist GC-1 was able to induce rhes expression in 17-day-old rats (Manzano et al. 2003). However, in adult mice, hypothyroidism was without effect on rhes expression in TRα-deficient mice, but decreased rhes in TRβ-deficient mice, suggesting considerable involvement of the highly expressed α receptor in thyroid hormone effects on rhes expression (Vallortigara et al. 2009). In agreement with these studies, thyroxine also induces rhes mRNA in RINm5F β-cells (Taylor et al. 2006).
Several lines of evidence indicate that dopamine input to the striatum is necessary for full expression of rhes mRNA. As animals with dopamine depletion of the striatum show dopamine receptor supersensitivity and loss of requisite dopamine D1/D2 receptor synergism (Mandel and Randall 1985; Marshall and Ungerstedt 1977; LaHoste and Marshall 1992), a link between dopamine input, rhes mRNA expression, and dopamine supersensitivity was examined. In situ hybridization studies with a radiolabeled rhes riboprobe showed a significant decrease in rhes mRNA expression throughout anterior, middle, and posterior striatum after dopamine depletion by 6-hydroxydopamine (6-OHDA) lesion. This effect was apparent as early as 2 weeks post-lesion and persisted up to 7 months post-lesion. Nucleus accumbens shell and olfactory tubercle were similarly affected. Total or near-total striatal lesion was necessary to see the decrease in rhes mRNA (Harrison and LaHoste 2006). Rhes protein in whole striatum was also significantly decreased by 6-OHDA lesion, as determined by Western blotting (Harrison et al. 2008). Furthermore, pharmacological dopamine depletion by acute high-dose (5 mg/kg) or repeated lower-dose (1 mg/kg) reserpine injection also decreased rhes mRNA expression (Harrison and LaHoste 2006). 5 days after recovery from acute reserpine injection, when synaptic dopamine levels should be recovering, rhes mRNA levels were restored to control (unpublished observations). Chronic treatment with the D2 antagonist eticlopride, which induces receptor up-regulation without causing receptor supersensitivity, did not affect rhes mRNA expression (Harrison and LaHoste 2006). All these manipulations of dopamine systems were performed in adult animals. It was further shown that removal of dopamine input on Day 4 of life does not affect rhes mRNA developmental expression (Harrison et al. 2008), in agreement with several other striatally expressed proteins such as DARPP-32 (Ehrlich et al. 1990; Luthman et al. 1990) and D2 receptors (Chen and Weiss 1991). Thus, in adult rats, there is a correlation between dopamine input and rhes mRNA and protein expression.
Rhes protein expression is affected by certain NMDA receptors. Low-dose memantine, which selectively targets extrasynaptic NMDA receptors, decreased Rhes protein in neuronal culture (Okamoto et al. 2009). This blockade of extrasynaptic NMDA receptors is neuroprotective in HD models (Okamoto et al. 2009) where Rhes is known to contribute to cell death by promoting disaggregation of mutant Huntingtin (mHtt) (Subramaniam et al. 2009).
In addition to these investigations in brain, regulation of Rhes in pancreas has also been examined, but the results are controversial. Initial studies employed anti-idiotypic antibodies that bound to proteins induced by efaroxan, an imidazoline compound that potentiates glucose-induced insulin secretion, and identified Rhes as being regulated by efaroxan (Chan et al. 2002). It was further suggested that Rhes is involved not only in the ability of efaroxan to induce insulin secretion but also in the desensitization of this response upon chronic treatment (18 h) to rat islets and BRIN-BD11 cells, as a decrease in rhes mRNA expression correlated with a desensitization of efaroxan-induced insulin secretion (Chan et al. 2002). The effect of efaroxan on rhes mRNA was later shown to be stereospecific and both concentration and time dependent. Indeed, rhes mRNA showed an increase after 4 h of efaroxan treatment, followed by a decline until 16 h (Taylor et al. 2006). Another group, however, used identical treatment conditions with efaroxan and did not detect changes in rhes mRNA by semi-quantitative RT-PCR (Sharoyko et al. 2005). Furthermore, over-expression or antisense knockdown of rhes mRNA in MIN6 cells did not affect insulin secretion, although no verification of alterations of Rhes protein expression was provided (Sharoyko et al. 2005). Thus, although studies on pancreatic cells and cell lines have reached the consensus that rhes mRNA is expressed in these cells, the ability of rhes to be physiologically regulated or to participate in insulin secretion remains unresolved. The regulation of rhes gene expression is summarized in Table 1.
Table 1.
Regulation of Rhes expression
| Treatment | Effect | References |
|---|---|---|
|
Hypothyroidism, neonatal rats T4 |
mRNA ↓, protein ↓ Reversed effect of hypothyroidism |
Falk et al. (1999) and Vargiu et al. (2001) |
|
Hypothyroidism, neonatal rats T3, GC-1 |
mRNA ↓ Reversed effect of hypothyroidism |
Manzano et al. (2003) |
| Hypothyroidism, adult rats | No change mRNA | Vargiu et al. (2001) |
|
Hypothyroidism, adult mice T3 |
mRNA ↓ No change mRNA |
Vallortigara et al. (2008, 2009) |
| 6-OHDA lesion, adult rat | mRNA ↓ | Harrison and LaHoste (2006) |
| Protein ↓ | Harrison et al. (2008) | |
| Eticlopride (0.5 mg/kg), adult rat | No change mRNA | Harrison and LaHoste (2006) |
| ICV 6-OHDA, neonatal rat | No change mRNA | Harrison et al. (2008) |
| Reserpine (5 mg/kg, 1 mg/kg), adult rat | mRNA ↓ | Harrison and LaHoste (2006) |
| Efaroxan, cultured rat pancreatic islets, BRIN-BD11 cells | mRNA ↓ | Chan et al. (2002) and Taylor et al. (2006) |
| Efaroxan, cultured rat pancreatic islets | No change mRNA | Chan et al. (2002) and Taylor et al. (2006) |
| Memantine (5–10 μM) in primary striatal cultures | Protein ↓ | Okamoto et al. (2009) |
Behavioral Findings in Rhes Mutant Mice
A mouse line with a constitutive null mutation of the rhes gene has been generated and tested for differences in basal and drug-induced behavior. Although rhes −/− mice weigh less than wild-type counterparts through adulthood, they are viable and fertile. Initial characterization of these mice on a CD1 background showed only slight behavioral anomalies among the rhes −/− mice, including decreased locomotor activity during the first 5 min of open field exploration, increased tendency to fall from a rotarod, and increased anxiety among female mutant mice relative to wild-type mice. There were no differences in passive avoidance or Morris water maze tests, and the authors concluded that behavioral deficits were “modest” (Spano et al. 2004). All these tests were without drug challenge.
A separate strain of rhes mutant mice was derived from the original strain by backcrossing for ten generations to a C57BL/6J background (Errico et al. 2008). Both basal and drug-stimulated behaviors have been tested in these animals. Mutant mice show no differences in striatal or cortical content of dopamine, DOPAC, or HVA relative to wild-type mice. Furthermore, there are no genotype differences in dopamine D1 receptor mRNA. Slight but significant genotype differences were observed in locomotor activity in novelty-induced exploration and open field tests, with rhes −/− mice showing more activity than rhes +/+ mice. There was no genotype difference in the rotarod test. These effects are opposite to those observed in mice on the CD1 background (Errico et al. 2008). Possible reasons for the discrepancy include gender differences (males and females tested in the CD1 group and only females tested in the C57BL/6 group) or differences in methodology (time course measured in open field). However, overall these findings suggest that any differences in basal behavior among the genotypes are not robust, and drug challenge may be required to determine the role played by Rhes protein in striatal signaling and behavior.
An inhibitory role for Rhes in several dopamine receptor-mediated behaviors has been suggested by studies using exogenous drug administration in rhes mutant mice. For example, rhes −/− mice show significantly greater locomotor activation than rhes +/+ mice in response to the D1 agonist SKF81297 (Errico et al. 2008) and more stereotypy in response to the D1/D2 agonist apomorphine (Quintero et al. 2008). Among D2 receptor responses, rhes −/− mice show more catalepsy in response to the D2 antagonists haloperidol (Errico et al. 2008) and eticlopride (Quintero et al. 2008), and more of the brief early stereotypy response characteristic of D2 receptor activation (Quintero et al. 2008). Although all these findings suggest an inhibitory role for Rhes in dopamine-mediated behaviors, Rhes has a facilitative role in one behavioral response tested so far. The grooming response to the D1 agonist SKF38393 is decreased in a gene-dose-dependent manner in rhes mutant mice (Quintero et al. 2008). This difference in the directional effect of rhes gene knockout on behavior may be due to the intracellular pathways subserving the behaviors. Whereas locomotor activity is thought to be mediated by AC and GSK3 pathways (Undie et al. 2000; Beaulieu et al. 2005), grooming is activated by drugs known to promote phospholipase C (PLC) activation (Clifford et al. 1999). Thus, Rhes may differentially affect dopamine receptor-stimulated intracellular pathways, but this hypothesis has not yet been directly tested.
Although initial behavioral studies focused on dopamine systems, no specific link between dopamine receptors and Rhes has been demonstrated (e.g., Harrison and He 2011). If Rhes functions are similar to its closest relative, AGS1/Dexras1, it is likely to interact with general signaling proteins, such as heterotrimeric G proteins (Cismowski et al. 2000), rather than with specific receptors. Thus, the effects on behaviors mediated by opioid receptors, another striatally enriched GPCR, have been investigated. Rhes −/− mice show enhanced morphine analgesia in both tail flick and formalin tests relative to rhes +/+ mice. Furthermore, rhes −/− show no tolerance and significantly reduced naloxone-precipitated withdrawal after repeated morphine administration (Lee et al. 2011). These results are surprising in light of the findings of increased signaling through AC in rhes −/− mice (Errico et al. 2008; see below). The findings may be best interpreted in light of opioid signaling through multiple pathways, including AC and PLC–Protein kinase C (PKC). As PLC–PKC pathways have been shown to be important in promoting tolerance and dependence (Mathews et al. 2008; Shukla et al. 2006; Smith et al. 1999; Zeitz et al. 2001), and inhibiting acute analgesia (Bonacci et al. 2006; Newton et al. 2007; Xie et al. 1999), the results may suggest a role for Rhes in promoting these pathways, analogous to the results with dopamine D1 receptors and grooming. However, thus far this mechanism has not been directly tested. The effects of Rhes on behavior are summarized in Table 2.
Table 2.
Behavior in Rhes mutant mice
| Behavior | Effect | References |
|---|---|---|
| Open field, drug-naïve | rhes −/− locomotion ↓ first 5 min | Spano et al. (2004) |
| Rotarod, drug-naïve | rhes −/− ↓latency to fall | Spano et al. (2004) |
| Elevated plus-maze, drug-naïve | rhes −/− females ↑ anxiety | Spano et al. (2004) |
| Morris water maze, drug-naive | No genotype difference | Spano et al. (2004) |
| Passive avoidance, drug-naïve | No genotype difference | Spano et al. (2004) |
| Novelty-induced exploration, drug-naïve | rhes −/− ↑ activity | Errico et al. (2008) |
| Open field, drug-naïve | rhes −/− ↑ activity | Errico et al. (2008) |
| Rotarod, drug-naïve | No genotype difference | Errico et al. (2008) |
| Locomotor activity, SKF 81297 | rhes −/− ↑activity | Errico et al. (2008) |
| Stereotypy, apomorphine | rhes −/−, rhes +/− ↑ response | Quintero et al. (2008) |
| Catalepsy, haloperidol | rhes −/− ↑ response | Errico et al. (2008) |
| Catalepsy, eticlopride | rhes −/− ↑ response | Quintero et al. (2008) |
| Grooming, SKF 38393 | rhes −/− ↓ response | Quintero et al. (2008) |
| Tail flick analgesia, morphine | rhes −/− ↑ analgesia | Lee et al. (2011) |
| Formalin analgesia, morphine | rhes −/− ↑ analgesia | Lee et al. (2011) |
| Morphine analgesia tolerance | rhes −/− no tolerance | Lee et al. (2011) |
| Opiate withdrawal (naloxone-precipitated) | rhes −/− ↓ response | Lee et al. (2011) |
Cellular Actions of Rhes
Although Rhes is known to affect several striatally mediated behaviors, the exact cellular mechanism by which it exerts these actions is currently unknown. Several clues have been gained, however, from both cell culture and in vivo studies. Some basic characteristics of Rhes expression were determined in PC12 cells. In these cells, Rhes is localized to the plasma membrane through farnesylation. Furthermore, 30 % of Rhes was bound to GTP, as opposed to 18 % of H-Ras. This finding suggests some constitutive activity of Rhes. The authors suggested that this constitutive activity is due to decreased intrinsic GTPase activity, based on certain amino acid substitutions in Rhes versus H-Ras. For example, both Rhes and AGS1/Dexras1 have amino acid substitutions in positions corresponding to amino acids 12 and 59 of H-Ras, suggesting low GTPase activity (Vargiu et al. 2004).
The ability of Rhes to regulate the activity of several intracellular enzymes has been examined. When expressed in HeLa cells, Rhes did not bind appreciably to the Ras-binding domain of Raf1 in pull-down assays or did it activate ERK2 when expressed in COS-7 cells (Vargiu et al. 2004). It is not known whether Rhes binds to the striatally enriched B-Raf isoform (Morice et al. 1999) or whether it affects ligand-stimulated ERK activation. Rhes did, however, affect the activity of another Ras effector, PI3 kinase. It bound to the Ras-binding domain of the catalytic p110 subunit, and membrane-bound Rhes promoted phosphorylation of histone H2B, indicating that Rhes promotes activation of Akt under basal conditions (Vargiu et al. 2004). The finding of Rhes involvement in Akt signaling could have wide implications as this pathway has been shown to be involved in dopamine D2 receptor signaling (Beaulieu et al. 2005), schizophrenia etiology (Emamian et al. 2004), and the mechanism of action of anti-psychotic drugs (Masri et al. 2008). Indeed, an SNP in the human rasd2 gene has been highly significantly associated with a subgroup of schizophrenics without deficit in sustained attention (Liu et al. 2008), and a rasd2 SNP was also shown to be likely to legitimately control mRNA expression and affect disease state (Ciobanu et al. 2010). Thus, it will be important to determine if Rhes is involved in the critical alteration of Akt signaling mediated by dopamine receptors.
Several lines of evidence, both in vitro and in vivo, demonstrate that Rhes affects signaling through GPCRs. Initial experiments in PC12 cells, using a reporter gene, indicated that although Rhes had no effect on signaling through the Gi/o-coupled M2 muscarinic receptor, it inhibited signaling through the Gs-coupled thyroid-stimulating hormone and β2-adrenergic receptors. It was additionally demonstrated that the locus of action of Rhes is upstream of heterotrimeric G protein activation in that it did not affect reporter gene activation mediated by forskolin or a constitutively active Gs mutant (Vargiu et al. 2004). These findings suggested that Rhes preferentially targets signaling mediated by Gs-coupled receptors. Such a mechanism would be complementary to AGS1/Dexras1, which only interacts with Gi/o to affect signaling (Cismowski et al., 2000). However, more recent findings suggest a more complicated picture, with Rhes interacting with Gi/o as well. The first indication of Rhes interaction with Gi/o signaling was an effect on N-type calcium channels, Cav2.2, expressed in HEK cells (Thapliyal et al. 2008). Both Rhes and AGS1/Dexras1 decreased basal calcium current density and attenuated the ability of the muscarinic agonist carbachol to decrease Cav2.2 currents. The effects were blocked by pertussis toxin (PTX) and could be attributed to βγ subunits of PTX-sensitive heterotrimeric G proteins. Interestingly, the ability of the Gs-coupled β2-adrenergic receptor to inhibit Cav2.2 was not affected by either Rhes or AGS1/Dexras1, indicating that the effect was specific to Gi/o-coupled receptors. The authors concluded that Rhes, like AGS1/Dexras1, can act as a guanine nucleotide exchange factor for Gαi and thus tonically activate Gβγ. As for the ability of Rhes and AGS1/Dexras1 to attenuate agonist-mediated Gβγ activation, two hypotheses were proposed: they activate Gβγ to a point near saturation before receptor activation; they bind to heterotrimeric Gαβγ to promote βγ signaling and prevent receptor-mediated activation of the heterotrimer (Thapliyal et al. 2008).
Further support for Rhes interaction with Gi/o comes from studies on AC. When heterologously expressed in CHO cells along with the dopamine D1 receptor, Rhes inhibits the production of cAMP by the D1 receptor agonist SKF 83822. This agonist preferentially targets D1 receptors that are coupled only to AC and cAMP production (Undie et al. 1994). This finding would initially seem to support the notion that Rhes preferentially targets Gs to inhibit signaling through Gs-coupled receptors such as the D1 receptor. However, several characteristics of this phenomenon suggest a less straightforward interpretation: (1) the effect was blocked by PTX, suggesting Gi/o involvement; (2) AGS1/Dexras1, which is known to bind to and activate Gi/o but not Gs (Cismowski et al. 2000), showed a similar inhibition of D1-mediated cAMP accumulation; (3) like AGS1/Dexras1, Rhes bound to Gi but not Gs in pull-down assays (Harrison and He 2011). Rhes has also been shown to bind to Gβ1, Gβ2, and Gβ3 (Hill et al. 2009), further suggesting the possibility that Rhes can bind to and perhaps stabilize a heterotrimeric G protein.
It has been shown that in COS-7 cells, co-expression of Rhes with Gs-coupled receptors decreases the amount of cAMP produced and the level of radioligand receptor binding (Agretti et al. 2007). However, as this effect only occurred when Rhes was transfected at a level at least double that of the receptors, this is not likely to account for the effects with D1 receptors in CHO cells. Nevertheless, these studies raise an important question about the effect of Rhes on GPCR surface expression that has not yet been examined in vivo.
In vivo work has also indicated that Rhes normally inhibits signaling through AC. Rhes −/− mice showed increased basal and drug-stimulated phosphorylation of GluR1 at Ser845, which is phosphorylated by protein kinase A, relative to rhes +/+ mice. Treatment with both the D1 agonist SKF 81297 and the D2 antagonist haloperidol resulted in relatively higher phosphorylation in mutant mice (Errico et al. 2008). As the D2 effect is mediated through adenosine A2A receptors (Hakansson et al. 2006), this finding suggests a more general mechanism of action of Rhes on the AC system, rather than a specific effect on dopamine systems. Indeed, this study also demonstrated a slight but significant increase in Gαs/olf in the absence of Rhes that could contribute to the increased signaling through AC in the rhes −/− mice (Errico et al. 2008).
In addition to effects on GPCR signaling, Rhes has recently been shown to be a critical mediator of striatal-specific cell death in HD (Subramaniam et al. 2009). Despite the ubiquitous presence of Huntingtin protein, degeneration is restricted to the corpus striatum in HD. Recent work has shown that Rhes is required for mHtt to be cytotoxic, thus finally offering an explanation for the selective striatal vulnerability. Rhes binds to mHtt and, to a lesser extent, wild-type Huntingtin (Htt), causing sumoylation of mHtt. This enhanced sumoylation promotes disaggregation of mHtt and increased cytotoxicity (Subramaniam et al. 2009). A more general role for Rhes in sumoylation was demonstrated in that Rhes regulates this process physiologically, with rhes −/− mice showing decreased sumoylation of multiple striatal proteins, in comparison with rhes +/+ mice. Interestingly, this phenomenon was present in 1-year-old, but not 6-month-old, mice. Furthermore, Rhes can regulate sumoylation at several levels, including promotion of “cross-sumoylation” of E1 and Ubc9 (Subramaniam et al. 2010).
Another role for Rhes in HD pathology was also recently elucidated. Rhes was found to bind to and activate mTOR (Subramaniam and Snyder 2011; Subramaniam et al. 2012). Because mTOR is crucial for trophic factor signaling, a decrease in mTOR activation would lead to degeneration of striatal cells, as seen to a high degree in HD. Rhes binds to mHtt with greater affinity than to wild-type Htt, and thus the presence of mHtt could sequester Rhes and result in a loss of function (Subramaniam and Snyder 2011). It has also been recently demonstrated that rhes mRNA expression is decreased in a primary striatal neuronal model of HD, possibly as an autocompensatory mechanism (Seredenina et al. 2011). The combination of decreased expression and sequestration by mHtt would result in a loss of functional Rhes and subsequent decreased activation of mTOR in striatum, leading to cellular degeneration. The ability of Rhes to activate mTOR is also involved in L-DOPA-induced dyskinesia in the 6-OHDA lesion model of Parkinson’s disease, with rhes −/− mice displaying less drug-induced dyskinesia than rhes +/+ mice, while retaining the therapeutic efficacy of the L-DOPA (Subramaniam et al. 2012).
There is a precedent for proteins functioning in both G protein signaling and in mTOR pathways. The guanine nucleotide dissociation inhibitor (GDI) AGS3, which can bind up to four Gαi–GDP and is necessary for cocaine sensitization (Bowers et al. 2004), also affects mTOR and autophagy. The key player connecting these functions in this case is Gαi3. Gαi3 inhibits autophagy by promoting PI3 kinase and mTOR signaling through growth factors (Ghosh et al. 2010). It has recently been shown that AGS3 and the guanine nucleotide exchange factor GIV compete for binding to Gαi3, with binding to AGS3 causing retention of the GDP-bound state and promotion of autophagy, and binding to GIV promoting Gαi3 activation and inhibition of autophagy (Garcia-Marcos et al. 2011). It is possible that the abilities of Rhes to bind to PI3 kinase (Vargiu et al. 2004) and to bind to activated Gαi (Harrison and He 2011) enable Rhes to perform a similar function in promoting mTOR signaling and inhibiting autophagy, but this remains speculative. The cellular actions of Rhes are summarized in Table 3.
Table 3.
Cellular actions of Rhes
| Model | Effect | References |
|---|---|---|
| HeLa cells | No Raf1 binding in pull-down assay | Vargiu et al. (2004) |
| COS-7 cells | No ERK activation | Vargiu et al. (2004) |
| COS-7 cells | Binds and activates PI3 Kinase | Vargiu et al. (2004) |
| PC12 cells | No effect on muscarinic M2 receptor- mediated reporter gene activation | Vargiu et al. (2004) |
| PC12 cells | Inhibits TSH receptor-stimulated reporter gene activation | Vargiu et al. (2004) |
| PC12 cells | Inhibits β-adrenergic receptor-stimulated reporter gene activation | Vargiu et al. (2004) |
| PC12 cells | No effect on forskolin-stimulated reporter gene activation | Vargiu et al. (2004) |
| PC12 cells | No effect on mutant Gαs-stimulated reporter gene activation | Vargiu et al. (2004) |
| HEK cells | Inhibits basal Ca2+ current density; inhibits muscarinic decrease of Ca2+ current | Thapliyal et al. (2008) |
| Mouse | rhes −/− ↑ GluR1 phosphorylation | Errico et al. (2008) |
| Mouse | rhes −/− ↓ GTP binding to Gαi/o | Errico et al. (2008) |
| CHO cells | Inhibits dopamine D1 receptor-stimulated cAMP | Harrison and He (2011) |
| Mouse | Binds to Gαi in pull-down assay | Harrison and He (2011) |
| Yeast 2-hybrid | Binds Gβ1, Gβ2, Gβ3 | Hill et al. (2009) |
| HEK293 cells | Binds wild type and mutant Htt | Subramaniam et al. (2009) |
| HEK293 cells | Sumoylation of mHtt | Subramaniam et al. (2009) |
| Mouse | rhes −/− ↓ sumoylation of multiple proteins | Subramaniam et al. (2010) |
| In vitro | Promotes cross-sumoylation of E1 and Ubc9 | Subramaniam et al. (2010) |
| Mouse, striatal cells | Binds to and activates mTOR | Subramaniam and Snyder (2011) and Subramaniam et al. (2012) |
Conclusions
As described in this brief review, the GTP-binding protein Rhes is emerging as an important player in striatal physiology and pathology. Rhes is likely to subserve diverse functions in the striatum, with substantial evidence for some roles (e.g., HD), and roles in other functions still emerging (e.g., GPCR signaling and behavior). Even the role of Rhes in HD pathology may be quite complex, with new results indicating that Rhes interaction with mTOR is critical for maintaining striatal trophic signaling (Subramaniam and Snyder, 2011). As most people do not carry the Huntingtin mutation, it is likely that functions such as mTOR activation, which does not involve Rhes binding to mHtt, are important for striatal physiology. There may also be some intersection between functions of Rhes in mTOR signaling and in G protein signaling. As GTP-bound Gαi promotes mTOR signaling and inhibits autophagy (Ghosh et al. 2010), Rhes may also promote mTOR activation indirectly by stabilizing a GTP bound Gαi. Figure 2 depicts models for our current knowledge of Rhes functioning in GPCR signaling and in HD.
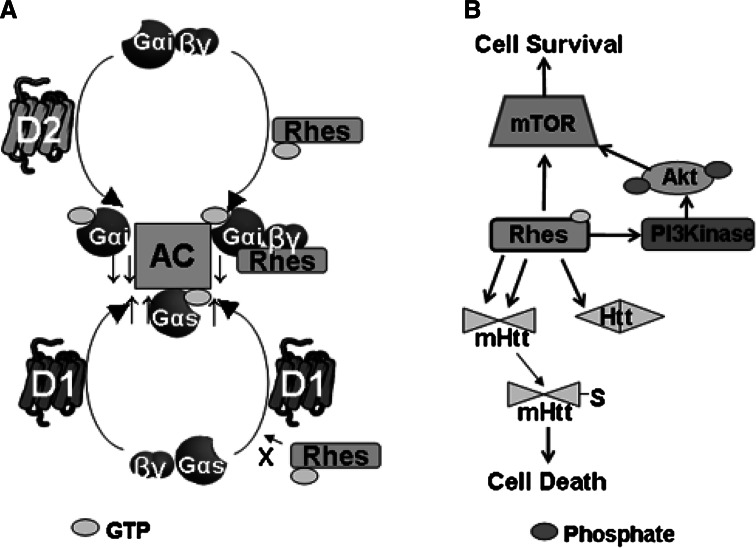
Fig. 2.
Schematic of effects of Rhes on cellular signaling. a Working model of Rhes and GPCR signaling. In the absence of Rhes (upper left), dopamine D2 receptors promote activation of Gαi/o and thus inhibition of AC. Rhes itself (upper right) is postulated to activate Gαi/o and also inhibit AC, but to a lesser extent than D2 receptors. As such, it can have a partial agonist-like effects when competing with D2 for Gαi/o. D1 receptors (bottom left) activate Gαs/olf to provide robust stimulation of AC. Rhes is hypothesized to inhibit this effect in two ways: by decreasing the ability of D1 to activate Gαs/olf (bottom right) and by providing increased inhibitory tone on AC through its interaction with a Gαi/o heterotrimer (upper right). b Rhes can affect the balance of cell survival and death through interaction with Huntingtin and other proteins in striatum (see Subramaniam and Snyder 2011). By activating mTOR either by direct binding or through its ability to bind to PI3 kinase, Rhes can promote cell survival. However, by binding to mHtt more readily than to wild-type Htt, Rhes can promote cell death both by causing sumoylation and solubility of mHtt and by being sequestered and prevented from binding to mTOR
Many questions remain about Rhes function in both physiology and pathology of the striatum. Based on the information that we currently have about Rhes, the following will be fruitful areas of future study: (1) What is the exact mechanism of Rhes action in HD pathology and in trophic factor signaling in the striatum? (2) What is the mechanism of Rhes action in GPCR signaling? The extent of Rhes binding to components of GPCR signaling is currently unknown, and there are very little data about such interactions in vivo. Does Rhes bind to a G protein heterotrimer? Does Rhes regulate which pathway a GPCR activates, e.g., AC versus PLC? Which striatal behaviors are regulated through Rhes participation in GPCR signaling? (3) Does Rhes affect signaling through the Akt pathway? Although an early report indicated that Rhes binds to PI3 kinase (Vargiu et al. 2004), there has been no further information on this action of Rhes. This may be another means for Rhes to interact with mTOR signaling. Also, in light of recent results showing that dopamine D2 receptors can signal through Akt and that this mechanism is associated with schizophrenia, further investigation into the role of Rhes in regulating the ability of the striatally enriched D2 receptor to affect Akt signaling is warranted.
Acknowledgments
The author is supported by NIH Grant P20RR016816 and Louisiana Board of Regents Grant LEQSF(2009-11)-RD-A-11.
References
- Agretti P, De Marco G, Pinchera A, Vitti P, Bernal J, Tonacchera M (2007) Ras homolog enriched in striatum inhibits the functional activity of wild type thyrotropin, follicle-stimulating hormone, luteinizing hormone receptors and activating thyrotropin receptor mutations by altering their expression in COS-7 cells. J Endocrinol Invest 30:279–284 [DOI] [PubMed] [Google Scholar]
- Altar CA, Walter RJ, Neve KA, Marshall JF (1984) Computer assisted video analysis of [3H] spiroperidol binding autoradiographs. J Neurosci Methods 10:173–188 [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005) An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273 [DOI] [PubMed] [Google Scholar]
- Bernal J, Nunez J (1995) Thyroid hormones and brain development. Eur J Endocrinol 133:390–398 [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV (2006) Differential targeting of Gβγ-subunit signaling with small molecules. Science 312:443–446 [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW (2004) Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42:269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali JJ, Balcueva EA, Rybalkin I, Robishaw JD (1992) Selective tissue distribution of G protein γ subunits, including a new form of the γ subunits identified by cDNA cloning. J Biol Chem 267:24023–24027 [PubMed] [Google Scholar]
- Chan SLF, Monks LK, Gao H, Deaville P, Morgan NG (2002) Identification of the monomeric G-protein, Rhes, as an efaroxan-regulated protein in the pancreatic β-cell. Br J Pharmacol 136:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Weiss B (1991) Ontogenetic expression of D2 dopamine receptor mRNA in rat corpus striatum. Dev Brain Res 63:95–104 [DOI] [PubMed] [Google Scholar]
- Ciobanu DC, Lu L, Mozhui K, Wang X, Jagalur M, Morris JA, Taylor WT, Dietz K, Simon P, Williams RW (2010) Detection, validation, and downstream analysis of allelic variation in gene expression. Genetics 184:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski MJ, Ma C, Ribas C, Xie X, Spruyt M, Lizano JS, Lanier SM, Duzic E (2000) Activation of heterotrimeric G-protein signaling by a ras-related protein: implications for signal integration. J Biol Chem 275:23421–23424 [DOI] [PubMed] [Google Scholar]
- Clifford JJ, Tighe O, Croke DT, Kinsella A, Sibley DR, Drago J, Waddington JL (1999) Conservation of behavioral topography to dopamine D1-like receptor agonists in mutant mice lacking the D1A receptor implicates a D1-like receptor not coupled to adenylyl cyclase. Neuroscience 93:1483–1489 [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Rosen NL, Kurihara T, Shalaby IA, Greengard P (1990) DARPP-32 development in the caudate nucleus is independent of afferent input from the substantia nigra. Dev Brain Res 54:257–263 [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004) Convergent evidence for impaired AKT1-GSK3β signaling in Schizophrenia. Nat Genet 36:131–137 [DOI] [PubMed] [Google Scholar]
- Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De Chiara V, Prosperetti C, Spano D, Herve D, Pasqualetti M, Di Lauro R, Fisone G, Usiello A (2008) The GTP-binding protein Rhes modulates dopamine signaling in striatal medium spiny neurons. Mol Cell Neurosci 37:335–345 [DOI] [PubMed] [Google Scholar]
- Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG (1999) Rhes: a striatal-specific ras homolog related to Dexras1. J Neurosci Res 57:782–788 [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH (2000) Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron 28:183–193 [DOI] [PubMed] [Google Scholar]
- Finlan BS, Andres DA (1997) Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem 272:21982–21988 [DOI] [PubMed] [Google Scholar]
- Garcia-Marcos M, Ear J, Farquhar MG, Ghosh P (2011) A GDI (AGS3) and a GEF (GIV) regulate autophagy by balancing G protein activity and growth factor signals. Mol Biol Cell 22:673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Beas AO, Bornheimer SJ, Garcia-Marcos M, Forry EP, Johannson C, Ear J, Jung BH, Cabrera B, Carethers JM, Farquhar MG (2010) A Gαi-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Cell Biol 21:2338–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt CE, Snyder SH (1993) Cloning and expression of adenylyl cyclase localized to the corpus striatum. Nature 361:536–538 [DOI] [PubMed] [Google Scholar]
- Graham TE, Key TA, Kilpatrick K, Dorin RI (2001) Dexras1/AGS1, a steroid hormone-induced guanosine triphosphate-binding protein, inhibits 3′,5′-cyclic adenosine monophosphate-stimulated secretion in AtT-20 corticotroph cells. Endocrinology 142:2631–2640 [DOI] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G (2006) Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem 96:482–488 [DOI] [PubMed] [Google Scholar]
- Han I, You Y, Kordower JH, Brady ST, Morfini GA (2010) Differential vulnerability of neurons in Huntington’s disease: the role of cell type-specific features. J Neurochem 113:1073–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, He YE (2011) Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res 89:874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ (2006) Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience 137:483–492 [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ, Ruskin DN (2008) Ontogeny and dopaminergic regulation in brain of Ras homolog enriched in striatum (Rhes). Brain Res 1245:16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin J-P, Glowinski J, Girault J-A (1993) Golf and Gs in rat basal ganglia: possible involvement of Golf in coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci 13:2237–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Goddard A, Ladds G, Davey J (2009) The cationic region of Rhes mediates its interactions with specific Gβ subunits. Cell Physiol Biochem 23:01–08 [DOI] [PubMed] [Google Scholar]
- Joyce JN, Loeschen SK, Marshall JF (1985) Dopamine D-2 receptors in rat caudate-putamen: the lateral to medial gradient does not correspond to dopaminergic innervation. Brain Res 338:209–218 [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Behrend EN (1998) Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem 273:3129–3131 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF (1992) Dopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor density. Synapse 12:14–26 [DOI] [PubMed] [Google Scholar]
- Lee FA, Baiamonte BA, Spano D, LaHoste GJ, Soignier RD, Harrison LM (2011) Mice lacking rhes show altered morphine analgesia, tolerance, and dependence. Neurosci Lett 489:182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-L, Fann CS-J, Liu C-M, Chen WJ, Wu J-Y, Hung S-l, Chen C-H, Jou Y-S, Liu S-K, Hwang T-J, Hsieh MH, Chang CC, Yang W-C, Lin J-J, Chou FH-C, Faraone SV, Tsuang MT, Hwa H-G (2008) RASD2, MYH9, and CACNG2 genes at chromosome 22q12 associated with the subgroup of schizophrenia with non-deficit in sustained attention and executive function. Biol Psychiatry 64:789–796 [DOI] [PubMed] [Google Scholar]
- Luthman J, Lindqvist E, Young D, Cowburn R (1990) Neonatal dopamine lesion in the rat results in enhanced adenylate cyclase activity without altering dopamine receptor binding or dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein (DARPP-32) immunoreactivity. Exp Brain Res 83:85–95 [DOI] [PubMed] [Google Scholar]
- Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K (1994) Gem: an induced, immediate early protein belonging to the Ras family. Science 265:241–244 [DOI] [PubMed] [Google Scholar]
- Mandel RL, Randall PK (1985) Quantification of lesion-induced dopaminergic supersensitivity using the rotational model in the mouse. Brain Res 330:358–363 [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ (1990) Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in rat brain and pituitary: and in situ hybridization-receptor autoradiographic analysis. J Neurosci 10:2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ (1992) A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience 46:959–971 [DOI] [PubMed] [Google Scholar]
- Manzano J, Morte B, Scanlan TS, Bernal J (2003) Differential effects of triiodothyronine and the thyroid hormone receptor β-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144:5480–5487 [DOI] [PubMed] [Google Scholar]
- Marshall JF, Ungerstedt U (1977) Supersensitivity to apomorphine following destruction of the ascending dopamine neurons: quantification using the rotational model. Eur J Pharmacol 41:361–367 [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu J-M, Gainetdinov RR, Caron MG (2008) Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci 105:13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews JL, Smrcka A, Bidlack JM (2008) A novel Gβγ-subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci 28:12183–12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice C, Nothias F, Konig S, Vernier P, Baccarini M, Vincent J-D, Barnier JV (1999) Raf-1 and B-Raf proteins have similar regional distributions but differential subcellular localization in adult rat brain. Eur J Neurosci 11:1995–2006 [DOI] [PubMed] [Google Scholar]
- Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ et al (2007) Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav 6:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M (2008) Functional organization of the basal ganglia: therapeutic implications for Parkinson’s Disease. Mov Disord 23:S548–S559 [DOI] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Chen H-SV, Tong G, Hayden MR, Lipton SA (2009) Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant Huntingtin. Nat Med 15:1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Roberts RC (2010) Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem 113:287–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE (1993) The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 14:94–106 [DOI] [PubMed] [Google Scholar]
- Quintero GQ, Spano D, LaHoste GJ, Harrison LM (2008) The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. NeuroReport 19:1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenina T, Gokce O, Luthi-Carter R (2011) Decreased striatal RGS2 expression is neuroprotective in Huntington’s disease (HD) and exemplifies a compensatory aspect of HD-induced gene regulation. PLoS ONE 6:e22231. doi:10.1371/journal.pone.0022231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharoyko VV, Zaitseva II, Varsanyi M, Portwood N, Leibiger B, Leibiger I, Berggren P-O, Efendic S, Zaitsev SV (2005) Monomeric G-protein, Rhes, is not an imidazoline-regulated protein in pancreatic β-cells. Biochem Biophys Res Comm 338:1455–1459 [DOI] [PubMed] [Google Scholar]
- Shukla PK, Tang L, Wang ZJ (2006) Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent kinase II in opioid tolerance and dependence. Neurosci Lett 404:266–269 [DOI] [PubMed] [Google Scholar]
- Smith FL, Lohmann AB, Dewey WL (1999) Involvement of phospholipid signal transduction pathways in morphine tolerance in mice. Br J Pharmacol 128:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano D, Branchi I, Rosica A, Pirro MT, Riccio A, Mithbaokar P, Affuso A, Arra C, Campolongo P, Terracciano D, Macchia V, Bernal J, Alleva E, Di Lauro R (2004) Rhes is involved in striatal function. Mol Cell Biol 24:5788–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW (2000) Genes expressed in human tumor endothelium. Science 289:1197–1202 [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Snyder SH (2011) Huntington’s disease is a disorder of the corpus striatum: focus on Rhes (Ras homologue enriched in striatum). Neuropharmacology 60:1187–1192 [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH (2009) Rhes, a striatal specific protein, mediates mutant-Huntingtin cytotoxicity. Science 324:1327–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH (2010) Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation among the basic sumoylation enzymes E1 and UBC9. J Biol Chem 285:20428–20432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A (2012) Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci. doi:10.1038/nn.2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Jackson DA, Morgan NG, Chan SLF (2006) Rhes expression in pancreatic β-cells is regulated by efaroxan in a calcium-dependent process. Biochem Biophys Res Comm 349:809–815 [DOI] [PubMed] [Google Scholar]
- Thapliyal A, Bannister RA, Hanks C, Adams BA (2008) The monomeric G proteins AGS1 and Rhes selectively influence Gαi-dependent signaling to modulate N-type (Cav2.2) calcium channels. Am J Physiol Cell Physiol 295:C1417–C1426 [DOI] [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Freidman E (1994) Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem 62:2045–2048 [DOI] [PubMed] [Google Scholar]
- Undie AS, Berki AC, Beardsley K (2000) Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology 39:75–87 [DOI] [PubMed] [Google Scholar]
- Usui H, Falk JD, Dopazo A, de Lecea L, Erlander MG, Sutcliffe JG (1994) Isolation of clones of rat striatum-specific mRNAs by directional tag PCR subtraction. J Neurosci 14:4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara J, Alfos S, Micheau J, Higueret P, Enderlin V (2008) T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol Dis 31:378–385 [DOI] [PubMed] [Google Scholar]
- Vallortigara J, Chassande O, Higueret P, Enderlin V (2009) Thyroid hormone receptor alpha plays an essential role in the normalisation of adult-onset hypothyroidism-related hypoexpression of synaptic plasticity target genes in striatum. J Neuroendocrinol 21:49–56 [DOI] [PubMed] [Google Scholar]
- Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Sutcliffe JG, Bernal J (2001) Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Mol Brain Res 94:1–8 [DOI] [PubMed] [Google Scholar]
- Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J (2004) The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene 23:559–568 [DOI] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P (1983) A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature 301:69–71 [DOI] [PubMed] [Google Scholar]
- Watson JB, Coulter PM, Margulies JE, de Lecea L, Danielson PE, Erlander MG, Sutcliff JG (1994) G protein gamma 7 subunit is selectively expressed in medium-sized neurons and dendrites of the rat neostriatum. J Neurosci Res 39:108–116 [DOI] [PubMed] [Google Scholar]
- Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinckle PM et al (1999) Genetic alteration of phospholipase C β3 expression modulates behavioral and cellular responses to μ opioids. Proc Natl Acad Sci USA 96:10385–10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKCγ mutant mice. Pain 94:245–253 [DOI] [PubMed] [Google Scholar]