Abstract
The intestinal epithelium sits at the interface between an organism and its luminal environment, and as such is prone to oxidative damage induced by luminal oxidants. Mucosal integrity is maintained by the luminal redox status of the glutathione/glutathione disulfide (GSH/GSSG) and cysteine/cystine (Cys/CySS) couples which also support luminal nutrient absorption, mucus fluidity, and a diverse microbiota. The epithelial layer is uniquely organized for rapid self-renewal that is achieved by the well-regulated processes of crypt stem cell proliferation and crypt-to-villus cell differentiation. The GSH/GSSG and Cys/CySS redox couples, known to modulate intestinal cell transition through proliferation, differentiation or apoptosis, could govern the regenerative potential of the mucosa. These two couples, together with that of the thioredoxin/thioredoxin disulfide (Trx/TrxSS) couple are the major intracellular redox systems, and it is proposed that they each function as distinctive redox control nodes or circuitry in the control of metabolic processes and networks of enzymatic reactions. Specificity of redox signaling is accomplished in part by subcellular compartmentation of the individual redox systems within the mitochondria, nucleus, endoplasmic reticulum, and cytosol wherein each defined redox environment is suited to the specific metabolic function within that compartment. Mucosal oxidative stress would result from the disruption of these unique redox control nodes, and the subsequent alteration in redox signaling can contribute to the development of degenerative pathologies of the intestine, such as inflammation and cancer.
Keywords: intestinal oxidative stress, intestinal redox biology, luminal redox and microbiota, GSH compartmentation, redox status and intestinal cell transition, GSH/GSSG redox couple, Cys/CySS redox couple
1. Organization of the intestinal epithelium and regenerative potential
1.1 Structural and functional organization
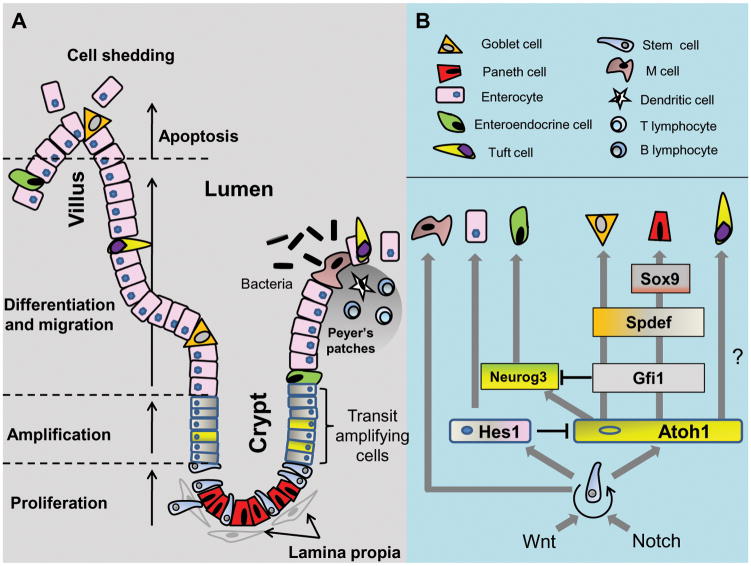
The mammalian gastrointestinal epithelium is capable for self-renewal every 4–5 days and is one of the highest proliferative tissue in an organism [1]. The small intestinal epithelium is extensively folded into crypts (of Lieberkühn) and villi, a structural feature that maximizes the absorptive surface area for nutrients, electrolytes and water. Within the epithelium the five major cell types are derived from an absorptive lineage, i.e, enterocytes, or a secretory lineage, i.e., mucus-secreting globlet cells, hormone-secreting enteroendocrine cells, Paneth cells, and tuft cells. The multi-potent intestinal stem cell (ISC) and progenic transit-amplifying cell reside in the crypt and proliferate into progenitor cells that migrate to the villus tip. The stem cell niche is supported by surrounding stromal or mesenchymal cells known as the lamina propria that regulates stem cell behavior through secretion of growth factors and cytokines [2]. During crypt-to-villus transition, the progenitor cells differentiate into goblet, enteroendocrine, tuft, or epithelial (enterocyte) cells; Paneth cells differentiate and remain within the crypt [3]. The villus tip enterocytes at 4–5 days post differentiation undergo spontaneous apoptosis and are shed into the intestinal lumen, thus completing one renewal cycle (Figure 1). Likewise, colonic crypts house proliferative cells and differentiated cells reside at epithelial surfaces, but the colonic epithelium is distinctly devoid of villi and Paneth cells.
Figure 1. Structural organization (A) and epithelial differentiation (B) in the small intestine.
A. The intestinal epithelium is organized into the villus and crypt regions each containing specialized cell types with distinct functions. Within the crypt, intestinal stem cell (ISC) progenitors differentiate into absorptive or secretory lineages along the crypt-to-villus axis. Differentiated Paneth cells remain in the crypt. Gut immune response is mediated by specialized M cells from GALT in Peyer’s patches. Apical enterocytes undergo apoptosis and are shed into the lumen. B. Intestinal self-renewal and ISC proliferation is regulated by signals from Paneth cells and the surrounding lamina propria. Notch-dependent activation of Hes1 or Atoh1 genes respectively determines the secretory and absorptive lineages. The mechanisms of ISC-to-M cell or Atoh-1-dependent Tuft cell differentiation are unknown.
Enterocytes are most abundant, at >80%, and they function in apical nutrient and water absorption. Secretory goblet cells comprise ~16% to 50% of the small intestinal and colonic epithelium, respectively. The functions of goblet cells include the secretion of mucus and a supportive environment for the gut microbiota while the other secretory enteroendocrine, Paneth, and tuft cells each perform essential functions in gastrointestinal hormone secretion, host defense, and bicarbonate secretion, respectively [4, 5]. Tuft cells also uniquely express cyclooxygenase 1 and 2 (COX1 and COX2), which suggests their involvement in intestinal pathobiology [4]. The gastrointestinal tract is the hub for the gut-associated lymphoid tissue (GALT) [6], organized as Peyer’s patches scattered along the intestine and are surrounded by a specialized follicle-associated epithelium (FAE). The FAE contains invaginated microfold cells (M cells), a class of specialized enterocytes which function in luminal antigen presentation to the dendritic cells, B- and T-lymphocytes within the lamina propria, and trigger an immune response [7].
The sequential processes of proliferation, migration, differentiation, and apoptosis within the intestinal epithelium are dynamic. At base crypts, asymmetric division of ISCs gives rise to two daughter cells, one remaining as a stem cell (self-renew) at the base, and the other becomes a transient amplifying cell that undergo differentiation (Figure 1A). The “stemness” of ISCs is maintained by signals from adjacent Paneth cells. Wnt3, Notch ligand Dll4, and EGF signals support ISC growth but also limit ISC number [8], thereby maintaining a define number of crypt ISCs. ISC cell number is also regulated by mesenchymal cell-derived bone morphogenic proteins (BMP) which inhibit ISC proliferation [9].
1.2 Signaling of cell proliferation and differentiation
The signaling mechanisms that govern ISC proliferation and differentiation into mature intestinal epithelial cells are incompletely understood. Among the key candidates are the Wnt/β-catenin, Notch, and BMP pathways. The Wnt/β-catenin pathway is known to drive ISC proliferation and renewal within the intestinal crypt, participating in the maintenance of stem/progenitor cells, inhibition of stem cell differentiation, regulation of crypt-to-villus cell migration, early development of the secretory lineage, and terminal differentiation of Paneth cells [10, 11]. A highly expressed Wnt-activated gene, Lgr5 (leucine-rich repeat containing G protein-coupled receptor 5) in ISCs under basal conditions is a widely accepted stem cell marker [12]. The maintenance of ISCs in an undifferentiated, proliferative state is further supported by Notch signaling while BMP-dependent suppression of Wnt signaling promotes ISC quiescence [13].
Crypt-to-villus migration of progenitor cells is characterized by a decrease in Wnt signaling. Concurrent gradient increase in BMP activation was associated with increased cell quiescence and differentiation [14]. Cell fate decision between differentiating into a secretory or an absorptive lineage is the function of the Notch signaling pathway [15] (Figure 1B) wherein depletion of Hes-1 (hairy/enhancer of split 1), a direct Notch target gene, was associated with excessive number of goblet, enteroendocrine, and Paneth cells [16]. Conversely, the Atoh1 (Atonal homologue 1) gene, which is repressed by Hes-1 transcription factor, is required for progenitor cell differentiation into a secretory lineage; reportedly all intestinal secretory cell types are derived from a single Atoh1-dependent secretory progenitor [17]. The finding that Atoh1 expression was influenced by Wnt signaling [15] suggests an interaction between the Wnt and Notch pathways. Other transcription factors, Gfi1, Neurogenin3 (Neurog3), SAM pointed domain containing Ets transcription factor (Spdef), and SRY-box containing gene 9 (Sox9), support differentiation into specific secretory cell types (Figure 1B). Gfi1 represses Neurog3 and is critical for progenitor cell-to-Paneth/goblet cell differentiation [18]. Spdef promotes terminal differentiation of goblet cells and maturation of Panel cells [19], whereas Sox9 is necessary for Paneth cell differentiation [20]. Tuft cell differentiation is modulated by the Lgr5-expressing ISC which acquired secretory characteristics in an Atoh1-dependent way [4]. While the mechanisms for M cell differentiation are yet unknown, these cells have been shown to derive from Lgr5 progenitor cells [21].
2. Redox biology of the intestine
2.1 Concept of cellular redox environment
The glutathione (GSH), cysteine (Cys), and thioredoxin (Trx) couples are the major cellular redox systems in cells [22], and the cellular redox state of the individual couples is described by its inter-convertible reduced and oxidized forms, i.e., GSH/GSSG, Cys/CySS, or Trx/TrxSS. The collective product of their reducing potential and reducing capacity constitute the intracellular redox environment [23]. The ratio of GSH-to-GSSG approximates the intracellular redox environment given the large cellular GSH pool size [23], and the tendency of GSH for electron donation or acceptance is defined by its redox potential, Eh. Under physiological conditions, Eh for GSH/GSSG is between between −260 to −200mV [24].
An emerging view is that in biologic systems, the GSH/GSSG redox couple, together with those of Trx/TrxSS, and Cys/CySS serves as distinctive redox control nodes or circuitry in the redox control of cellular metabolism and growth [24]. The fact that each of the redox couples exists far from equilibrium and can function as on-off sulfur switches support such a mode for independent regulation of single protein or protein sets in normal physiology [25]. This viewpoint provides a novel metabolic framework to redefine cellular oxidative stress as the disruption of biological redox signaling events [22] as opposed to the classical view of oxidative stress as simply an imbalance between pro- and anti-oxidant systems [26]. The extent to which the mechanisms of sulfur switches and redox circuitry control intestinal cell function and growth is an open question.
2.2. Cellular redox systems and compartmentation
Glutathione and GSH-dependent enzymes
GSH is present in millimolar concentrations in the intestinal epithelium [27–29], a level that is similar to other cells types (2–10mM) [30]. Intracellular GSH exists as the biologically active reduced-thiol form, and its oxidation to GSSG is oft associated with oxidative stress. Mucosal GSH redox homeostasis is maintained by de novo synthesis [31], regeneration from GSSG [32], and GSH uptake [28, 33]. Mucosal GSH uptake across the apical membrane occurs independently of intracellular GSH synthesis [31], and is stimulated by monovalent cations [33, 34], a characteristic that is shared by renal proximal tubular cells [35]. An important aspect of intestinal GSSG reduction is the supply of NADPH for the function of the GSH redox cycle [36].
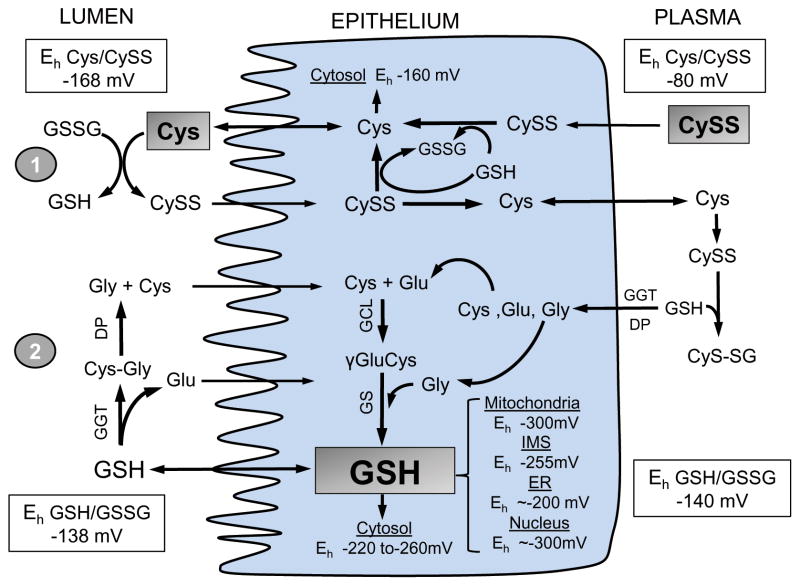
Compartmental distribution of GSH likely exists within all cell types, including enterocytes. Cellular GSH is distributed among mitochondria, endoplasmic reticulum (ER) and nucleus as distinct GSH redox pools [37] (Figure 2). GSH levels are high in the mitochondrial, cytosolic and nuclear compartments with relatively reduced with Eh, between −260mV and −300 mV [24]. In contrast, the ER matrix exhibits Eh between −170mV and −205mV [38]. The vast difference in redox potential within the different organelles is well suited to the specific biological or metabolic function within that compartment. For example, the oxidized environment of the ER supports proper folding of nascent proteins [39]. A GSH/GSSG Eh of −255mV in the mitochondrial intermembrane space (IMS) supports disulfide bond formation of imported cytosolic proteins [40], even though the matrix exhibits a more reduced GSH Eh (~300mV) [41]. While matrix GSH balance is achieved through carrier-mediated cytosol-to-mitochondria GSH import [42], it remains unclear how the IMS maintains an oxidized Eh despite free access of cytosolic GSH through porin channels [43]. Cytosolic-nuclear GSH interaction is dynamic, notably during cell cycle wherein nuclear GSH increased 4-fold [44]. An unresolved dilemma is the supposed notion that nuclear GSH is maintained independently from that of cytosolic GSH [24] despite the fact that cytosol-to-nuclear GSH import occurs by passive diffusion through nuclear pores [45].
Figure 2. Contribution of the Cys/CySS and GSH/GSSG systems to intestinal redox homeostasis.
At intestinal brush-border the Cys/CySS shuttle (1) and γ-glutamyl cycle (2) support luminal thiol/disulfide homeostasis. Within the intestinal epithelium, CySS reduction and de novo GSH synthesis maintain intracellular Eh of the Cys/CySS and GSH/GSSG couples, respectively. Cytosolic GSH is exported or compartmentalized within subcellular compartments as distinct GSH redox pools. CySS predominates within plasma, Cys status is controlled by thiol/disulfide exchange with liver-derived GSH, and CyS-SG is hydrolyzed to constituent amino acids for enterocyte re-uptake. Shaded boxes for luminal Cys, cellular GSH, and plasma CySS represent the major determinant of the Eh in these compartments. Dietary GSH or Cys sources and basal-lateral GSH efflux or influx to-and-from plasma are not shown for purposes of simplicity.
Intra-intestinal antioxidant defense is mediated by GSH-dependent enzymes that are compartmentalized within the cytosol, mitochondria and nucleus. The glutaredoxin (Grx) isoenzymes, Grx1 and Grx2, are localized to cytosol and mitochondria, respectively where they participate in the reduction of GSSG or GSH-mixed disulfides through thiol-disulfide exchanges [46]. The monothiol Grx3, which is devoid of oxidoreductase activity, serves as a redox sensor during signaling induced by reactive oxygen or nitrogen species (ROS/RNS) [47]. The localization and high expression of Grx2 in mouse duodenal enterocytes [48] suggest a role in redox reactions. Glutathione peroxidase 1 (Gpx1) and the intestinal specific Gpx2 (formerly known as GSHpx-GI [49]) are the major hydrogen peroxide (H2O2) reducing intestinal selenoproteins [50] with a kinetic rate of ~ 5×107 M−1 s−1. Gpx1 exhibits uniform crypt-to-villus distribution while Gpx2 predominates in the crypt region [50, 51], and is highly expressed in the ileum and cecum [52]. Interestingly, Gpx1 and Gpx2 are differentially sensitive to selenium (Se) deprivation, the former being more vulnerable to Se deficiency [53], underscoring its importance in intestinal cell survival. The targeting of Gpx4, which catabolizes phospholipid hydroperoxides, to the cytosol and nucleus of human small intestine and colon maybe physiologically meaningful in antioxidant protection [54]. An extracellular Gpx3 isoform functions in mucosal cytoprotection against luminal oxidants [55]. GSH S-transferase (GST) catalyzes GSH-dependent detoxication of luminal electrophiles and carcinogens, and GST expression levels are oft used as an index of intestinal tumorigenic potential. The abundance and varied expression of intestinal GST is sensitive to luminal diet and drug exposure [56]. Among the human GST multigenic family of isoenzymes, [57], GST Pi and Mu are highly expressed in the small intestine with lower abundance in the colon [56], consistent with rare neoplastic occurrences in the small intestine [58]. The decrease in proximal-to-distal GST in the colon [56] suggests reduced colonic xenobiotic detoxication and increased cancer risk.
Thioredoxin and redox proteins
Intestinal thioredoxin (Trx) proteins function in antioxidant defense and redox regulation [48] through reduction of cysteine disulfides within the Cys-XX-Cys (CGPC) motif in proteins. Trx1 and Trx2 are independently regulated isoforms that are specific to cytosol/nucleus and mitochondria [59]. The Eh of Trx1 and Trx2 are highly reduced, between −300mV and −330mV [37]. Accompanying the subcellular distribution of Trx are the seleno-containing Trx reductase isoenzymes that catalyze NADPH-dependent reduction of oxidized Trx. The selenium-Cys residue in the C-terminal motif of the enzyme is essential for catalytic activity. Gut immune response and innate immunity is a recognized role for intestinal Trx. The mucosa expresses high levels of Trx [60] which participates in the antimicrobial function of human β-defensin 1 (hBD-1) [61]. High Trx expression in unstimulated and stimulated lamina propria T lymphocytes (LP-T) reportedly contributes to the maintenance of intracellular redox homeostasis or proinflammatory responses, respectively [62].
Peroxiredoxins (Prxs) are a class of poorly understood redox proteins in the intestine. Prxs are known to be highly expressed in cells (1% of total proteins) with high catalytic rates (~107M−1s−1) in H2O2 reduction [63], a function that is shared with GPxs. The all essential peroxidatic Cys participates in H2O2-catalyzed formation of Cys-sulfenic acid (Cys-SOH) and disulfide bond with a C-terminal Cys residue, a reversible mechanism implicated in redox signaling [64]. Specific inactivation of membrane-associated Prx1 suggests a novel mechanism for H2O2 accumulation and signal propagation initiated at the receptor site [65]. The biological roles for all the known Prx isoforms (Prx 1–6) [66], including in the intestine are incompletely characterized. The finding that Prx exhibits specific subcellular localizations, namely Prx1, 2 in the cytosol, Prx4 in the extracellular space, and Prx3 within the mitochondria suggests specific metabolic functions within these respective compartments [66].
2.3 Homeostasis of luminal and extracellular thiol redox status
Source and function of luminal GSH
Luminal GSH, derived from dietary intake and biliary output [27, 67], is a dynamic pool that is important in absorptive and detoxication functions and mucus protection [68]. It is estimated that biliary GSH in duodenal lumen accounted for 50% of hepatic GSH [27, 67]. Dietary contribution is more varied, and depends on the consumption of GSH-rich or GSH-deficient foods [69]. Significant roles for luminal GSH includes reduction of dietary disulfides [70], conjugation of electrophiles/xenobiotics [71], scavenging of divalent metals [72], and maintenance of mucus fluidity through assembly/disassembly of mucin oligomers [73]. Moreover, elevated luminal GSH was shown to promote luminal lipid peroxide uptake and reduce lymphatic peroxide transport [29, 74]. Apart from mucosal uptake, luminal GSH can be hydrolyzed by the apical membrane γ-glutamyl-transpeptidase [75]. In rat small intestine, substantial GSH hydrolysis occurred preferentially at submillimolar luminal levels [33], the reason yet unknown.
Cysteine redox couple and extracellular thiol/disulfide balance
The extracellular/luminal redox environment is largely determined by the Cys/CySS redox couple, with contributions from the GSH system [70]. The plasma Cys/CySS and GSH/GSSG redox couples are displaced from equilibrium with Eh values tightly regulated at −80mV and −140mV, respectively [24, 76]. The actual extracellular Cys and CySS concentrations are low, 40μM and 8–10μM, respectively, and are modulated by dietary Cys/CySS [77], GSH hydrolysis [78], thiol-disulfide exchange reactions [79], and the Cys/CySS shuttle [80]. An oxidized plasma Cys/CySS redox state has been shown to be associated with vascular pathologies like diabetes, cardiovascular disease, and atherosclerosis [76]; thus, plasma Cys/CySS changes could be predictive of health or disease [81]. Luminal Cys/CySS contributes majorly to maintaining the thiol-disulfide redox state of extracellular proteins [82] and the lumen [68]. In rat intestine, ~40% of luminal Cys was from GSH hydrolysis which participated in nutrient absorption [83] and mucus preservation [84]. Luminal thiol-disulfide redox status is regulated through the Cys/CySS shuttle [70] involving luminal Cys export [68], GSSG reduction and CySS formation [70], followed by CySS uptake [85], intracellular GSH-mediated CySS reduction, and Cys re-release into the lumen (Figure 2). In polarized Caco-2 cells, Eh for Cys/CySS at the basal and apical surfaces are regulated at different rates [86], implying independent redox signaling mechanisms at opposite polar membrane surfaces.
Role of gut microbiota
A complex intestinal luminal microflora is represented by 500–1,000 species of bacteria [87], and a reducing environment supports a microflora of 100–1000 times greater anaerobes to aerobes in adult gut lumen [88]. The gut microbiota prevents pathogen colonization, supports intestinal nutrition and regulates the mucosal immune system [89]. An over abundance of pathogenic species, a “dysbiotic” flora, contributes to aberrant mucosal immune response and chronic intestinal inflammation [90]. The redox biology of bacteria-intestinal host interaction is incompletely understood. Significantly, gut bacteria produces millimolar levels of hydrogen sulfide (H2S) [91]; however, high catabolism [92] maintains low luminal H2S shown to avert the inhibition of mitochondrial cytochrome oxidase [93], ROS production, GSH redox imbalance, and tissue oxidative stress. In colonic HT-29 cells, mitochondrial respiratory rate was actually stimulated by mitochondrial sulfide quinone reductase (SQR)-catalyzed formation of sulfide, an oxidation product of H2S [94]. The reverse electron flow between SQR and complex I yielded NADH [95]. An antioxidant role for H2S akin to that in neuronal cells [96], is yet to be defined in intestinal cells.
3. Intestinal oxidative stress and gut pathobiology
3.1 Intestinal oxidative stress and cell fate
Redox modulation of cell transition and growth
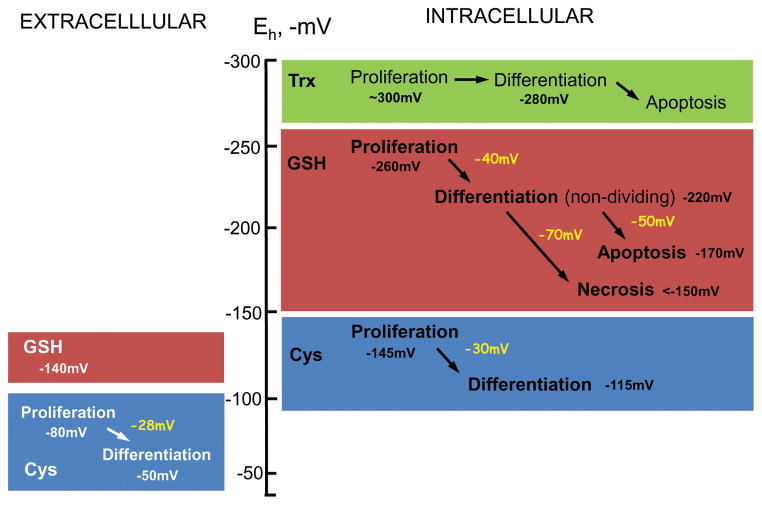
Phenotypic transitions of normal intestinal cells from proliferation to differentiation or apoptosis are associated with increasing oxidation of the Eh of intracellular GSH/GSSG or extracellular Cys/CySS redox systems [22, 25] (Figure 3). Significantly, cell transition is unrelated to the Eh of the Trx/TrxS couple [97]. The Eh of intracellular GSH/GSSG varies between −260mV to −240mV at proliferation, −220mV to −200mV at differentiation, and −170mV to −150mV at apoptosis [98]. In neuronal PC-12 cells, exit from quiescence and entry into proliferative, growth arrested, or apoptotic states depended on the severity of the GSH/GSSG imbalance [99]. As for extracellular Cys/CySS, Eh between −80mV to −50mV was associated with cell transition from a proliferative to a non-dividing differentiated state [100]. Moreover, the proliferative potential in CaCo-2 cells was modulated by the extracellular Cys-to-CySS ratio [74, 99, 101, 102]. Since the GSH and Cys redox systems are displaced from equilibrium, possible synergy between the two couples in controlling cell fate remains an unresolved issue. Notably, CaCo-2 proliferative response to changes in Cys/CySS status occurred independently of cellular GSH or GSH synthesis [103], and extracellular Cys oxidation can induce CaCo-2 proliferation through redox activation of growth receptors without altering cellular GSH [100]. Moreover, Cys/CySS (Eh, −150mV) was able to increase EGFR phosphorylation, p44/42 MAPK signaling and cell growth [104].
Figure 3. Association of GSH/GSSG, Cys/CySS and Trx/TrSS redox potential (Eh) with normal intestinal phenotypic transitions.
Under physiological conditions, intestinal cell transition from proliferation to differentiation or apoptosis is associated with quantitative changes in the Eh of the GSH/GSSG or Cys/CySS redox couples. A 40mV oxidation in intracellular GSH/GSSG Eh elicits cell transition from a proliferative to differentiated, non dividing state. The apoptotic or necrotic states result from an additional 50mV or 70mV oxidation. Additionally, intestinal cell progression from proliferation to differentiation is accompanied by ~ 30mV oxidation of the Eh of extracellular/intracellular Cys/CySS redox couple. Cell transition is unrelated to the Eh of the Trx/TrxS redox couple. A role for extracellular GSH/GSSG in intestinal phenotypic change is unknown.
Lipid hydroperoxides have been shown to elicit CaCo-2 proliferation at low levels and apoptosis at high levels in association with GSH/GSSG disruption [105]. This means that a change in phenotypic outcome can readily be modulated by an imposed transient [74, 99] or sustained [105, 106] shift in the GSH/GSSG redox potential such as would occur during acute or chronic intestinal oxidative stress. In rat small intestine, normal epithelial growth and apoptosis subscribed to a circadian rhythm corresponding to the animal’s feeding and post-prandial periods [107, 108]. Sustained consumption of a peroxidized lipid diet disrupted mucosal GSH/GSSG status and caused mucosal cytostasis, while GSH supplementation restored normal mucosal GSH levels and turnover activity [109, 110]. Surprisingly, mucosal growth following massive small bowel resection was associated with GSH depletion and an oxidized GSH/GSSG status [111]. This increased proliferative activity may, in part be mediated by elevated plasma Cys/CySS [111], consistent with origination of redox-dependent-growth signals from the basal membrane (Figure 3).
Mitochondrial GSH, oxidative DNA damage and cell apoptosis
The mitochondrion is an oxidant-prone compartment. The mitochondrial genome, comprising a circular double-stranded DNA organized in nucleoids and lacking histones, is vulnerable to oxidative damage [112]. Early studies demonstrated that the depletion of mitochondrial GSH (mtGSH) sensitized cells to oxidant-induced cell injury [113] and apoptosis [114–116]. Loss of mtGSH was shown to induce transition pore opening [117], inhibition of respiratory complexes [118], decreased ATP [119], and increased ROS production [120]. A proposed vicious cycle of ROS-mtDNA damage through disrupted transcription of electron transport proteins, exaggerated ROS production, and decreased mtGSH also contributed to mitochondrial failure and apoptotic initiation [121]. In colonic HT-29 cells, an added perturbation of cellular NAD+/NADH and NADP+/NADPH redox status further compromised mitochondrial respiratory function and attenuated cellular NADPH availability [122].
Matrix GSH homeostasis is controlled by cytosol-to-matrix GSH transport [42], dicarboxylate (DIC) and oxoglutarate (OGC) carrier function [42, 118, 123], and mitochondrial substrates and bioenergetics [124]. MtGSH is a major player in colonic cell survival, and its preservation is critical to mtDNA integrity [125]. In colonic HT-29 cells, mtDNA damage induced by the redox cycling agent, menadione (MQ) was preceded by mtGSH imbalance [116]. The findings that increased or decreased mtGSH import, respectively, attenuated or exacerbated MQ-mediated oxidative mtDNA damage [116] support the hypothesis that mtDNA vulnerability to oxidative stress corresponded to the mtGSH status. Whether mtGSH quenches ROS or promotes mtDNA repair or both remains an open question.
3.2 Oxidative stress, altered redox status and intestinal pathology
Excessive inflammatory cell-induced ROS generation and tissue oxidative stress are central to the onset and development of chronic gut inflammation. Elevated tissue GSSG [126, 127] and/or decreased GSH synthesis [128] have been correlated with the severity of mucosal inflammation or diminished mucosal GSH contents in IBD (intestinal bowel disease) patients [126]. It remains unclear whether intestinal oxidative stress is secondary to the inflammatory process or vice versa. Our finding that impaired mucosal GSH/GSSG preceded the onset of colonic inflammation and clinical colitis in the CD4+CD45RBhigh T-lymphocyte-reconstituted SCID mouse model of ulcerative colitis is consistent with redox-dependent mechanisms in disease development (Aw, unpublished data). Decreased disease manifestations by N-acetylcysteine support this contention [129, 130]. However, the jury is still out on the causal role of mucosal GSH/GSSG disruption in IBD pathogenesis since antioxidant therapies with vitamin C, E, and GSH were without effect in the HLA-B27 transgenic rat model of IBD [131].
Redox signaling in gut inflammation is complex and poorly understood. Attenuated lamina propria T-lymphocyte (LP-T) proliferation is associated with low intracellular GSH [132] and hyporeactivity to luminal microflora [133]. A reduced lamina propria redox milieu can signal LP-T activation [134], accomplished through recruitment of cysteine-secreting blood-borne macrophages that elevated LP-T GSH levels [132]. The perpetuation of an inflammatory phenotype within the lamina propria was thus initiated and sustained by T cell transition from a bacteria-tolerant to a reactive state [135]. Indeed, CD14+, cysteine positive macrophages and CD3+ LP-T cells with increased cellular GSH are associated with the IBD gut [132].
NF-κB activation can be attenuated by the constitutively high LP-T Trx1 status [62]. Moreover, p65 nuclear translocation was shown to be prevented by bacteria-induced NADPH oxidase-derived H2O2 via oxidation of the redox-active Cys of Ubc12, a ubiquitin-like conjugating enzyme [136]. Bacteria-derived ROS can also transiently alter the cytosolic and mitochondrial Trx status and inhibit NF-κB activity [137]. Interestingly, intestinal susceptibility or resistance to luminal pathogens is influenced by the bacterial composition. The vulnerability of C3H/HeOuJ susceptible mice to Citrobacter rodentium-induced colitis was prevented by transfer of an enriched Bacteroides microbiota from C57BL/6 resistant mice; GSH/GSSG-mediated changes in inflammatory cytokines and systemic pathogen load was suggested to play a protective role [138]. In IBD, the fragile balance among the luminal bacterial microenvironment, mucosal GSH/GSSG status, and epithelial survival were notably disrupted by increased pathogenic bacterial strains [139], suggesting that probiotic bacteria intervention could enhance epithelial GSH and attenuate mucosal inflammation [140].
A persistent state of tissue oxidative stress is a common link between chronic gut inflammation and increased cancer incidence. Modulation of GSH- and/or Trx-dependent functions were shown to enhance cancer cell proliferation, migration, metastasis, and apoptosis evasion [141, 142]. Elevated Trx1 was associated with aggressive growth of primary colorectal cancer cells [143], and is characteristic of colon cancer [144]. Consistent with Grx3 function in tumor growth and survival, protein expression levels were increased ~50-fold [145], and the knock down of Grx3 attenuated NF-κB survival signaling and inhibited tumor progression [146]. Hence, the targeting of intestinal Grx3 expression and NF-κB signaling could underpin colon cancer therapy. The finding that expression of intestinal specific Gpx2 was linked to cancer cell proliferation and early tumor growth supports its role in cancer biology [50]. Colonic susceptibility to oxidative stress and increased cancer risk is further supported by reduced or aberrant Gpx1, Gpx3, and selenoprotein P expressions [147]. Elucidating the role of Gpxs in intestinal inflammation and cancer pathogenesis has been advanced by the creation of genetic mouse model deficient in Gpx genes. Interestingly, mice containing Gpx2 gene deficiency exhibited minimal gut pathological [148] or IBD [49] symptoms. A compensatory increase in Gpx1 could explain this lack of pathology [149]. However, the Gpx1/2 double knockout mice exhibited severe ileocolitis and distal ileal inflammation [148], consistent with Gpx relevance in gut cancer biology.
4. Concluding remarks and Perspective
Loss of intestinal homeostasis is underpinned by mucosal oxidative stress and associated tissue redox imbalance. We now know that homeostatic control of the intestinal epithelial redox environment is central to the functions of the organ in nutrient digestion and absorption, stem cell proliferation, apical enterocyte apoptosis, and immune response. However, the redox mechanisms controlling the fundamental processes of crypt cell signaling and genesis or bacteria-host immunological responses that are critical to intestinal regeneration or survival within the defined microenvironment of the gut are as yet ill-defined. Recent advances in our understanding of intestinal redox biology supports a novel conceptual view that distinct distribution of individual GSH/GSSG, Trx/TrxSS and Cys/CySS redox systems within subcellular organelles can function as unique independent redox control nodes of metabolic pathways. This means that during oxidative stress, a disrupted control node would result in altered protein function through oxidation of catalytic site redox active cysteines. The precise mechanism of how redox-mediated dysregulation of the function of a single protein or protein sets collectively signals mucosal inflammation or aberrant cell turnover are unanswered questions of relevance to intestinal degenerative disorders, such as IBD and cancer.
Highlights.
Intestinal oxidative stress is associated with loss of mucosal redox balance
The Cys/CySS redox couple largely governs the luminal redox environment
The GSH/GSSG couple determines the intra-mucosal redox environment
Distinct GSH redox pools exist in subcellular organelles within cells
Phenotypic cell transition subscribes to changes in GSH and Cys redox potentials
Acknowledgments
Research in the author’s laboratory was supported by a grant from National Institute of Health, DK 44510.
Abbreviations
- Cys
cysteine
- CySS
cysteine
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- Trx
reduced thioredoxin
- TrxSS
oxidized thioredoxin
Footnotes
Conflict of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipkin M. Proliferation and differentiation of gastrointestinal cells. Physiol Rev. 1973;53:891–915. doi: 10.1152/physrev.1973.53.4.891. [DOI] [PubMed] [Google Scholar]
- 2.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141:503–19. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- 4.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–80. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouellette AJ. Paneth cell alpha-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215–29. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons DL, Spencer J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 2011;4:148–57. doi: 10.1038/mi.2010.85. [DOI] [PubMed] [Google Scholar]
- 7.Jung C, Hugot JP, Barreau F. Peyer’s Patches: The Immune Sensors of the Intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 10.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 11.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 12.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 13.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuya-Oka A, Hasebe T. Sonic hedgehog and bone morphogenetic protein-4 signaling pathway involved in epithelial cell renewal along the radial axis of the intestine. Digestion. 2008;77 (Suppl 1):42–7. doi: 10.1159/000111487. [DOI] [PubMed] [Google Scholar]
- 15.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 16.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 17.Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–9. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 18.Bjerknes M, Cheng H. Cell Lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev Biol. 2010;345:49–63. doi: 10.1016/j.ydbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–28. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–96. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 23.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 24.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–90. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab. 2010;12 (Suppl 2):116–25. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sies H. Oxidative stress: Introductory remarks. In: Sies H, editor. Oxidative stress. London: Academic Press; 1985. pp. 1–8. [Google Scholar]
- 27.Aw TY. Biliary glutathione promotes the mucosal metabolism of luminal peroxidized lipids by rat small intestine in vivo. J Clin Invest. 1994;94:1218–25. doi: 10.1172/JCI117439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aw TY, Williams MW. Intestinal absorption and lymphatic transport of peroxidized lipids in rats: effect of exogenous GSH. Am J Physiol. 1992;263:G665–72. doi: 10.1152/ajpgi.1992.263.5.G665. [DOI] [PubMed] [Google Scholar]
- 29.Aw TY, Williams MW, Gray L. Absorption and lymphatic transport of peroxidized lipids by rat small intestine in vivo: role of mucosal GSH. Am J Physiol. 1992;262:G99–106. doi: 10.1152/ajpgi.1992.262.1.G99. [DOI] [PubMed] [Google Scholar]
- 30.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 31.Aw TY, Wierzbicka G, Jones DP. Oral glutathione increases tissue glutathione in vivo. Chem Biol Interact. 1991;80:89–97. doi: 10.1016/0009-2797(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Shan XQ, Aw TY, Jones DP. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47:61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 33.Hagen TM, Jones DP. Transepithelial transport of glutathione in vascularly perfused small intestine of rat. Am J Physiol. 1987;252:G607–13. doi: 10.1152/ajpgi.1987.252.5.G607. [DOI] [PubMed] [Google Scholar]
- 34.Vincenzini MT, Iantomasi T, Favilli F. Glutathione transport across intestinal brush-border membranes: effects of ions, pH, delta psi, and inhibitors. Biochim Biophys Acta. 1989;987:29–37. doi: 10.1016/0005-2736(89)90451-3. [DOI] [PubMed] [Google Scholar]
- 35.Hagen TM, Aw TY, Jones DP. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988;34:74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- 36.Aw TY, Rhoads CA. Glucose regulation of hydroperoxide metabolism in rat intestinal cells. Stimulation of reduced nicotinamide adenine dinucleotide phosphate supply. J Clin Invest. 1994;94:2426–34. doi: 10.1172/JCI117610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–37. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon BM, Heath SH, Kim R, Suh JH, Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal. 2008;10:963–72. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bass R, Ruddock LW, Klappa P, Freedman RB. A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem. 2004;279:5257–62. doi: 10.1074/jbc.M304951200. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann JM, Riemer J. The intermembrane space of mitochondria. Antioxid Redox Signal. 2010;13:1341–58. doi: 10.1089/ars.2009.3063. [DOI] [PubMed] [Google Scholar]
- 41.Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol. 2004;39:1513–9. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–18. [PubMed] [Google Scholar]
- 43.Koehler CM, Beverly KN, Leverich EP. Redox pathways of the mitochondrion. Antioxid Redox Signal. 2006;8:813–22. doi: 10.1089/ars.2006.8.813. [DOI] [PubMed] [Google Scholar]
- 44.Markovic J, Borras C, Ortega A, Sastre J, Vina J, Pallardo FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–24. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 45.Ho YF, Guenthner TM. Isolation of liver nuclei that retain functional trans-membrane transport. J Pharmacol Toxicol Methods. 1997;38:163–8. doi: 10.1016/s1056-8719(97)00082-8. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 47.Haunhorst P, Berndt C, Eitner S, Godoy JR, Lillig CH. Characterization of the human monothiol glutaredoxin 3 (PICOT) as iron-sulfur protein. Biochem Biophys Res Commun. 2010;394:372–6. doi: 10.1016/j.bbrc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Godoy JR, Funke M, Ackermann W, Haunhorst P, Oesteritz S, Capani F, et al. Redox atlas of the mouse Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993;268:2571–6. [PubMed] [Google Scholar]
- 50.Chu FF, Esworthy RS, Doroshow JH. Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic Biol Med. 2004;36:1481–95. doi: 10.1016/j.freeradbiomed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Chu FF, Esworthy RS, Ho YS, Bermeister M, Swiderek K, Elliott RW. Expression and chromosomal mapping of mouse Gpx2 gene encoding the gastrointestinal form of glutathione peroxidase, GPX-GI. Biomed Environ Sci. 1997;10:156–62. [PubMed] [Google Scholar]
- 52.Chu FF, Esworthy RS. The expression of an intestinal form of glutathione peroxidase (GSHPx-GI) in rat intestinal epithelium. Arch Biochem Biophys. 1995;323:288–94. doi: 10.1006/abbi.1995.9962. [DOI] [PubMed] [Google Scholar]
- 53.Wingler K, Bocher M, Flohe L, Kollmus H, Brigelius-Flohe R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem. 1999;259:149–57. doi: 10.1046/j.1432-1327.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 54.Speckmann B, Bidmon HJ, Pinto A, Anlauf M, Sies H, Steinbrenner H. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J Biol Chem. 2011;286:10764–10772. doi: 10.1074/jbc.M110.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tham DM, Whitin JC, Kim KK, Zhu SX, Cohen HJ. Expression of extracellular glutathione peroxidase in human and mouse gastrointestinal tract. Am J Physiol. 1998;275:G1463–71. doi: 10.1152/ajpgi.1998.275.6.G1463. [DOI] [PubMed] [Google Scholar]
- 56.Hoensch H, Peters WH, Roelofs HM, Kirch W. Expression of the glutathione enzyme system of human colon mucosa by localisation, gender and age. Curr Med Res Opin. 2006;22:1075–83. doi: 10.1185/030079906X112480. [DOI] [PubMed] [Google Scholar]
- 57.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–48. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annual Review of Pharmacology and Toxicology. 2006;46:215–34. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 60.Gasdaska JR, Gasdaska PY, Gallegos A, Powis G. Human thioredoxin reductase gene localization to chromosomal position 12q23-q24.1 and mRNA distribution in human tissue. Genomics. 1996;37:257–9. doi: 10.1006/geno.1996.0554. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 62.Sido B, Giese T, Autschbach F, Lasitschka F, Braunstein J, Meuer SC. Potential role of thioredoxin in immune responses in intestinal lamina propria T lymphocytes. Eur J Immunol. 2005;35:408–17. doi: 10.1002/eji.200424500. [DOI] [PubMed] [Google Scholar]
- 63.Cox AG, Pearson AG, Pullar JM, Jonsson TJ, Lowther WT, Winterbourn CC, et al. Mitochondrial peroxiredoxin 3 is more resilient to hyperoxidation than cytoplasmic peroxiredoxins. Biochem J. 2009;421:51–8. doi: 10.1042/BJ20090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonsson TJ, Lowther WT. The peroxiredoxin repair proteins. Subcell Biochem. 2007;44:115–41. doi: 10.1007/978-1-4020-6051-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger HO, and protein chaperones. Antioxid Redox Signal. 2011;15:781–94. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 67.Ballatori N, Rebbeor JF. Roles of MRP2 and oatp1 in hepatocellular export of reduced glutathione. Semin Liver Dis. 1998;18:377–87. doi: 10.1055/s-2007-1007171. [DOI] [PubMed] [Google Scholar]
- 68.Dahm LJ, Jones DP. Secretion of cysteine and glutathione from mucosa to lumen in rat small intestine. Am J Physiol. 1994;267:G292–300. doi: 10.1152/ajpgi.1994.267.2.G292. [DOI] [PubMed] [Google Scholar]
- 69.Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, et al. Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- 70.Dahm LJ, Jones DP. Rat jejunum controls luminal thiol-disulfide redox. J Nutr. 2000;130:2739–45. doi: 10.1093/jn/130.11.2739. [DOI] [PubMed] [Google Scholar]
- 71.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–64. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 72.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudson VM. New insights into the pathogenesis of cystic fibrosis: pivotal role of glutathione system dysfunction and implications for therapy. Treat Respir Med. 2004;3:353–63. doi: 10.2165/00151829-200403060-00003. [DOI] [PubMed] [Google Scholar]
- 74.Aw TY. Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility. Toxicol Appl Pharmacol. 2005;204:320–8. doi: 10.1016/j.taap.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Hagen TM, Wierzbicka GT, Bowman BB, Aw TY, Jones DP. Fate of dietary glutathione: disposition in the gastrointestinal tract. Am J Physiol. 1990;259:G530–5. doi: 10.1152/ajpgi.1990.259.4.G530. [DOI] [PubMed] [Google Scholar]
- 76.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 77.Park Y, Ziegler TR, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, et al. Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J Nutr. 2010;140:760–5. doi: 10.3945/jn.109.116764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 79.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 80.Lash LH, Jones DP. Characteristics of cysteine uptake in intestinal basolateral membrane vesicles. Am J Physiol. 1984;247:G394–401. doi: 10.1152/ajpgi.1984.247.4.G394. [DOI] [PubMed] [Google Scholar]
- 81.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, et al. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–23. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 82.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 83.Scharrer E, Senn E, Wolffram S. Stimulation of mucosal uptake of selenium from selenite by some thiols at various sites of rat intestine. Biol Trace Elem Res. 1992;33:109–20. doi: 10.1007/BF02783999. [DOI] [PubMed] [Google Scholar]
- 84.Snary D, Allen A, Pain RH. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 1970;40:844–51. doi: 10.1016/0006-291x(70)90980-0. [DOI] [PubMed] [Google Scholar]
- 85.Neil MW. The absorption of cystine and cysteine from rat small intestine. Biochem J. 1959;71:118–24. doi: 10.1042/bj0710118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mannery YO, Ziegler TR, Hao L, Shyntum Y, Jones DP. Characterization of apical and basal thiol-disulfide redox regulation in human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G523–30. doi: 10.1152/ajpgi.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 88.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 90.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 2004;229:586–97. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 91.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 92.Weisiger RA, Pinkus LM, Jakoby WB. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980;29:2885–7. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- 93.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta. 2005;1725:201–12. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. Faseb J. 2007;21:1699–706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 95.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–11. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–8. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 97.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–9. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 98.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 99.Aw TY. Cellular redox: a modulator of intestinal epithelial cell proliferation. News Physiol Sci. 2003;18:201–4. doi: 10.1152/nips.01448.2003. [DOI] [PubMed] [Google Scholar]
- 100.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 101.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radical Biology and Medicine. 2002;33:1499–506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 102.Jonas CR, Gu LH, Nkabyo YS, Mannery YO, Avissar NE, Sax HC, et al. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1421–9. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- 103.Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY. Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif. 2002;35:117–29. doi: 10.1046/j.1365-2184.2002.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289:G70–8. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 105.Gotoh Y, Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY. Lipid peroxide-induced redox imbalance differentially mediates CaCo-2 cell proliferation and growth arrest. Cell Prolif. 2002;35:221–35. doi: 10.1046/j.1365-2184.2002.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. Faseb J. 2001;15:2131–9. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- 107.Tabata K, Johnson LR. Ornithine decarboxylase and mucosal growth in response to feeding. Am J Physiol. 1986;251:G270–4. doi: 10.1152/ajpgi.1986.251.2.G270. [DOI] [PubMed] [Google Scholar]
- 108.Iwakiri R, Gotoh Y, Noda T, Sugihara H, Fujimoto K, Fuseler J, et al. Programmed cell death in rat intestine: effect of feeding and fasting. Scand J Gastroenterol. 2001;36:39–47. doi: 10.1080/00365520150218048. [DOI] [PubMed] [Google Scholar]
- 109.Tsunada S, Iwakiri R, Fujimoto K, Aw TY. Chronic lipid hydroperoxide stress suppresses mucosal proliferation in rat intestine: potentiation of ornithine decarboxylase activity by epidermal growth factor. Dig Dis Sci. 2003;48:2333–41. doi: 10.1023/b:ddas.0000007872.66693.6c. [DOI] [PubMed] [Google Scholar]
- 110.Tsunada S, Iwakiri R, Noda T, Fujimoto K, Fuseler J, Rhoads CA, et al. Chronic exposure to subtoxic levels of peroxidized lipids suppresses mucosal cell turnover in rat small intestine and reversal by glutathione. Dig Dis Sci. 2003;48:210–22. doi: 10.1023/a:1021775524062. [DOI] [PubMed] [Google Scholar]
- 111.Tian J, Washizawa N, Gu LH, Levin MS, Wang L, Rubin DC, et al. Stimulation of colonic mucosal growth associated with oxidized redox status in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1081–91. doi: 10.1152/ajpregu.00050.2006. [DOI] [PubMed] [Google Scholar]
- 112.Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Genet. 2005;6:815–25. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S3–6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 114.Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Circu ML, Aw TY. Redox biology of the intestine. Free Radic Res. 2011;45:1245–66. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Circu ML, Moyer MP, Harrison L, Aw TY. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med. 2009;47:1190–8. doi: 10.1016/j.freeradbiomed.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kamga CK, Zhang SX, Wang Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am J Physiol Cell Physiol. 2010;299:C497–505. doi: 10.1152/ajpcell.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–78. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, et al. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–36. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst) 2006;5:145–52. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 122.Circu ML, Maloney RE, Aw TY. Disruption of pyridine nucleotide redox status during oxidative challenge at normal and low-glucose states: implications for cellular adenosine triphosphate, mitochondrial respiratory activity, and reducing capacity in colon epithelial cells. Antioxid Redox Signal. 2011;14:2151–62. doi: 10.1089/ars.2010.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coll O, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 124.Martensson J, Lai JC, Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990;87:7185–9. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Green RM, Graham M, O’Donovan MR, Chipman JK, Hodges NJ. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–90. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- 126.Iantomasi T, Marraccini P, Favilli F, Vincenzini MT, Ferretti P, Tonelli F. Glutathione metabolism in Crohn’s disease. Biochem Med Metab Biol. 1994;53:87–91. doi: 10.1006/bmmb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 127.Holmes EW, Yong SL, Eiznhamer D, Keshavarzian A. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43:1088–95. doi: 10.1023/a:1018899222258. [DOI] [PubMed] [Google Scholar]
- 128.Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–92. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–15. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsunada S, Iwakiri R, Ootani H, Aw TY, Fujimoto K. Redox imbalance in the colonic mucosa of ulcerative colitis. Scand J Gastroenterol. 2003;38:1002–3. doi: 10.1080/00365520310005055. [DOI] [PubMed] [Google Scholar]
- 131.Schepens MA, Vink C, Schonewille AJ, Roelofs HM, Brummer RJ, van der Meer R, et al. Supplemental antioxidants do not ameliorate colitis development in HLA-B27 transgenic rats despite extremely low glutathione levels in colonic mucosa. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21584. in press. [DOI] [PubMed] [Google Scholar]
- 132.Sido B, Lasitschka F, Giese T, Gassler N, Funke B, Schroder-Braunstein J, et al. A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation. Gastroenterology. 2008;134:179–91. doi: 10.1053/j.gastro.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Qiao L, Schurmann G, Meuer SC, Wallich R, Schirren A, Autschbach F. Regulation of T cell reactivities by intestinal mucosa. Adv Exp Med Biol. 1995;371A:31–4. doi: 10.1007/978-1-4615-1941-6_6. [DOI] [PubMed] [Google Scholar]
- 134.Yan Z, Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–66. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reyes BM, Danese S, Sans M, Fiocchi C, Levine AD. Redox equilibrium in mucosal T cells tunes the intestinal TCR signaling threshold. J Immunol. 2005;175:2158–66. doi: 10.4049/jimmunol.175.4.2158. [DOI] [PubMed] [Google Scholar]
- 136.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. Embo J. 2007;26:4457–66. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–46. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00509.2010. [DOI] [PubMed] [Google Scholar]
- 139.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–93. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, et al. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Colorectal Dis. 2006;21:737–46. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- 141.Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–68. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 142.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29:312–22. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 143.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, et al. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 144.Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–66. [PubMed] [Google Scholar]
- 145.Ohayon A, Babichev Y, Galperin M, Altman A, Isakov N. Widespread expression of PICOT in mouse and human tissues with predominant localization to epithelium. J Histochem Cytochem. 2010;58:799–806. doi: 10.1369/jhc.2010.956532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest. 2011;121:212–25. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murawaki Y, Tsuchiya H, Kanbe T, Harada K, Yashima K, Nozaka K, et al. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–30. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 148.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–55. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 149.Florian S, Krehl S, Loewinger M, Kipp A, Banning A, Esworthy S, et al. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic Biol Med. 2010;49:1694–702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]