Abstract
Background
Maintenance therapy is a new treatment paradigm for advanced non-small cell lung cancer (NSCLC). We conducted a meta-analysis of randomized studies with single agent maintenance therapy.
Methods
An electronic literature search of public databases (MEDLINE, EMBASE, Cochrane library) and manual search of relevant conference proceedings was performed. A formal meta-analysis was conducted using Comprehensive Meta Analysis software (Version 2.0). Outcome data were pooled and reported as hazard ratio (HR). The primary outcome of interest was overall survival (OS) and secondary outcome was progression free survival (PFS).
Results
Twelve studies were included (5 meeting abstracts, 7 full manuscripts) with a total of 4286 patients (maintenance arm/control arm- 2449/1837, median age 61 years, males -69 %). The OS (HR 0.86, 95% confidence intervals [CI] 0.80-0.92; P=0.0003) and PFS (HR 0.80, 95% CI 0.77-0.84; P<0.0001) were superior with maintenance therapy. ‘Switch’ maintenance was associated with significant OS and PFS improvement (OS HR 0.84, 95% CI 0.77-0.91; P=0.00026; PFS HR 0.62, 95% CI 0.57-0.67; P<0.0001). Despite a modest improvement in PFS (HR 0.90, 95%CI 0.85-0.95; P=0.007), “continuation” maintenance was not associated with survival benefit (HR 0.927, 95%CI 0.78-1.09; P=0.33). Improvements in OS and PFS were observed with both EGFR-targeted agents (HR 0.83, 95% CI 0.74-0.92; P=0.004; HR 0.64, 95% CI 0.58-0.71 P<0.0001) and cytotoxic agents (HR 0.89, 95% CI 0.80-0.98; P=0.018; HR 0.85, 95% CI 0.80-0.89; P < 0.0001).
Conclusions
Single agent maintenance therapy improves overall survival, though statistical significance was only noted with ‘switch’ maintenance.
Keywords: Maintenance therapy, non-small cell lung cancer, NSCLC, EGFR, pemetrexed, erlotinib, gefitinib
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths globally1. The majority of the patients have advanced stage disease at the time of diagnosis, for which there are no curative treatment options. Non-small cell lung cancer (NSCLC) accounts for nearly 85% of all cases of lung cancer in the United States. For patients with advanced stage NSCLC, systemic therapy is the recommended treatment. Platinum-based combination chemotherapy regimens have demonstrated modest improvements in overall survival and in quality of life for advanced NSCLC patients who have a good performance status2. Four to six cycles of combination chemotherapy are considered the standard of care for advanced NSCLC. The median progression-free survival with standard combination chemotherapy regimens is approximately 4 months with a median overall survival of approximately 8 to 11 months for patients with a good performance status3.
Maintenance therapy, also referred to as consolidation therapy, refers to the use of systemic therapy in patients who benefit from combination chemotherapy in order to extend the duration of disease control4. The initial studies employed prolongation or continuation of combination chemotherapy until progression of disease5, 6. When compared to chemotherapy for a defined duration, there was no improvement in overall survival with the use of prolonged combination chemotherapy. In addition, the increased incidence of cumulative toxicities was a major impediment. Therefore, combination chemotherapy is usually not recommended beyond 4 to 6 cycles for advanced NSCLC. In recent years, administration of a single agent as maintenance therapy following 4 cycles of combination chemotherapy has been studied in randomized clinical trials. Pemetrexed, an anti-folate compound and erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR) have both demonstrated modest improvements in overall survival compared to placebo in the maintenance setting7, 8. Both of these agents have now been approved by the United States Food and Drug Administration for maintenance therapy of advanced NSCLC. Other agents that have been evaluated in the maintenance setting such as gemcitabine, docetaxel, vinorelbine, paclitaxel and gefitinib have not demonstrated improvement in overall survival9-13. Therefore, a number of questions remain open regarding the use of maintenance therapy in advanced NSCLC.
Soon and colleagues reported on a meta-analysis of randomized clinical trials that reported on the role of maintenance therapy for advanced NSCLC14. The analysis included some clinical trials that utilized combination chemotherapy as maintenance and others that employed single agent therapy. There was a statistically significant improvement in progression-free survival and overall survival for maintenance therapy. However, the analysis did not specifically address the role of single agent maintenance therapy that is now the commonly utilized approach in routine practice. Furthermore, the data from recent randomized studies not included in the meta-analysis by Soon et al has shed further light on the benefits of maintenance therapy for advanced NSCLC8. With the availability of both targeted agents and chemotherapy for maintenance therapy, it has now become important to determine personalized treatment based on individual patient characteristics. Another relevant issue regarding optimizing maintenance therapy is whether the agent used has or has not been given as part of the first-line combination regimen. Continuing one of the agents used as front-line therapy (continuation maintenance) vs. incorporating an agent that the patient has not previously received (switch maintenance) are the two utilized strategies 7, 15. In order to understand the impact of each of these strategies on the outcomes for patients with advanced NSCLC, we conducted a meta-analysis of randomized studies that evaluated only single agent maintenance therapy.
METHODS
Search Methodology
We conducted an electronic literature search of public databases (MEDLINE, EMBASE, Cochrane library) and a manual search of conference proceedings. Relevant search terms, such as “non-small cell lung cancer, maintenance therapy, anti-neoplastic agents” were included in the search strategy. The list of retrieved studies was then manually searched and reviewed for eligible trials. The annual meeting proceedings of ASCO and the World Conference on Lung Cancer from 1994-2011 were hand searched for eligible trials. Prospective trials registers were explored for relevant ongoing trials.
Inclusion Criteria, Selection of Trials and Data Collection
Randomized controlled trials that reported the effect of single agent maintenance therapy on survival or progression-free survival in histologically or cytologically proven stage IIIB or IV NSCLC patients were included. Studies were considered for this review if they measured clinical outcomes such as, overall survival (OS), progression-free survival (PFS), objective response (OR), treatment-related morbidity, treatment-related mortality, or quality of life (QOL) measures. The list of references was reviewed and studies were identified by three co-authors. Abstracts that seemed eligible were screened for further review. The full-text of any abstract that appeared to be eligible was carefully examined for inclusion. Any disagreements were resolved by consensus.
Two reviewers independently extracted the data from the selected articles using standardized data compilation forms. Original data from meeting presentations for qualifying abstracts were accessed through virtual meeting. The name of the first author and the publication year has been used to identify the article in the review. We extracted data related to the clinical outcomes, and also on the methodological quality of the trials.
Outcome Measures
A formal meta-analysis was conducted using Comprehensive Meta Analysis software (Version 2.0). The outcome data were pooled and reported as hazard ratio (HR). The primary outcome of interest was overall survival (OS) and secondary outcomes included progression free survival (PFS), objective response rate and toxicities. All the included studies, except one reported OS data.
Statistical Analysis
The appropriate outcome data was extracted from individual published reports of the included trials using methods described in the literature16, 17. The data was then pooled for meta-analysis and the final summary statistics is reported as hazard ratios (HR), where the HR of less than 1 signifies an advantage for maintenance therapy. The meta-analysis results are displayed as forest plots. The statistical heterogeneity of trial results was determined from the forest plot statistics. A p-value of greater than 0.1 for χ2 test and I2 value of less than 0.25 reflects the low level of heterogeneity18. A fixed-effect model was used to perform the primary analyses.
Subgroup Analyses
The trials were stratified into four different categories for subgroup analyses to evaluate the effect of- ‘continuation’ maintenance (5 trials); ‘switch’ maintenance (8 trials); cytotoxic agents (9 trials); EGFR targeted agents (4 trials). The OS and PFS are reported as summary results from each subgroup analysis.
Objective Response Rate, Quality of Life and Adverse Events
The data for these outcome measures have been reported diversely across the trials, which made it complicated for statistical pooling. A qualitative assessment of these outcomes is provided in this report.
RESULTS
Search Strategy/Study selection Results
The search strategy and selection steps for the eligible studies are summarized in the consort diagram shown in Figure 1. We included 12 trials for final analysis with a total of 4286 patients. Of the included studies, 5 are meeting abstracts and 7 are peer-reviewed full journal articles.
Figure 1.
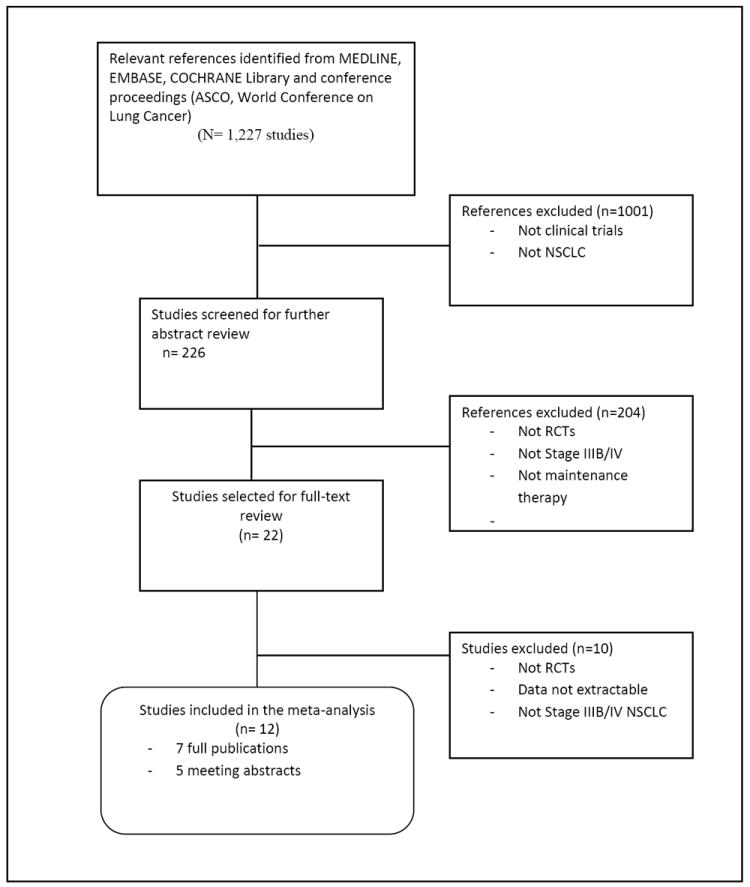
CONSORT diagram- Search Strategy and Study Selection
Characteristics of Included Studies
A summary of characteristics of the 12 included studies is provided in Table 1. The median sample size was 275.5 patients ranging from 130 to 663. The median age of all included patients was 61 years. Continuation maintenance was evaluated in 5 trials and ‘switch’ maintenance was evaluated in 8 trials. Nine of the trials evaluated cytotoxic agents for maintenance and 4 of the trials used EGFR targeted agents. Males (69%), Caucasians (75%), adenocarcinoma/squamous cell carcinoma (51%/28%), Stage III/IV (21%/78%), smokers (69%), performance status 0/1/2 (34%/69%/8%) are some of the characteristics of the patients included in this analysis.
Table 1.
Trial Characteristics
| Study | Type of article | Total enrolled | Median age of participants | Maintenance arm | Control | Type of maintenance | Primary end point | Significant in primary end point | Methodologic quality of trial |
|---|---|---|---|---|---|---|---|---|---|
| Perol 201026 | Abstract | 464 | Not reported | Gemcitabine; Erlotinib | Observation | Cytotoxic agent/continuation maintenance; EGFR targeted agent/switch maintenance | PFS | Yes | Study was statistically powered; Data about double blinding, allocation concealment, ITT principle not available |
| Belani 201027 | Abstract | 255 | 67 | Gemcitabine | BSC | Cytotoxic agent/continuation maintenance | OS | No | Study was powered; Data about blinding allocation concealment, ITT principle not reported. |
| Gaafar 201028 | Full Manuscript | 173 | 61 | Gefitinib | Placebo | EGFR targeted therapy/Switch maintenance | OS | No | Study was powered; double-blind design |
| Belani 200311 | Full Manuscript | 130 | 65.5 | Paclitaxel | Observation | Cytotoxic agent/continuation maintenance | TTP | No | Phase II design |
| Brodowicz10 2006 | Full Manuscript | 206 | 57 | Gemcitabine | BSC | Cytotoxic agent/continuation maintenance | TTP | Yes | Study was powered |
| Westeel 20059 | Full Manuscript | 181 | 62.5 | Vinorelbine | Observation | Cytotoxic agent/Switch maintenance | OS | No | Study was powered; Central randomization |
| Fidias 200912 | Full Manuscript | 309 | 65 | Immediate Docetaxel | Delayed Docetaxel | Cytotoxic agent/Switch maintenance | OS | No | Study was powered; central randomization; ITT analysis; QOL assessed |
| Ciuleanu 200912 | Full Manuscript | 663 | 60.5 | Pemetrexed | Placebo | Cytotoxic agent/Switch maintenance | PFS | yes | Study was powered; central randomization; double blinded; ITT analysis. |
| Johnson 200829 | Full Manuscript | 186 | 66 | CAI | Placebo | Cytotoxic agent/Switch maintenance | OS | No | Study was powered; central randomization; double blinded; QOL assessed |
| Cappuzzo 20108 | Full Manuscript | 884 | 60 | Erlotinib | Placebo | EGFR targeted therapy/Switch maintenance | PFS | Yes | Study was powered; adaptive randomization via third party IVRS; ITT analysis. |
| Paz-Ares 201115 | Abstract | 539 | 61 | Pemetrexed | Placebo | Cytotoxic agent/continuation maintenance | PFS | Yes | Study was powered; double blinded |
| Zhang 201113 | Abstract | 296 | 54 | Gefitinib | Placebo | EGFR targeted therapy/Switch maintenance | PFS | Yes | Study was powered; 1:1 randomization; parallel design; ITT analysis |
Overall Survival
Single agent maintenance therapy was superior in improving OS (HR 0.86, 95%CI 0.80-0.92; P= 0.0003; Figure 2). There was no significant heterogeneity in the HRs of individual trials (P= 0.92, I2 < 0.05). Switch maintenance was found to be significantly better (HR 0.84, 95% CI 0.77-0.91; P =0.00026; Figure 3) whereas ‘continuation’ maintenance was not associated with a statistically significant survival benefit (HR 0.92, 95% CI 0.78-1.09; P= 0.33; Figure 3). Pooled data from trials that evaluated cytotoxic agents as maintenance therapy showed significant improvement in OS (HR 0.89, 95% CI 0.80-0.98; P=0.018; Figure 4). EGFR-targeted therapy, evaluated in 4 trials, was associated with significant improvement in OS (HR 0.83, 95% CI 0.74-0.92; P= 0.004; Figure 4).
Figure 2.
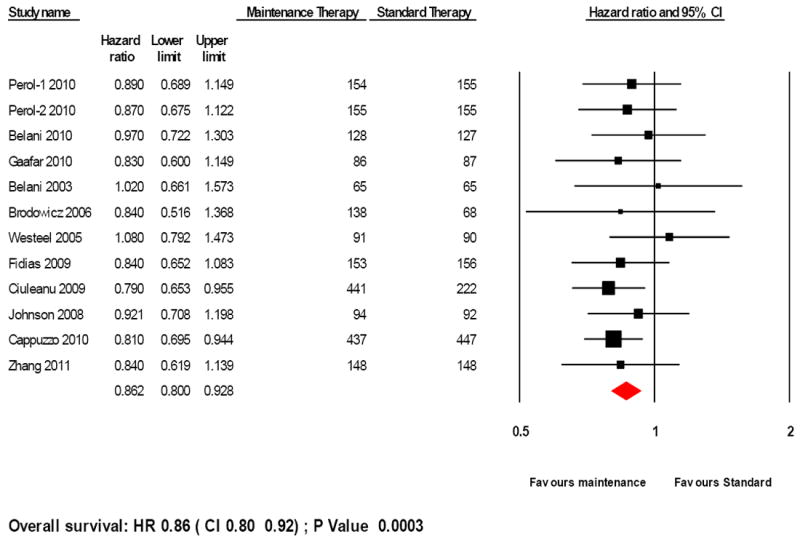
Forest plot for overall survival with maintenance therapy
Test of heterogeneity: P-value= 0.92, I2 < 0.05
Figure 3.
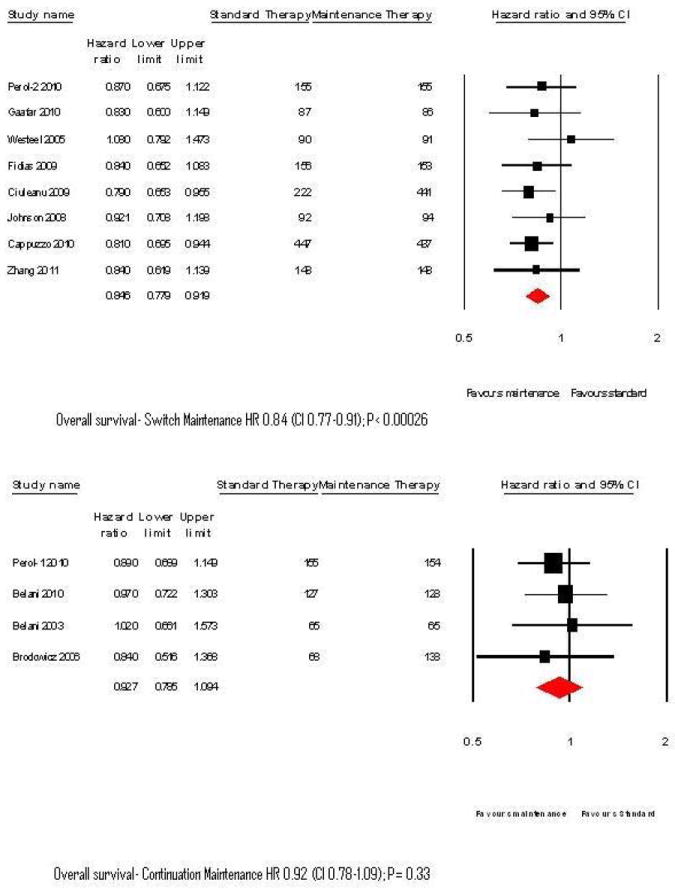
Forest plots for overall survival with Switch and continuation maintenance therapy
Figure 4.
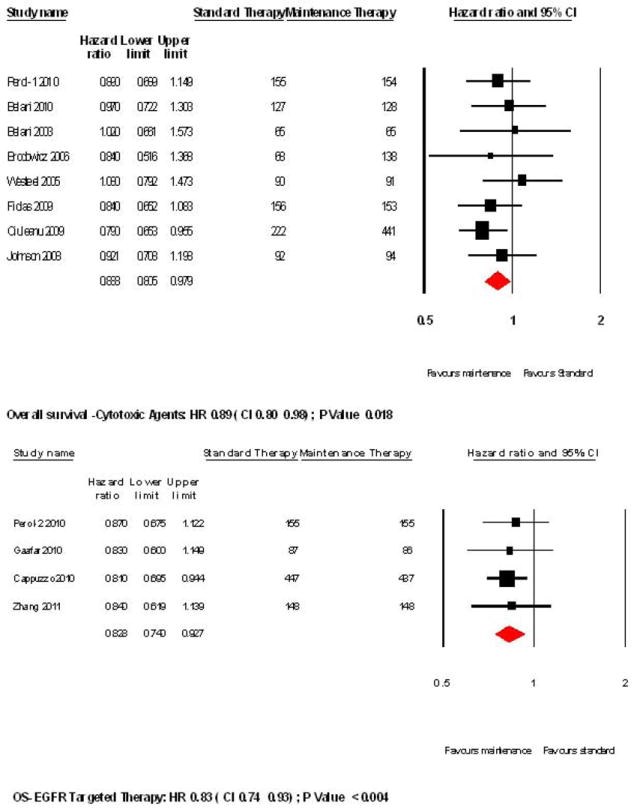
Forest plots for overall survival with cytotoxic and targeted therapy
Progression-free survival
A statistically significant improvement was seen in PFS in patients with maintenance therapy (HR 0.80, 95% CI 0.77-0.84; P < 0.0001; Figure 5). Switch maintenance was associated with significant improvement in PFS (HR 0.62, 95%CI 0.57- 0.67; P <0.0001) whereas continuation maintenance showed a relatively modest improvement in PFS (HR 0.90, 95% CI 0.85-0.95; P=0.007). Cytotoxic agents were associated with significant improvement in PFS (HR 0.85, 95% CI 0.80-0.89; P < 0.0001) and similar benefit was seen with EGFR-targeted maintenance therapy (HR 0.64, 95% CI 0.58-0.71, P< 0.0001).
Figure 5.
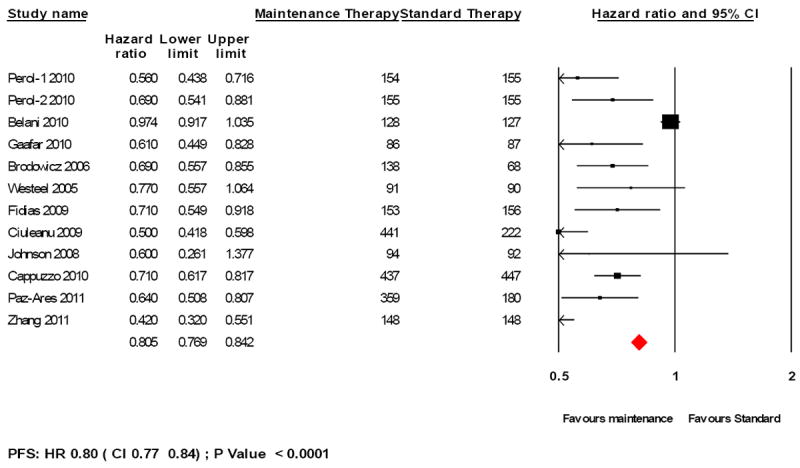
Forest plot for progression-free survival for maintenance therapy
Response Rate, QOL and Adverse events
The ORR in the maintenance arm was 21.25 % (7 trials; n= 1520) as compared to 7% in control arm (6 trials, n= 1110). In assessing AEs of grade 3 and above, 18% of the patients had toxicities in the maintenance arm (8 trials; n=2006) and 5% of patients in the control arm (7 trials; n=1400). Quality of life as assessed from 2 trials (Johnson 2008, Fidias 2009) was not significantly better in the maintenance arm.
DISCUSSION
This meta-analysis was conducted to define the optimal maintenance therapy for patients with advanced stage NSCLC. Single agents, rather than prolonged platinum-based combination therapy, are better suited for maintenance strategy to avoid cumulative toxicity and preserve quality of life. The results from single agent maintenance therapy trials have been varied, raising a number of questions regarding optimal strategy in this setting. This provided the rationale to conduct the meta-analysis. Salient limitations of the study include the fact that the present meta-analysis was not based on individual patient data. Furthermore, five of the twelve included studies have only been presented in abstract format at conferences but not yet published as full manuscripts in peer-reviewed journals. Though another recent publication evaluated this topic, it did not include five of the twelve trials included in our report19. Two of the studies in this analysis reported the time to progression (TTP), but not the PFS. Therefore, TTP was included in lieu of PFS for the analysis.
We observed a statistically significant benefit with single agent maintenance therapy, and this was observed with both cytotoxic agents and EGFR inhibitors. The hazard ratio for targeted agents was slightly better that than with chemotherapy. Our analysis of EGFR inhibitors did not account for the mutation status. In clinical trials, both erlotinib and gefitinib have demonstrated very robust PFS results for patients with an activating EGFR mutation8, 13. It is notable that the results with pemetrexed, the only approved cytotoxic agent in the maintenance setting demonstrated the best hazard ratio of 0.79 for overall survival 7. It appears that the inclusion in this analysis of other cytotoxic agents with more modest benefit diluted the overall risk reduction associated with cytotoxic agents. Among the studies included in our analysis, Johnson et al evaluated the role of carboxyaminoimidazole as maintenance therapy. Since this is not an agent with established anti-cancer effects in NSCLC, we conducted an analysis excluding this trial and found that no significant difference in the hazard ratio for PFS or OS.
Another aspect of the pemetrexed switch maintenance study that merits consideration is that it included patients with all sub-histologies of NSCLC rather than the non-squamous subset where the magnitude of benefit is higher. It is important to emphasize that pemetrexed is only approved for use in the non-squamous subset based on its efficacy. In the past few years, subset analyses from several randomized studies have documented the lack of efficacy with pemetrexed in squamous histology20, 21. Therefore, we conducted an additional analysis excluding the squamous patients enrolled to the pemetrexed study. There was a minor favorable change in the hazard ratio with the use of single agent cytotoxic chemotherapy in the maintenance setting, as would be expected. The PARAMOUNT study included only patients with non-squamous histology and therefore a separate analysis was not required15.
Switch maintenance therapy was associated with survival benefit, but continuation therapy was not in this analysis. A potential caveat to this observation is the fact that the survival data from the PARAMOUNT study have not been reported15. The PARAMOUNT study demonstrated significant improvement in progression-free survival with pemetrexed as continuation maintenance therapy following initial therapy in combination with cisplatin. Continuation maintenance strategy is currently being used in routine clinical practice with bevacizumab, a monoclonal antibody against the vascular endothelial growth factor. It is usually continued as maintenance therapy following initial administration in combination with platinum-based chemotherapy22. Similarly, cetuximab, an antibody to the epidermal growth factor receptor, is also given as continuation maintenance therapy following combination administration with chemotherapy23. This paradigm emerged from the design of the pivotal studies that utilized these agents as maintenance therapy in the investigational arm in patients with responding or stable disease. To date, the role of these agents in the maintenance therapy setting has not been definitively studied. Therefore, our meta-analysis was not able to evaluate the role of continuation maintenance therapy with these targeted agents.
Another important consideration with the use of maintenance therapy is the potential impact on the patient’s quality of life. Given the modest degree of benefit with certain agents, the decision to use maintenance therapy in routine practice depends on a variety of patient-specific factors. Prime among these are quality of life and symptomatic benefits. Only two studies included in our meta-analysis provided detailed reports on quality of life. In the recently reported PARAMOUNT study, there was no difference in quality of life parameters between pemetrexed and placebo, but the actual results were not reported. Taken together, we believe that current evidence is insufficient to make conclusions regarding the effect of maintenance therapy on quality of life.
In patients that receive maintenance therapy, the survival outcomes are influenced by the use of post-study therapy. In many of the studies included in the analysis, detailed information regarding the effect of post-study therapy on survival has not been reported9, 11, 12. Therefore, this analysis could not determine whether the survival benefit with maintenance therapy was altered to a significant extent with post-study therapy compared to those who did not receive any treatment after progression. In the phase III study by Fidias et al, though maintenance docetaxel was associated with a favorable outcome, the median survival was similar for patients that received docetaxel either as maintenance therapy or second line therapy12. In this instance, the observed improvement in overall survival (not statistically significant) was attributed to a higher proportion of patients in the maintenance arm receiving an active agent after first line therapy. This observation was also supported by the Perol study that had similar post-study therapy use for patients in the maintenance setting and control arms24. Though the progression-free survival was improved, there was no improvement in overall survival with maintenance therapy. In routine practice, approximately two-thirds of the patients receive second line therapy after first-line combination therapy25. Since it is not possible to identify a priori the patients that will not be able to receive second line therapy, the use of maintenance therapy provides the opportunity to administer an active anti-cancer therapy in the aftermath of favorable response with front-line combination chemotherapy. However, this has to be weighed in parallel to the wishes of many patients to have a ‘treatment-holiday’ after completion of combination chemotherapy.
Our knowledge of maintenance therapy is bound to expand when the results of several ongoing clinical trials mature over the next few years. The present meta-analysis provides clear evidence in support of the use of maintenance therapy as a ‘standard of care’ for patients with advanced NSCLC. The decision to use maintenance therapy should be based on an individualized approach that includes patient-specific factors and tumor-specific biomarkers.
Supplementary Material
Footnotes
The study was presented at the 47th Annual Meeting of the American Society of Clinical Oncology held at Chicago, IL in June 2011.
CONFLICT OF INTEREST STATEMENT
Suresh Ramalingam has served on adhoc advisory board meetings for Lilly, Genentech, Astellas and Pfizer.
Chandra P Belani has served as a consultant for Lilly and Genentech.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Center MM, Desantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Bunn PA., Jr Chemotherapy for advanced non-small-cell lung cancer: who, what, when, why? J Clin Oncol. 2002;20(18 Suppl):23S–33S. [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Owonikoko TK, Ramalingam SS, Belani CP. Maintenance therapy for advanced non-small cell lung cancer: current status, controversies, and emerging consensus. Clin Cancer Res. 2010;16(9):2496–504. doi: 10.1158/1078-0432.CCR-09-2328. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002;20(5):1335–43. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- 6.Smith IE, O’Brien ME, Talbot DC, Nicolson MC, Mansi JL, Hickish TF, et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001;19(5):1336–43. doi: 10.1200/JCO.2001.19.5.1336. [DOI] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Westeel V, Quoix E, Moro-Sibilot D, Mercier M, Breton JL, Debieuvre D, et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2005;97(7):499–506. doi: 10.1093/jnci/dji096. [DOI] [PubMed] [Google Scholar]
- 10.Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155–63. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Belani CP, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;21(15):2933–9. doi: 10.1200/JCO.2003.02.563. [DOI] [PubMed] [Google Scholar]
- 12.Fidias P, Dakhil SR, Lyss A, Loesch D, Waterhouse D, Cunneen J, et al. Phase III study of immediate versus delayed docetaxel after induciton therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer: Updated report with survival. J Clin Oncol. 2007;24(18S) doi: 10.1200/JCO.2008.17.1405. Abs # 7516, 388s. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Shenglin M, Song X, BHan B, Chng Y, Huang C, et al. Efficacy, tolerability, and biomarker analyses from a phase III, randomized, placebo-controlled, parallel group study of gefitinib as maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC; INFORM; C-TONG 0804) J Clin Oncol. 2011;29(15S) doi: 10.1097/JTO.0000000000000445. Abstract # 7511. [DOI] [PubMed] [Google Scholar]
- 14.Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27(20):3277–83. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 15.Paz-Ares L, De Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo plus BSC immediately following induction treatment with pem plus cisplatin for advanced nonsquamous non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(15S) Abstract # 7510. [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(16) doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zang J, Xu J, Bai C, Qin Y, Liu K, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest. 2011;140(1):117–26. doi: 10.1378/chest.10-2745. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 21.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 22.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 23.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 24.Perol M, Chouaid C, Milleron B, Gervais R, Barlesi F, Westeel V, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol. 2010;28(15S) doi: 10.1200/JCO.2011.39.9782. Abs # 7505. [DOI] [PubMed] [Google Scholar]
- 25.Ramalingam S, Sandler AB. Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist. 2006;11(6):655–65. doi: 10.1634/theoncologist.11-6-655. [DOI] [PubMed] [Google Scholar]
- 26.Perol M, Chouaid C, Milleron B, Gervais R, Barlesi F, Westeel V, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol. 2010;28(15s) doi: 10.1200/JCO.2011.39.9782. Abs # 7507. [DOI] [PubMed] [Google Scholar]
- 27.Belani CP, Waterhouse D, Ghazal H, Ramalingam SS, Bordoni R, Greenberg R, et al. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(15s) Abs # 7506. [Google Scholar]
- 28.Gaafar R, Surmont V, Scagliotti G, Van Klaveren RJ, Papamichael D, Welch J, et al. A double-blind, randomized, placebo-controlled phase III intergroup study of gefitinib (G) in patients (pts) with advanced NSCLC, non-progressing after first-line platinum-based chemotherapy (EORTC 08021-ILCP 01/03) J Clin Oncol. 2010;28(15S) doi: 10.1016/j.ejca.2011.06.045. Abstract # 7518. [DOI] [PubMed] [Google Scholar]
- 29.Johnson EA, Marks RS, Mandrekar SJ, Hillman SL, Hauge MD, Bauman MD, et al. Phase III randomized, double-blind study of maintenance CAI or placebo in patients with advanced non-small cell lung cancer (NSCLC) after completion of initial therapy (NCCTG 97-24-51) Lung Cancer. 2008;60(2):200–7. doi: 10.1016/j.lungcan.2007.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


